The Effect of Curcin Protein and Jatropha Plantation on Soil Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil for Curcin Protein In Vitro Treatment Experiments
2.2. Sample Collection of Jatropha Cultivation Areas
2.3. Curcin Treatment Soil Experiment
2.4. DNA Extraction and Sequencing
2.5. Determination Method of Enzyme Activity
2.6. Determination of the Degradation Rate of the Jatropha Ribosome Inactivation Protein Curcin in Soil
- (1)
- Soil from Jinhexiang and Lvyangcun was taken and passed through a 20 mesh sieve.
- (2)
- A total of 0.1 g of Jatropha seeds ground into powder with liquid nitrogen was added to 0.5 g of soil; 0.4 mL of distilled water was added, mixed with a pellet pestle (RS-Sigma) for 5 s, centrifuged at 3000 rpm for 30 s at room temperature, and the supernatant was aspirated to obtain a homogeneous mixture of soil, seeds and water. Twenty-one replicates were performed for the soil at each site (set of seven time points, three replicates per set).
- (3)
- EP tubes were placed in a thermostat, maintained at 30 °C and 80% relative humidity and incubated in the dark. Samples were taken on days 0, 6, 12, 18, 24, 30 and 36 of degradation and three replicates were taken at each time point.
- (4)
- Soil curcin protein was extracted and ELISA assays were performed.
2.7. Data Analysis and Processing
3. Results
3.1. Degradation of Curcin Protein in Soil and the Effect on Enzyme Activities
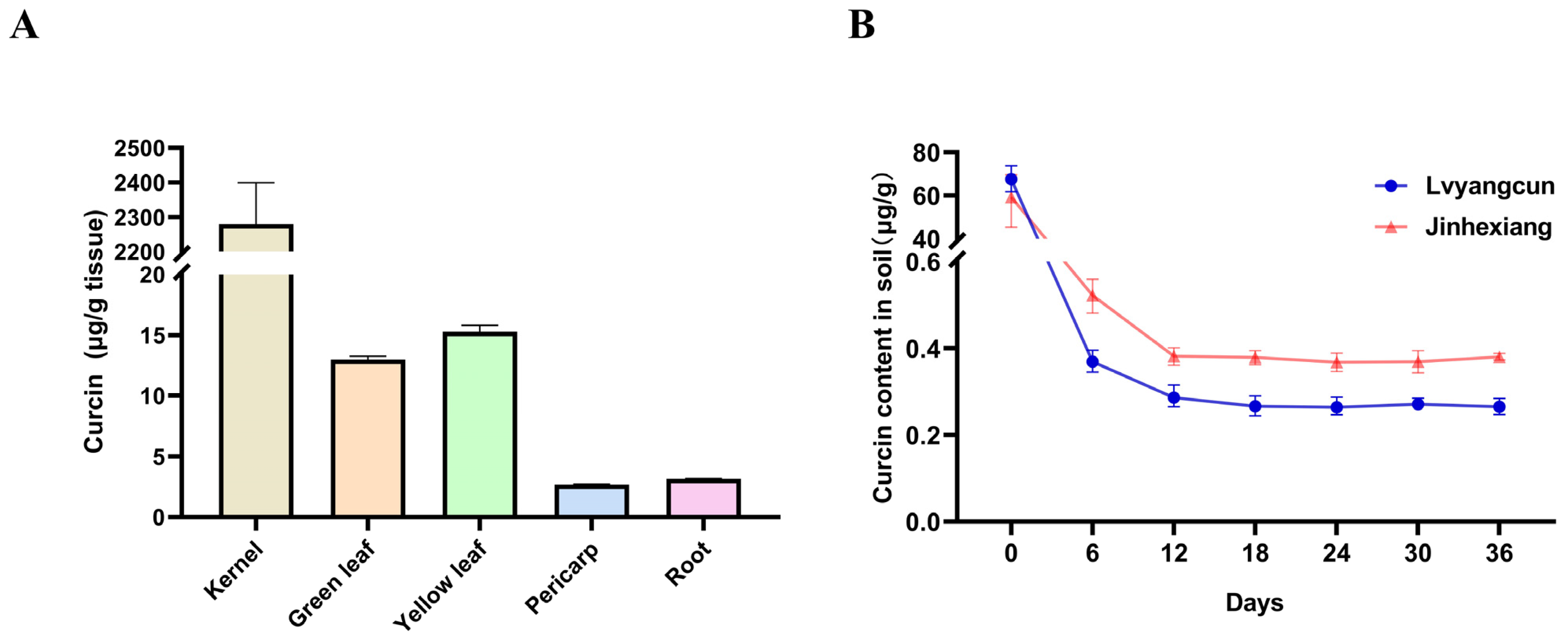
3.1.1. Possible Pathways of Curcin into the Soil and Its Degradation Dynamics in the Soil
3.1.2. Degradation Dynamics of Curcin Protein in the Soil
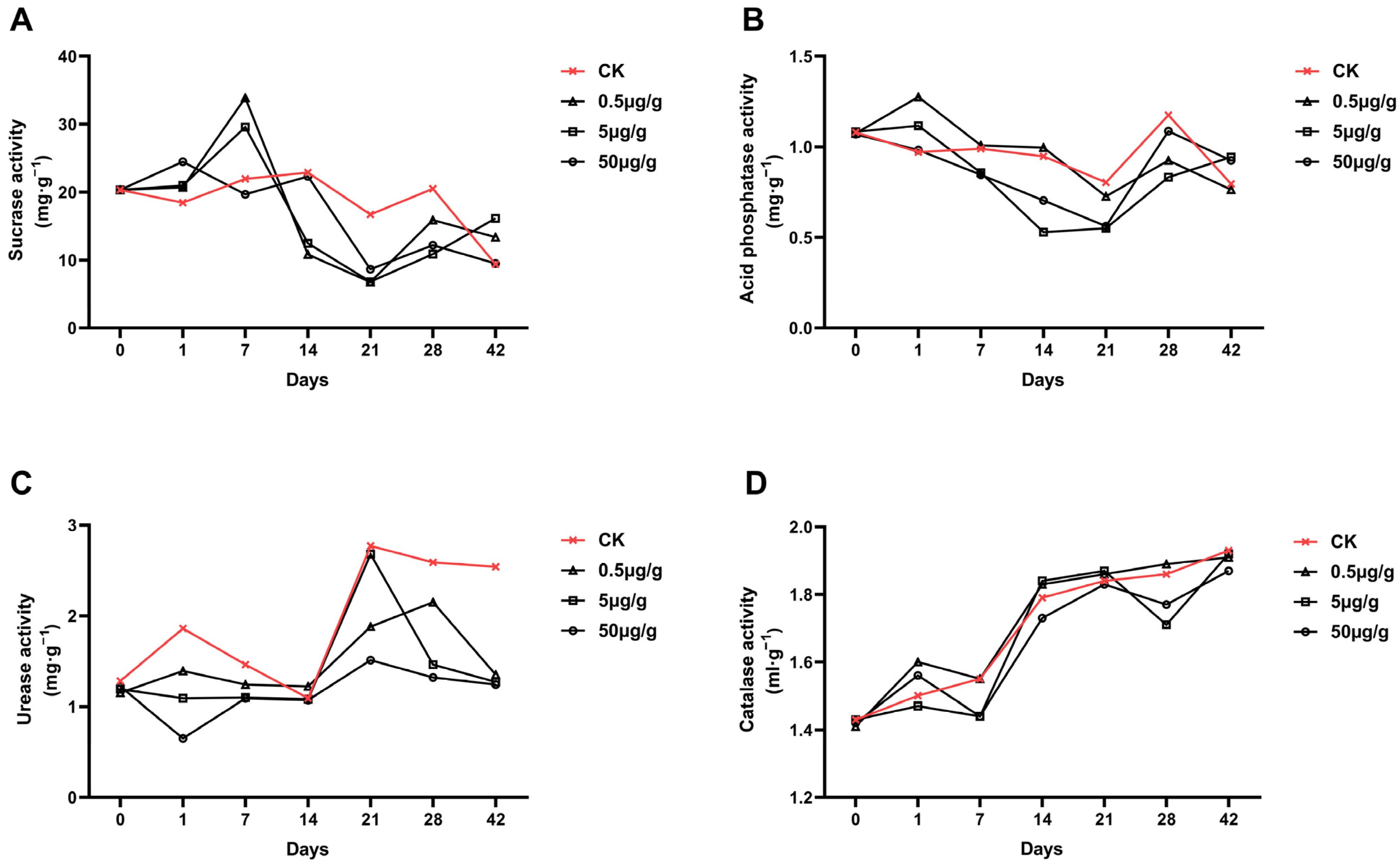
3.1.3. Effect of Curcin on Soil Enzyme Activities
3.2. Effect of Curcin Protein on Soil Fungi
3.2.1. Soil Fungi ITS Sequencing Quality Assessment Results
3.2.2. Effect of Different Concentrations of Curcin Treatment on Soil Fungal Diversity
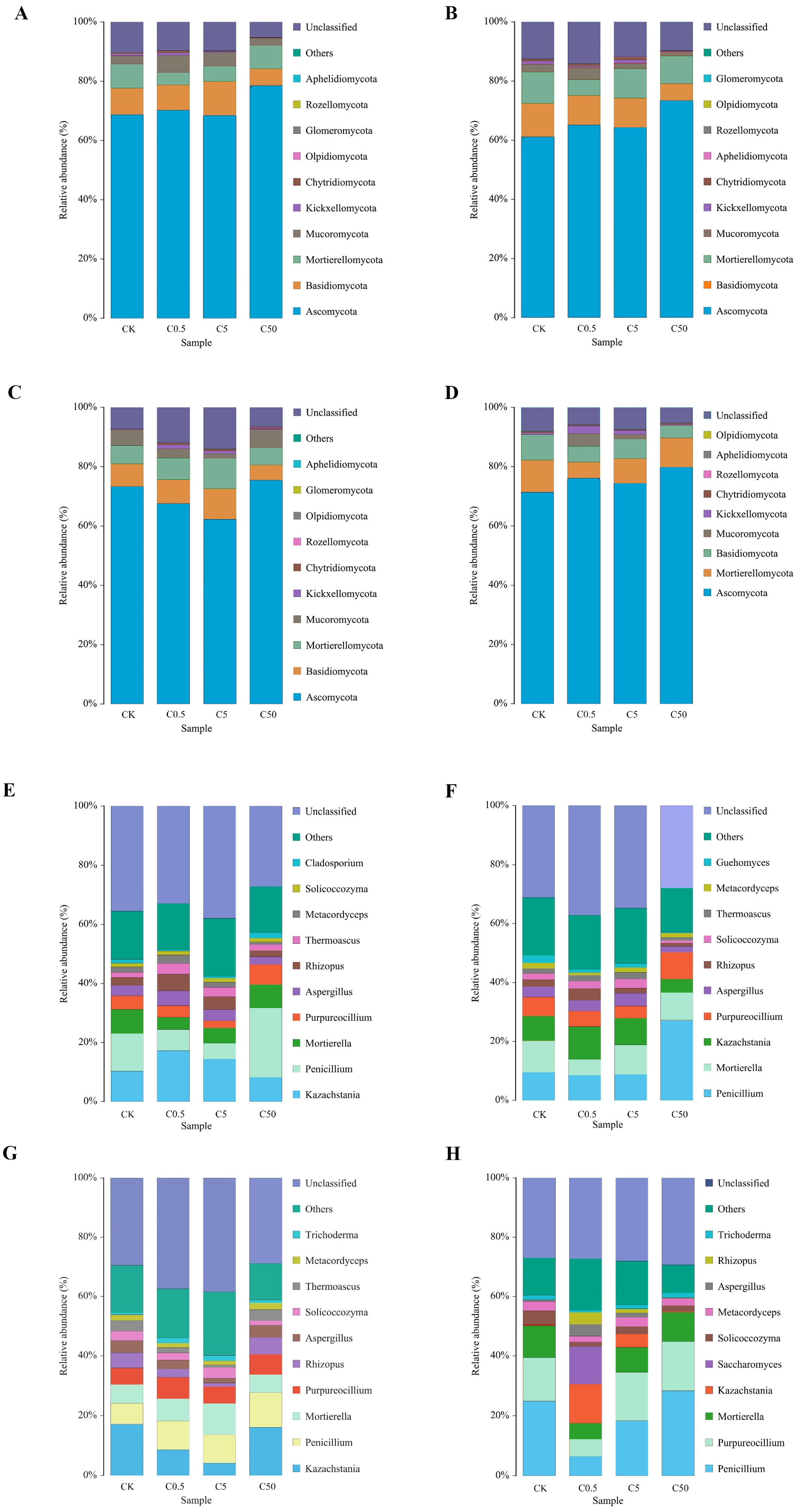
3.2.3. Effect of Different Concentrations of Curcin Treatment on the Composition of Soil Fungal Communities
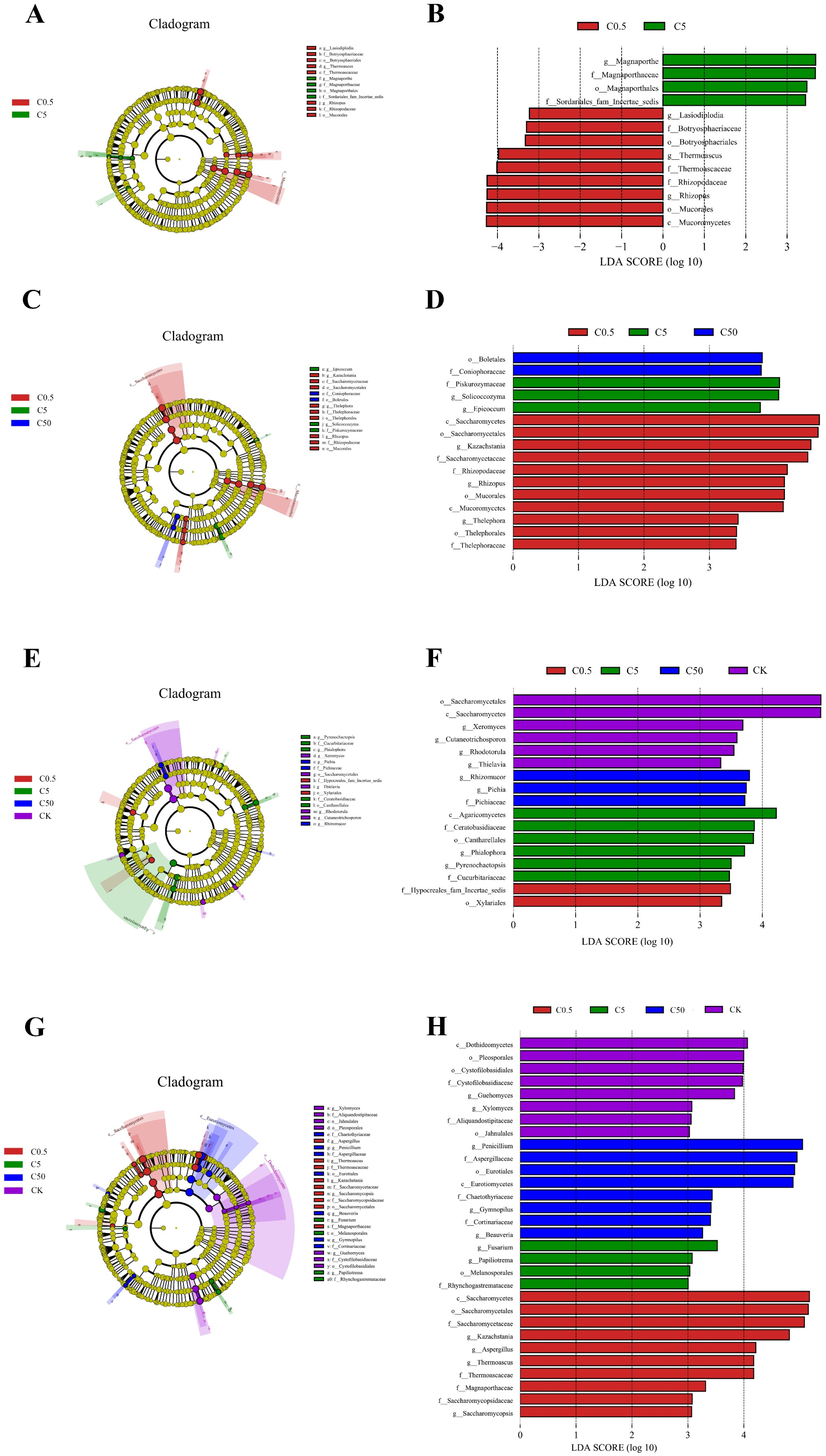
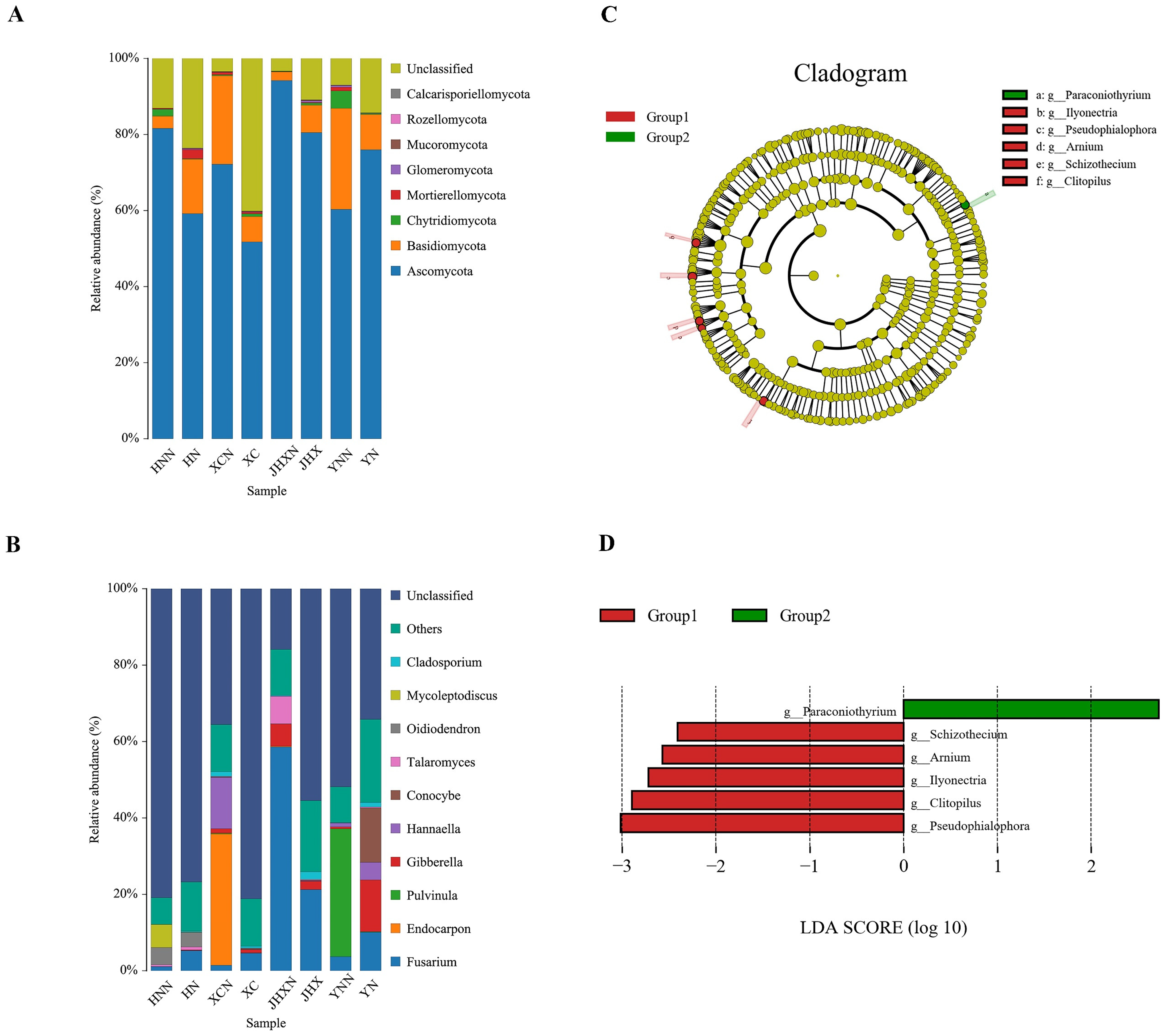
3.2.4. Indicator Groups of Soil Fungal Communities Treated with Different Concentrations of Curcin
3.3. Analysis of Soil Fungal Communities in Jatropha Planting and Non-Planting Areas in Different Climatic Regions
