Thinning Promotes Soil Phosphorus Bioavailability in Short-Rotation and High-Density Eucalyptus grandis × E. urophylla Coppice Plantation in Guangxi, Southern China
Abstract
:1. Introduction
2. Materials and Methods
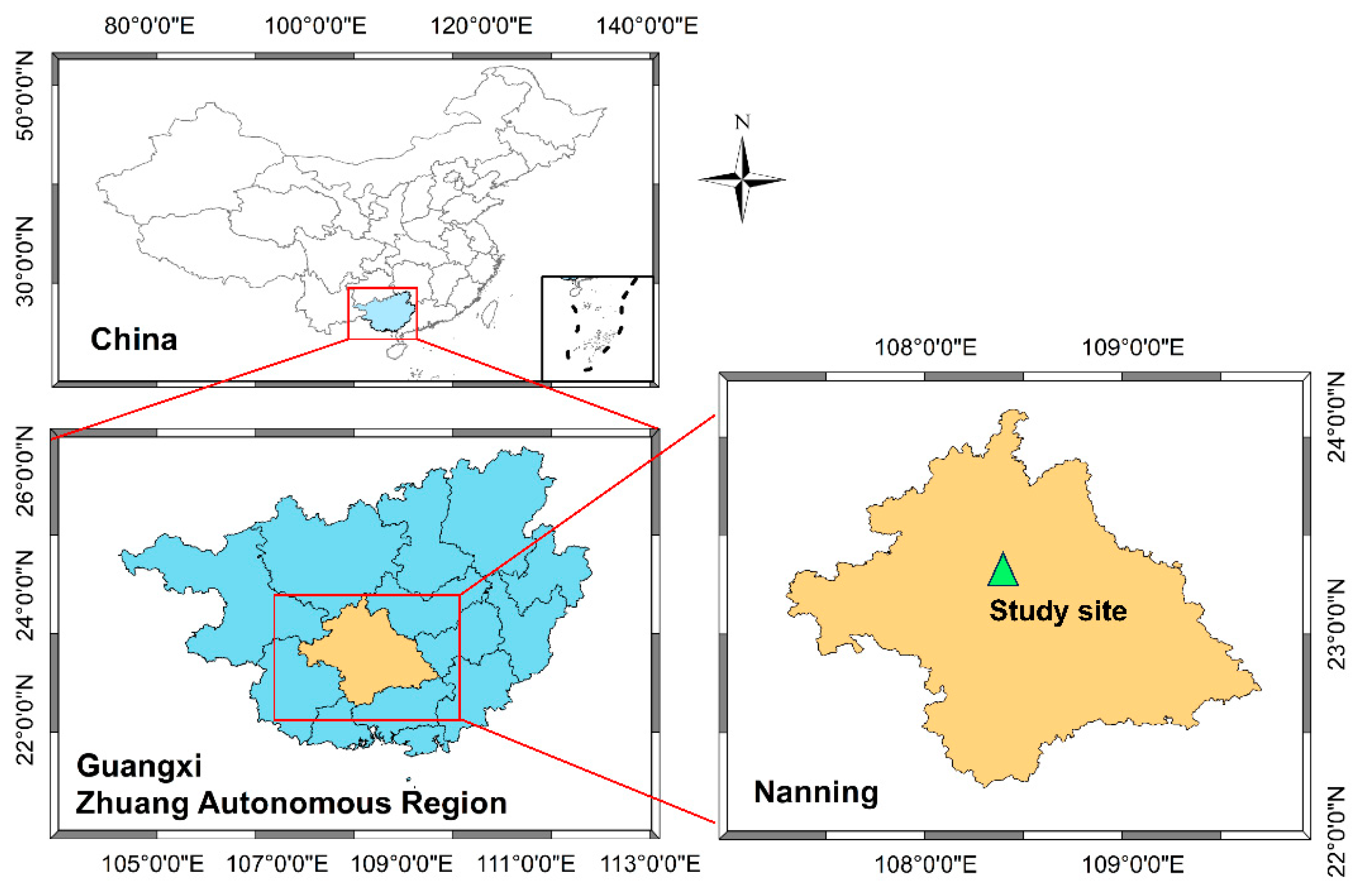
2.1. Study Site
2.2. Experimental Design
2.3. Sampling and Analysis
2.4. Statistical Analysis
3. Results
3.1. Response of Soil Nutrients Content to Thinning Intensity in E. urophylla × E. grandis Coppice Stands
3.2. Response of Soil Microbial Biomass to Thinning Intensity in E. urophylla × E. grandis Coppice Stands
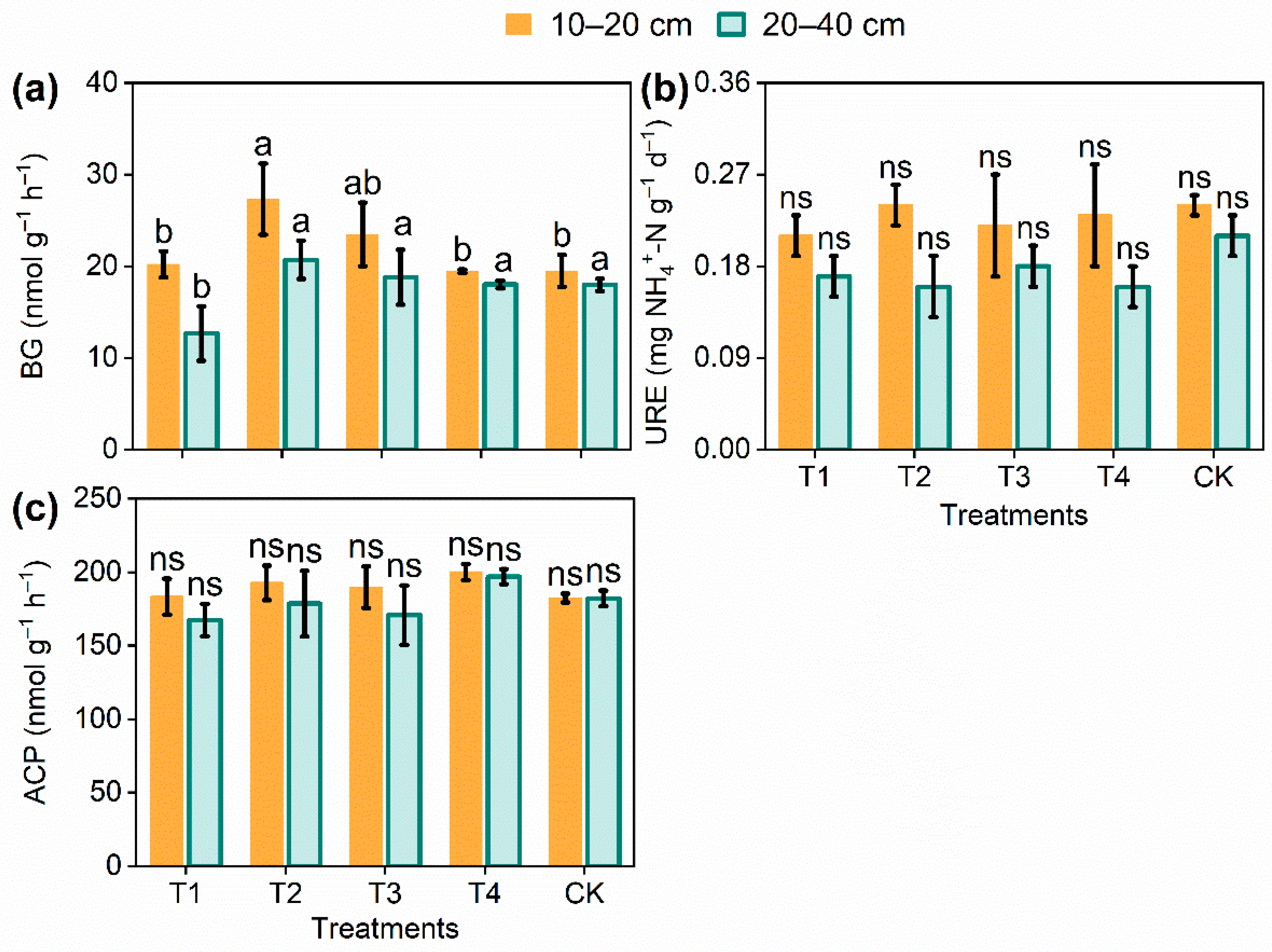
3.3. Response of Soil Enzyme Activities to Thinning Intensity in E. urophylla × E. grandis Coppice Stands
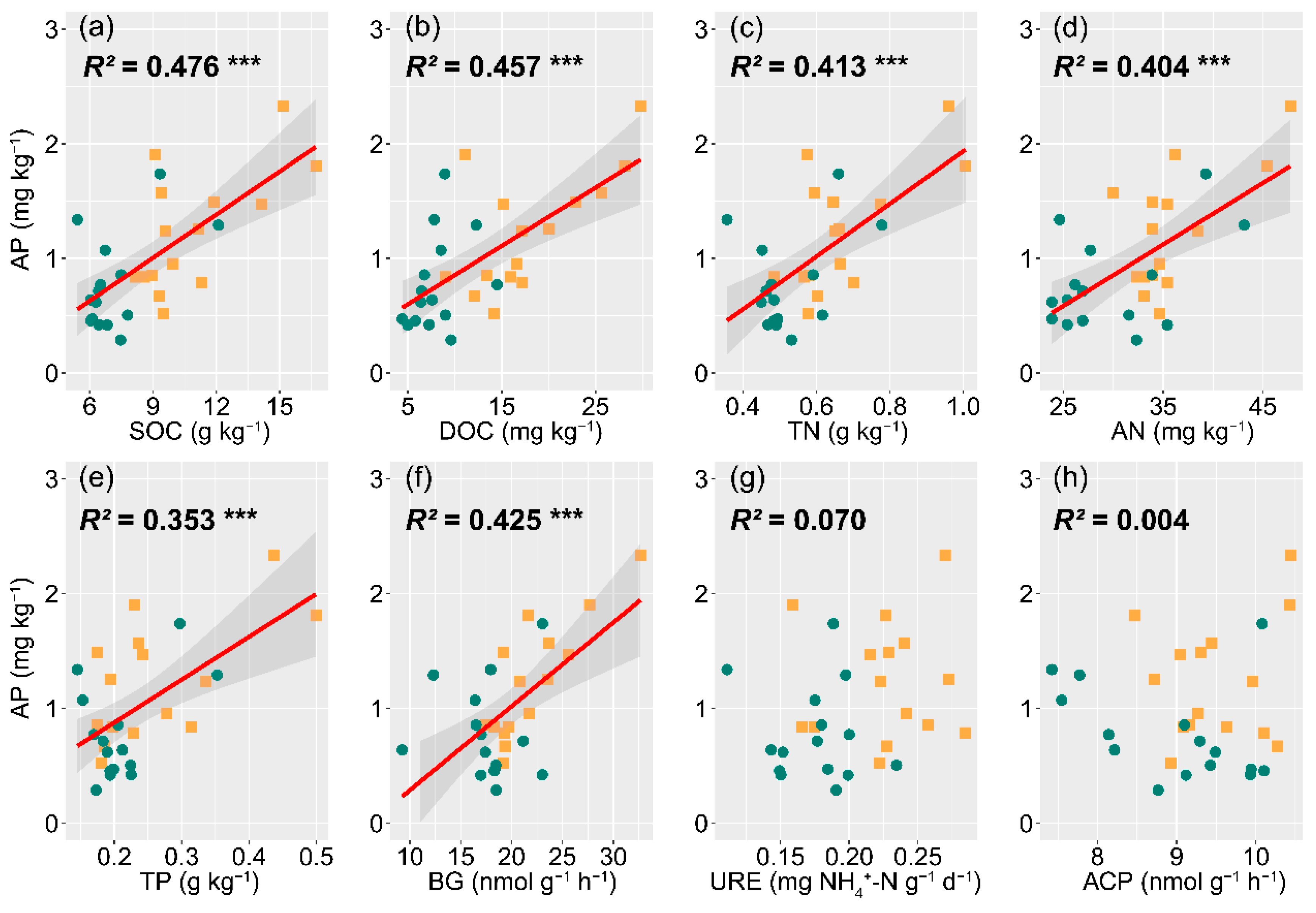
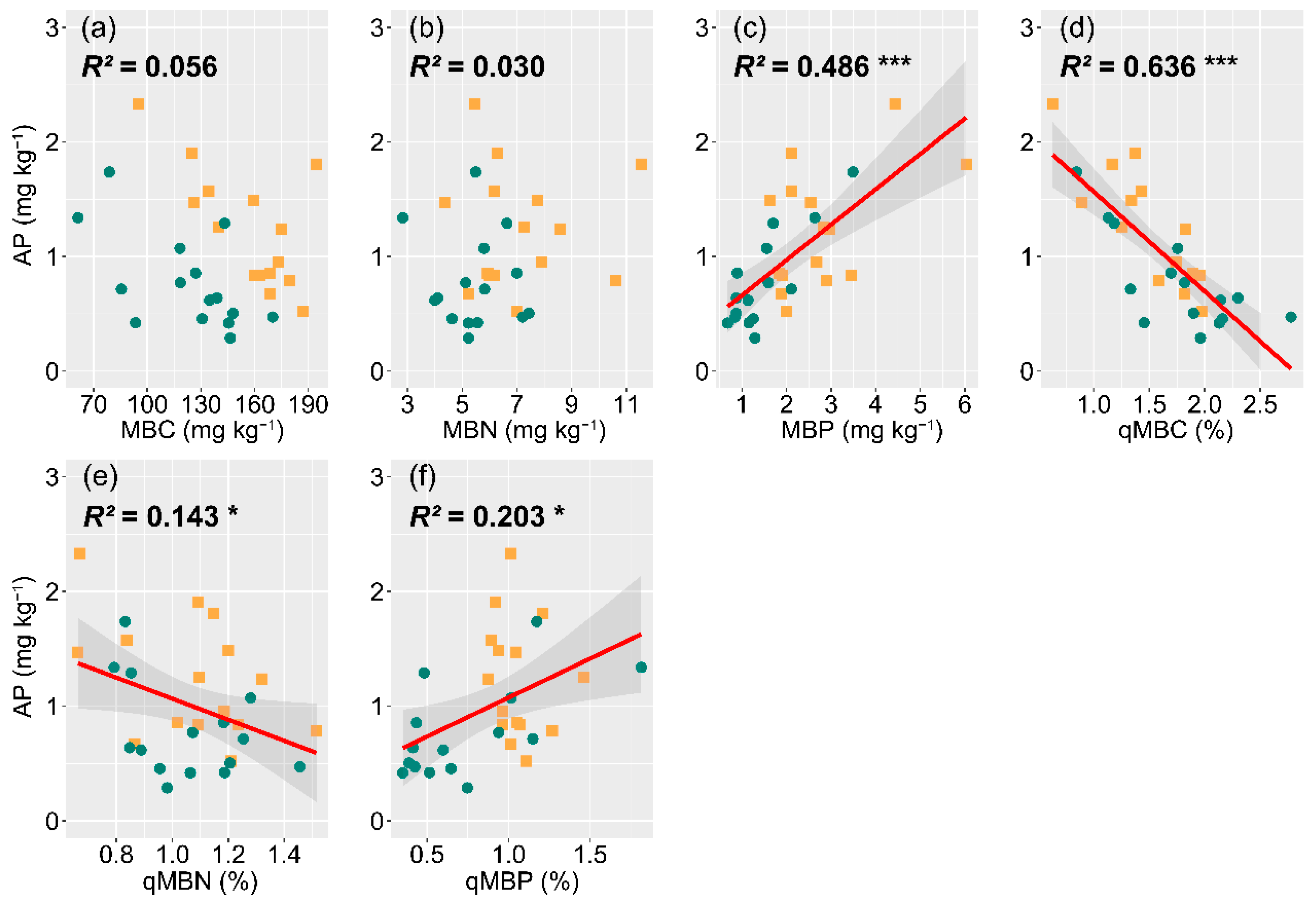
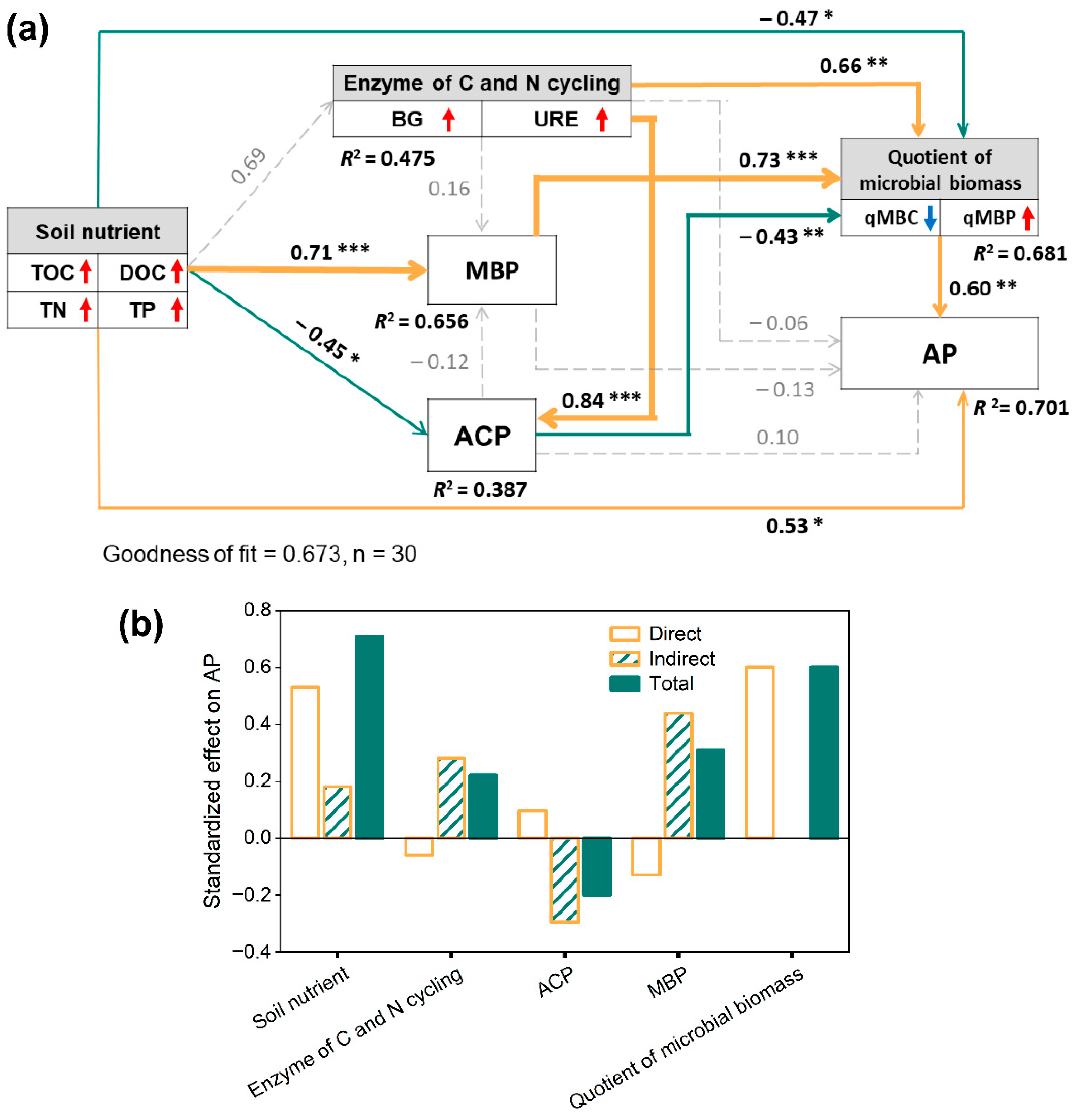
3.4. The Influence of Soil Nutrients, Microbial Biomass, and Enzyme Activity on P Availability
4. Discussion
4.1. Soil Nutrients of Different Intensity Thinned E. grandis × E. urophylla Coppice Plantations
4.2. Soil Microbial Biomass and Enzyme Activity of Different Intensity Thinned E. grandis × E. urophylla Coppice Plantations
4.3. Function of Soil Total Nutrient and DOC on P Bioavailability in E. grandis × E. urophylla Coppice Forest
4.4. Effects of Soil Microbial Biomass Turnover and Enzyme Activity on P Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.R.; Wen, M.J.; Deng, Y.S.; Su, X.L.; Jiang, D.H.; Wang, G.; Chen, Y.W.; Chen, G.J.; Yu, S.F. Occurrence patterns of black water and its impact on fish in cutover areas of Eucalyptus plantations. Sci. Total Environ. 2019, 693, 133393. [Google Scholar] [CrossRef] [PubMed]
- Lemessa, D.; Mewded, B.; Legesse, A.; Atinfau, H.; Alemu, S.; Maryo, S.; Tilahun, S. Do Eucalyptus plantation forests support biodiversity conservation. For. Ecol. Manag. 2022, 523, 120492. [Google Scholar] [CrossRef]
- Wen, Y.G.; Ye, D.; Chen, F.; Liu, S.R.; Liang, H.W. The changes of understory plant diversity in continuous cropping system of Eucalyptus plantations, South China. J. For. Res. 2010, 15, 252–258. [Google Scholar] [CrossRef]
- Wang, J.Y.; Deng, Y.S.; Li, D.Y.; Liu, Z.F.; Wen, L.L.; Huang, Z.G.; Jiang, D.H.; Lu, Y.P. Soil aggregate stability and its response to overland flow in successive Eucalyptus plantations in subtropical China. Sci. Total Environ. 2022, 807, 151000. [Google Scholar] [CrossRef]
- Xu, Y.X.; Li, C.; Zhu, Y.L.; Wang, Z.C.; Zhu, W.K.; Wu, L.C.; Du, A.P. The shifts in soil microbial community and association network induced by successive planting of Eucalyptus plantations. For. Ecol. Manag. 2022, 505, 119877. [Google Scholar] [CrossRef]
- Li, X.; Ye, D.; Liang, H.; Zhu, H.; Qin, L.; Zhu, Y.; Wen, Y. Effects of successive rotation regimes on carbon stocks in Eucalyptus plantations in subtropical China measured over a full rotation. PLoS ONE 2015, 10, e0132858. [Google Scholar] [CrossRef]