3.3.1. Comparison of Soil Fungal Diversity in Jatropha Planting and Non-Planting Areas in Different Climatic Regions
3.3.2. Soil Fungal Community Composition in Jatropha Planting and Non-Planting Areas in Different Climatic Regions
4. Discussion
4.1. Retention and Degradation of Curcin Protein in Soil
4.2. Assessment of the Effect of Exogenous Curcin Protein on Soil Enzyme Activity and Fungal Communities
4.3. Analysis of Soil Fungal Communities in Jatropha Planting and Non-Planting Areas in Different Regions
4.4. Shortcomings and Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lang, A.K.; Jevon, F.V.; Vietorisz, C.R.; Ayres, M.P.; Matthes, J.H. Fine roots and mycorrhizal fungi accelerate leaf litter decomposition in a northern hardwood forest regardless of dominant tree mycorrhizal associations. New Phytol. 2021, 230, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Liu, S.; García-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.A.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K.; et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022, 6, 900–909. [Google Scholar] [CrossRef]
- Lei, F.; Fu, J.; Zhou, R.; Wang, D.; Zhang, A.; Ma, W.; Zhang, L. Chemotactic response of Ginseng bacterial soft-rot to Ginseng root exudates. Saudi J. Biol. Sci. 2017, 24, 1620–1625. [Google Scholar] [CrossRef]
- Hannula, S.E.; de Boer, W.; van Veen, J.A. Do genetic modifications in crops affect soil fungi? a review. Biol. Fertil. Soils 2014, 50, 433–446. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef]
- Gebhard, F.; Smalla, K. Monitoring field releases of genetically modied sugar beets for persistence of transgenic plant dna and horizontal gene transfer. FEMS Microbiol. Ecol. 2019, 28, 261–272. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Silva, E.F.L.; Nunes, L.; Carneiro, R.F.V. The Effect of Converting Tropical Native Savanna to Eucalyptus Grandis Forest on Soil Microbial Biomass. Land Degrad. Dev. 2010, 21, 540–545. [Google Scholar] [CrossRef]
- Tian, W.H.; Yi, X.L.; Liu, S.S.; Zhou, C.; Wang, A.Y. Effect of transgenic cotton continuous cropping on soil bacterial community. Ann. Microbiol. 2020, 70, 61. [Google Scholar] [CrossRef]
- Ashutosh Kumar, S.; Xiao-Jin, J.; Bin, Y.; Junen, W.; Apurva, R.; Chunfeng, C.; Jitendra, A.; Pingyuan, W.; Wenjie, L.; Nandita, S. Biological indicators affected by land use change, soil resource availability and seasonality in dry tropics. Ecol. Indic. 2020, 115, 106369. [Google Scholar] [CrossRef]