- Laclau, J.P.; Deleporte, P.; Ranger, J.; Bouillet, J.P.; Kazotti, G. Nutrient dynamics throughout the rotation of Eucalyptus clonal stands in Congo. Ann. Bot. 2003, 91, 879–892. [Google Scholar] [CrossRef]
- Foltran, E.C.; Rocha, J.H.T.; Bazani, J.H.; Gonçalvesa, J.L.M.; Rodriguesd, M.; Pavinatod, P.; Valdugae, G.R.; Errof, J.; Garcia-Mina, J.M. Phosphorus pool responses under different P inorganic fertilizers for a eucalyptus plantation in a loamy Oxisol. For. Ecol. Manag. 2019, 435, 170–179. [Google Scholar] [CrossRef]
- McMahona, D.E.; Vergützb, L.V.; Valadares, S.V.; Silvab, I.R.D.; Jacksona, R.B. Soil nutrient stocks are maintained over multiple rotations in Brazilian Eucalyptus plantations. For. Ecol. Manag. 2019, 448, 364–375. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chiang, J.M.; McEwan, R.W.; Lin, T.C. Decadal effects of thinning on understory light environments and plant community structure in a subtropical forest. Ecosphere 2018, 9, e02464. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Li, J.L.; Yu, S.C.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Page-Dumroese, D.; Lv, R.H.; Wang, W.W.; Li, G.L.; Liu, Y. Interaction of initial litter quality and thinning intensity on litter decomposition rate, nitrogen accumulation and release in a pine plantation. Silva Fenn. 2014, 48, 1211. [Google Scholar] [CrossRef]
- Çömez, A.; Güner, Ş.T.; Tolunay, D. The effect of stand structure on litter decomposition in Pinus sylvestris L. stands in Turkey. Anna. For. Sci. 2021, 78, 19. [Google Scholar] [CrossRef]
- Camenzind, T.; Hattenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillic, A. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Qiu, X.C.; Wang, H.B.; Peng, D.L.; Liu, X.; Yang, F.; Lia, Z.; Cheng, S. Thinning drives C:N:P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Forrester, D.I. Growth responses to thinning, pruning and fertiliser application in Eucalyptus plantations: A review of their production ecology and interactions. For. Ecol. Manag. 2018, 310, 336–347. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; He, Z.; Wang, R.W.; Wu, P.F.; Ma, X.Q. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ. Sci. Pollut. Res. 2016, 23, 24135. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y.; Wang, N.; Zeng, L.X.; Lei, L.; Wang, X.R. Labile organic carbon pools and enzyme activities of Pinus massoniana plantation soil as affected by understory vegetation removal and thinning. Sci. Rep. 2018, 8, 573. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chang, S.X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef]