- Weise, C.; Schrot, A.; Wuerger, L.T.D.; Adolf, J.; Gilabert-Oriol, R.; Sama, S.; Melzig, M.F.; Weng, A. An unusual type I ribosome-inactivating protein from Agrostemma githago L. Sci. Rep. 2020, 10, 15377. [Google Scholar] [CrossRef] [PubMed]
- Amirzadeh, N.; Moghadam, A.; Niazi, A.; Afsharifar, A. Recombinant anti-HIV MAP30, a ribosome inactivating protein: Against plant virus and bacteriophage. Sci. Rep. 2023, 13, 2091. [Google Scholar] [CrossRef]
- Liu, J.; Wen, D.; Song, X.; Su, P.; Lou, J.; Yao, D.; Zhang, C. Evolution and natural selection of ribosome-inactivating proteins in bacteria, fungi, and plants. Int. J. Biol. Macromol. 2023, 248, 125929. [Google Scholar] [CrossRef]
- Citores, L.; Ferreras, J.M. Biological Activities of Ribosome-Inactivating Proteins. Toxins 2023, 15, 35. [Google Scholar] [CrossRef]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon Off. J. Int. Soc. Toxinol. 2004, 44, 371–383. [Google Scholar] [CrossRef]
- Citores, L.; Iglesias, R.; Gay, C.; Ferreras, J.M. Antifungal activity of the ribosome-inactivating protein BE27 from sugar beet (Beta vulgaris L.) against the green mould Penicillium digitatum. Mol. Plant Pathol. 2016, 17, 261–271. [Google Scholar] [CrossRef]