- Han, T.T.; Ren, H.; Hui, D.F.; Wang, J.; Lu, H.F.; Liu, Z.F. Light availability, soil phosphorus and different nitrogen forms negatively affect the functional diversity of subtropical forests. Glob. Ecol. Conserv. 2020, 24, e01334. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter—The unbutton model. Soil Biol. Biochem. 2015, 84, 168–176. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Xu, D.; Dell, B.; Malajczuk, N.; Gong, M. Effects of P fertilisation and ectomycorrhizal fungal inoculation on early growth of eucalypt plantations in southern China. Plant Soil 2001, 233, 47–57. [Google Scholar] [CrossRef]
- Kurth, J.K.; Albrecht, M.; Karsten, U.; Glaser, K.; Schloter, M.; Schulz, S. Correlation of the abundance of bacteria catalyzing phosphorus and nitrogen turnover in biological soil crusts of temperate forests of Germany. Biol. Fertil. Soils 2021, 57, 179–192. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z.Q. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef]
- Reichart, T.; Rammig, A.; Fuchslueger, L.; Lugli, L.F.; Quesada, C.A.; Fleischer, K. Plant phosphorus-use and -acquisition strategies in Amazonia. New Phytol. 2022, 234, 1126–1143. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Wang, J.P.; Chen, G.R.; Ji, S.H.; Zhong, Y.Q.; Zhao, Q.; He, Q.Q.; Wu, Y.H.; Bing, H.J. Close relationship between the gene abundance and activity of soil extracellular enzyme: Evidence from a vegetation restoration chronosequence. Soil Biol. Biochem. 2023, 177, 108929. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.Y.; Bao, W.K.; Tian, G.L. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, W.; Chen, Y. Heavy thinning temporally reduced soil carbon storage by intensifying soil microbial phosphorus limitation. Plant Soil 2023, 484, 33–48. [Google Scholar] [CrossRef]
- Zhou, X.G.; Zhu, H.G.; Wen, Y.G.; Goodale, U.M.; Zhu, Y.L.; Yu, S.F.; Li, C.T.; Li, X.Q. Intensive management and declines in soil nutrients lead to serious exotic plant invasion in Eucalyptus plantations under successive short-rotation regimes. Land Degrad. Dev. 2022, 31, 297–310. [Google Scholar] [CrossRef]
- Deng, X.; Cheng, F.; Li, M.; He, P.; Shen, L.; Liu, H.Y. Effect of different decay classes of Eucalyptus stump substrates on microbial resource limitation and carbon-use efficiency. Plant Soil 2022, 478, 651–669. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wu, P.N.; Qiao, Y.B.; Li, Y.M.; Liu, S.M.; Gao, C.K.; Liu, C.S.; Shao, J.; Yu, H.L.; Zhao, Z.H.; et al. The potential for soil C sequestration and N fixation under different planting patterns depends on the carbon and nitrogen content and stability of soil aggregates. Sci. Total Environ. 2023, 897, 165430. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Watanade, F.S.; Olsen, S.R. Test of an ascorbic acid methods for determination phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Wu, J.; He, Z.L.; Wei, W.X.; O’Donnell, A.G.; Syers, J.K. Quantifying microbial biomass phosphorus in acid soil. Bio. Fert. Soil 2000, 32, 500–507. [Google Scholar] [CrossRef]