- Montes, J.M.; Melchinger, A.E. Domestication and Breeding of Jatropha curcas L. Trends Plant Sci. 2016, 21, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Long, L. Review and prospects of Jatropha biodiesel industry in China. Renew. Sustain. Energy Rev. 2012, 16, 2178–2190. [Google Scholar]
- Qin, S.; Wang, X.; Han, P.; Lai, Z.; Ren, Y.; Ma, R.; Cheng, C.; Wang, T.; Xu, Y. LRP1-Mediated Endocytosis May Be the Main Reason for the Difference in Cytotoxicity of Curcin and Curcin C on U2OS Osteosarcoma Cells. Toxins 2022, 14, 771. [Google Scholar] [CrossRef]
- Qin, X.; Shao, C.; Hou, P.; Gao, J.; Lei, N.; Jiang, L.; Ye, S.; Gou, C.; Luo, S.; Zheng, X.; et al. Different functions and expression profiles of curcin and curcin-L in Jatropha curcas L. Z. Naturforschung C J. Biosci. 2010, 65, 355–362. [Google Scholar] [CrossRef]
- Hu, N.; Yang, Q.; Li, C.-Y.; Zhang, M.; Xu, Y.; Chen, F. Extraction of Total DNAs from Soil Samples of Jatropha curcas L. Available Regions and Validation by Multiplex-PCR Method. J. Sichuan Univ. Nat. Sci. Ed. 2016, 53, 683–688. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 420–428. [Google Scholar] [CrossRef]