- Buttler, A.; Teuscher, R.; Deschamps, N.; Gavazov, K.; Bragazza, L.; Mariotte, P.; Schlaepfer, R.; Jassey, V.E.J.; Freund, L.; Cuartero, J.; et al. Impacts of snow-farming on alpine soil and vegetation: A case study from the Swiss Alps. Sci. Total Environ. 2023, 903, 166225. [Google Scholar] [CrossRef]
- Saiya-Corka, K.R.; Sinsabaugha, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Fei, Y.F.; Huang, S.Y.; Zhang, H.B.; Tong, Y.Z.; Wena, D.S.; Xia, X.Y.; Han Wang, H.; Luo, Y.M.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Tian, S.Y.; Zhu, B.J.; Yin, R.; Wang, M.W.; Jiang, Y.J.; Zhang, C.Z.; Li, D.M.; Chen, X.Y.; Kardol, P.; Liu, M.J. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.K.; Zhou, Z.H. Thinning promotes the nitrogen and phosphorous cycling in forest soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Guan, D.X.; Li, W.B.; Sun, D.; Jin, C.G.; Yuan, F.H.; Wang, A.Z.; Wu, J.B. The effects of forest thinning on soil carbon stocks and dynamics: A meta-analysis. For. Ecol. Manag. 2018, 429, 36–43. [Google Scholar] [CrossRef]
- Melilloa, J.M.; Butlera, S.; Johnson, J.; Tang, J. Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proc. Natl. Acad. Sci. USA 2011, 108, 9508–9512. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Jin, Y.; Sun, Z.H. Impacts of thinning on soil carbon and nutrients and related extracellular enzymes in a larch plantation. For. Ecol. Manag. 2019, 450, 117523. [Google Scholar] [CrossRef]
- Xu, Y.X.; Du, A.P.; Wang, Z.C.; Zhu, W.K.; Li, C.; Wu, L.C. Effects of different rotation periods of Eucalyptus plantations on soil physiochemical properties, enzyme activities, microbial biomass and microbial community structure and diversity. For. Ecol. Manag. 2020, 456, 117683. [Google Scholar] [CrossRef]
- Liu, J.B.; Chen, J.; Chen, G.S.; Guo, J.F.; Li, Y.Q. Enzyme stoichiometry indicates the variation of microbial nutrient requirements at different soil depths in subtropical forests. PLoS ONE 2020, 15, e0220599. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Zeng, S.; Wu, D.M.; Jacobs, D.F.; Sloan, J.L. Soil pH, organic matter, and nutrient content change with the continuous cropping of Cunninghamia lanceolata plantations in South China. J. Soils Sediments 2017, 17, 2230–2238. [Google Scholar] [CrossRef]
- Yang, H.J.; Pan, C.; Wu, Y.; Qing, S.Q.; Wang, Z.B.; Wang, D.H. Response of understory plant species richness and tree regeneration to thinning in Pinus tabuliformis plantations in northern China. For. Ecosyst. 2023, 10, 100105. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Liu, H.M.; Huang, K.; Yang, Q.S.; Lu, R.L.; Yan, L.M.; Wang, X.H.; Xia, J.Y. Depth-dependent soil C-N-P stoichiometry in a mature subtropical broadleaf forest. Geoderma 2020, 370, 114357. [Google Scholar] [CrossRef]
- Berhe, A.A.; Barnes, R.T.; Six, J.; Marín-Spiotta, E. Role of soil erosion in biogeochemical cycling of essential elements: Carbon, nitrogen, and phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P.Q. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Lu, J.L.; Jia, P.; Feng, S.W.; Wang, Y.T.; Zheng, J.; Ou, S.N.; Wu, Z.H.; Liao, B.; Shu, W.S.; Liang, J.L. Remarkable effects of microbial factors on soil phosphorus bioavailability: A country-scale study. Glob. Chang. Biol. 2022, 28, 4459–4471. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Wang, B.; Wu, Y.; Chen, D.M. Regional-scale evidence that determinants of soil microbial biomass and N mineralization depend on sampling depth and layer on the Mongolian Plateau. Catena 2022, 213, 106180. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, C.X.; Ma, L.L.; Chu, G.W.; Liu, Z.F.; Tang, X.L. Plant community and soil properties drive arbuscular mycorrhizal fungal diversity: A case study in tropical forests. Soil Ecol. Lett. 2021, 3, 52–62. [Google Scholar] [CrossRef]
- Taki, H.; Inoue, T.; Tanaka, H. Responses of community structure, diversity, and abundance of understory plants and insect assemblages to thinning in plantations. For. Ecol. Manag. 2010, 259, 607–613. [Google Scholar] [CrossRef]
- See, C.R.; Keller, A.B.; Hobbie, S.E.; Pett-Ridge, J. Hyphae move matter and microbes to mineral microsites: Integrating the hyphosphere into conceptual models of soil organic matter stabilization. Glob. Chang. Biol. 2022, 28, 2527–2540. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.P.; Souza, R.S.C.; Jose, J.; Gerhardt, I.R.; Dante, R.A.; Mukherjee, S.; Huntemann, M.; Kyrpides, N.C.; Carazzolle, M.F.; Arruda, P. Plant microbiomes harbor potential to promote nutrient turnover in impoverished substrates of a Brazilian biodiversity hotspot. ISME J. 2022, 17, 354–370. [Google Scholar] [CrossRef]
- Xu, X.F.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Chu, S.S.; Ouyang, J.H.; Liao, D.D.; Zhou, Y.D.; Liu, S.S.; Shen, D.C.; Wei, X.H.; Zeng, S.C. Effects of enriched planting of native tree species on surface water flow, sediment, and nutrient losses in a Eucalyptus plantation forest in southern China. Sci. Total Environ. 2019, 675, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Zheng, H.; Zhang, K.; Ouyang, Z.Y.; Li, H.L.; Wu, B.; Shi, Q. Soil microbial community structure and function responses to successive planting of Eucalyptus. J. Environ. Sci. 2013, 25, 2102–2111. [Google Scholar] [CrossRef]
- Chen, X.L.; Chen, H.Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q.W. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, Z.J.; Zong, Y.Y.; Li, W.Q.; Chen, F.S.; Wang, G.G.; Li, J.J.; Fang, X.M. Mixing with coniferous tree species alleviates rhizosphere soil phosphorus limitation of broad-leaved trees in subtropical plantations. Soil Biol. Biochem. 2022, 175, 108853. [Google Scholar] [CrossRef]
- Lin, Y.; Xia, C.K.; Wu, G.Y.; Wang, F.C.; Wang, S.N.; Liu, Y.Q.; Chen, F.S. Replanting of broadleaved trees alters internal nutrient cycles of native and exotic pines in subtropical plantations of China. For. Ecosyst. 2022, 9, 100067. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Zhou, J.J.; Huang, G.H.; Yang, G.; Liu, G.F.; Liang, K.N. Effects of thinning intensity on soil quality and growth of teak plantation. For. Res. 2021, 34, 127–134. (In Chinese) [Google Scholar]
- Prommer, J.; Wanek, W.; Hofhansl, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C.; Sassmann, S.; Kitzle, B.; Soja, G.; et al. Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS ONE 2014, 9, e86388. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Chenua, C.; Angersb, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Liu, Q.; Guo, J.H.; Zhou, Q.C. Progress in the interaction of dissolved organic matter and microbes (1991–2020): A bibliometric review. Environ. Sci. Pollut. R. 2022, 29, 16817–16829. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, Z.J.; Liu, J.M.; Li, Q.H.; Yang, X.Y. Long-term effects of straw and manure on crop micronutrient nutrition under a wheat-maize cropping system. J. Plant Nutr. 2015, 38, 742–753. [Google Scholar] [CrossRef]
- Bolan, N.S.; Naidu, R.; Mahimairaja, S.; Baskaran, S. Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol. Fertil. Soils 1994, 18, 311–319. [Google Scholar] [CrossRef]
- Achat, D.L.; Morel, C.; Bakker, M.R.; Augusto, L.; Pellerin, S.; Gallet-Budynek, A.; Gonzalez, M. Assessing turnover of microbial biomass phosphorus: Combination of an isotopic dilution method with a mass balance model. Soil Biol. Biochem. 2010, 42, 2231–2240. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, B.; Zeng, H. Soil extracellular enzyme stoichiometry reflects the unique habitat of karst tiankeng and helps to alleviate the P-limitation of soil microbes. Ecol. Indic. 2022, 144, 109552. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhao, M.Y.; Yan, Z.B.; Yang, Y.H.; Niclas, K.J.; Huang, H.; Mipan, T.D.; He, X.J.; Hu, H.F.; Wright, J. Global patterns and predictors of soil microbial biomass carbon, nitrogen, and phosphorus in terrestrial ecosystems. Catena 2022, 211, 1060377. [Google Scholar] [CrossRef]
- Insam, H.; Domsch, K.H. Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microb. Ecol. 1988, 15, 177–188. [Google Scholar] [CrossRef]
- Kaschuk, G.; Alberton, Q.; Hungria, M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem. 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.L.; Yang, F. Critical review on soil phosphorus migration and transformation under freezing-thawing cycles and typical regulatory measurements. Sci. Total Environ. 2021, 751, 141614. [Google Scholar] [CrossRef]
- Zhang, D.S.; Kuzyakov, Y.; Zhu, H.T.; Alharbi, H.; Li, H.B.; Rengel, Z. Increased microbial biomass and turnover underpin efficient phosphorus acquisition by Brassica chinensis. Soil Tillage Res. 2022, 223, 105492. [Google Scholar] [CrossRef]
- Zhu, H.; Bing, H.J.; Wu, Y.H.; Sun, H.Y.; Zhou, J. Low molecular weight organic acids regulate soil phosphorus availability in the soils of subalpine forests, eastern Tibetan Plateau. Catena 2021, 203, 105328. [Google Scholar] [CrossRef]
- Hacker, M.; Ebeling, A.; Gessler, A.; Gleixner, G.; Macé, O.G.; Kroon, H.D.; Lange, M.; Mommer, L.; Eisenhauer, N.; Ravenek, J.; et al. Plant diversity shapes microbe-rhizosphere effects on P mobilisation from organic matter in soil. Ecol. Lett. 2015, 18, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, P.; Kong, C.H. Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 2009, 45, 436–440. [Google Scholar] [CrossRef]
- Vazqueza, E.; Benitoa, M.; Masaguera, A.; Espejo, R.; Díaz-Pinés, E.; Teutscherov, N. Long-term effects of no tillage and Ca-amendment on the activity of soil proteases and β-glucosidase in a Mediterranean agricultural field. Eur. J. Soil Biol. 2019, 95, 103135. [Google Scholar] [CrossRef]

| Indexes | T1 | T2 | T3 | T4 | CK |
|---|---|---|---|---|---|
| Slop gradient (°) | 25 | 24 | 23 | 27 | 25 |
| Slop aspect | Northwest | Northwest | Northwest | Northwest | Northwest |
| Elevation (m) | 302.1 | 299.8 | 304.5 | 303.9 | 304.6 |
| Stand density (tree ha−1) | 283 | 566 | 833 | 1116 | 1665 |
| Canopy density | 0.35 | 0.55 | 0.70 | 0.82 | 0.96 |
| Average tree height (m) | 26.23 | 23.87 | 23.58 | 25.37 | 24.67 |
| Average diameter at breast height (cm) | 24.17 | 19.53 | 19.60 | 19.43 | 16.77 |
| Stock volume (m3 ha) | 113.86 | 148.41 | 199.56 | 259.27 | 365.60 |
| Soil bulk density (g cm−3) | 1.28 | 1.31 | 1.38 | 1.41 | 1.47 |
| Soil total porosity (%) | 45.56 | 43.43 | 41.69 | 40.42 | 37.14 |
| Soil water content (%) | 14.14 | 15.18 | 12.08 | 11.96 | 12.60 |
| Soil pH | 4.29 | 4.09 | 4.17 | 4.11 | 4.25 |
| Indexes | Layers (cm) | T1 | T2 | T3 | T4 | CK | F | p |
|---|---|---|---|---|---|---|---|---|
| SOC (g kg−1) | 0–20 | 11.62 ± 3.62 ns | 12.90 ± 2.51 ns | 10.72 ± 1.18 ns | 9.59 ± 1.30 ns | 9.46 ± 0.42 ns | 0.917 | 0.491 |
| 20–40 | 8.55 ± 2.58 ns | 7.06 ± 1.66 ns | 6.56 ± 0.13 ns | 6.16 ± 0.10 ns | 7.37 ± 1.40 ns | 0.866 | 0.516 | |
| DOC (mg kg−1) | 0–20 | 20.40 ± 5.48 ns | 23.53 ± 5.17 ns | 18.01 ± 5.05 ns | 12.79 ± 3.37 ns | 17.74 ± 1.38 ns | 1.733 | 0.219 |
| 20–40 | 8.89 ± 2.43 ns | 7.75 ± 1.00 ns | 10.11 ± 3.16 ns | 5.54 ± 0.83 ns | 7.87 ± 2.05 ns | 1.307 | 0.332 | |
| TN (g kg−1) | 0–20 | 0.74 ± 0.19 ns | 0.78 ± 0.15 ns | 0.63 ± 0.04 ns | 0.60 ± 0.09 ns | 0.61 ± 0.04 ns | 0.987 | 0.457 |
| 20–40 | 0.62 ± 012 ns | 0.49 ± 0.13 ns | 0.47 ± 0.01 ns | 0.48 ± 0.02 ns | 0.55 ± 0.05 ns | 1.170 | 0.381 | |
| AN (mg kg−1) | 0–20 | 38.76 ± 5.35 ns | 37.73 ± 7.41 ns | 34.65 ± 1.09 ns | 33.88 ± 1.09 ns | 34.39 ± 0.36 ns | 0.564 | 0.694 |
| 20–40 | 34.14 ± 7.23 ns | 30.29 ± 6.42 ns | 26.44 ± 0.96 ns | 24.90 ± 1.45 ns | 33.11 ± 1.66 ns | 1.645 | 0.238 | |
| TP (g kg−1) | 0–20 | 0.38 ± 0.08 a | 0.31 ± 0.09 ab | 0.20 ± 0.02 b | 0.20 ± 0.02 b | 0.21 ± 0.05 b | 3.573 | 0.047 * |
| 20–40 | 0.26 ± 0.07 ns | 0.21 ± 0.06 ns | 0.18 ± 0.03 ns | 0.19 ± 0.00 ns | 0.20 ± 0.02 ns | 0.821 | 0.541 | |
| AP (mg kg−1) | 0–20 | 1.29 ± 0.40 bc | 1.79 ± 0.38 a | 1.55 ± 0.27 ab | 0.77 ± 0.07 c | 0.78 ± 0.19 c | 5.044 | 0.017 * |
| 20–40 | 0.93 ± 0.27 ab | 1.26 ± 0.42 a | 0.76 ± 0.27 ab | 0.52 ± 0.07 b | 0.40 ± 0.09 b | 3.498 | 0.049 * |
| Indexes | Layers (cm) | F | p |
|---|---|---|---|
| MBC | 0–20 | 8.528 | 0.003 ** |
| 20–40 | 15.242 | <0.001 ** | |
| qMBC | 0–20 | 5.202 | 0.016 * |
| 20–40 | 5.782 | 0.011 * | |
| MBN | 0–20 | 1.239 | 0.355 |
| 20–40 | 0.403 | 0.802 | |
| qMBN | 0–20 | 4.163 | 0.031 * |
| 20–40 | 0.613 | 0.663 | |
| MBP | 0–20 | 2.155 | 0.148 |
| 20–40 | 8.875 | 0.003 ** | |
| qMBP | 0–20 | 0.191 | 0.937 |
| 20–40 | 8.046 | 0.004 ** | |
| BG | 0–20 | 3.604 | 0.046 * |
| 20–40 | 3.892 | 0.037 * | |
| URE | 0–20 | 0.344 | 0.409 |
| 20–40 | 1.416 | 0.298 | |
| ACP | 0–20 | 0.989 | 0.457 |
| 20–40 | 1.232 | 0.357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Ali, I.; Du, X.; Xu, Y.; Ye, S.; Yang, M. Thinning Promotes Soil Phosphorus Bioavailability in Short-Rotation and High-Density Eucalyptus grandis × E. urophylla Coppice Plantation in Guangxi, Southern China. Forests 2023, 14, 2067. https://doi.org/10.3390/f14102067
Xiao X, Ali I, Du X, Xu Y, Ye S, Yang M. Thinning Promotes Soil Phosphorus Bioavailability in Short-Rotation and High-Density Eucalyptus grandis × E. urophylla Coppice Plantation in Guangxi, Southern China. Forests. 2023; 14(10):2067. https://doi.org/10.3390/f14102067
Chicago/Turabian StyleXiao, Xiangsheng, Izhar Ali, Xu Du, Yuanyuan Xu, Shaoming Ye, and Mei Yang. 2023. "Thinning Promotes Soil Phosphorus Bioavailability in Short-Rotation and High-Density Eucalyptus grandis × E. urophylla Coppice Plantation in Guangxi, Southern China" Forests 14, no. 10: 2067. https://doi.org/10.3390/f14102067
APA StyleXiao, X., Ali, I., Du, X., Xu, Y., Ye, S., & Yang, M. (2023). Thinning Promotes Soil Phosphorus Bioavailability in Short-Rotation and High-Density Eucalyptus grandis × E. urophylla Coppice Plantation in Guangxi, Southern China. Forests, 14(10), 2067. https://doi.org/10.3390/f14102067