- Alef, K.E.; Nannipieri, P.E. Methods in Applied Soil Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1995; pp. 569–576. [Google Scholar]
- Zhang, Y.; Zhou, G.; Wu, N. A Review of Studies on Soil Enzymology. J. Trop. Subtrop. Bot. 2004, 12, 83–90. [Google Scholar]
- He, W.; King, A.J.; Khan, M.A.; Cuevas, J.A.; Ramiaramanana, D.; Graham, I.A. Analysis of seed phorbol-ester and curcin content together with genetic diversity in multiple provenances of Jatropha curcas L. from Madagascar and Mexico. Plant Physiol. Biochem. 2011, 49, 1183–1190. [Google Scholar] [CrossRef]
- Crecchio, C.; Stotzky, G. Insecticidal activity and biodegradation of the toxin from Bacillus thuringiensis subsp. kurstaki bound to humic acids from soil. Soil Biol. Biochem. 1998, 30, 463–470. [Google Scholar] [CrossRef]
- Crecchio, C.; Stotzky, G. Biodegradation and insecticidal activity of the toxin from Bacillus thuringiensis subsp kurstaki bound on complexes of montmorillonite-humic acids-Al hydroxypolymers. Soil Biol. Biochem. 2001, 33, 573–581. [Google Scholar] [CrossRef]
- Stotzky, G. Persistence and biological activity in soil of insecticidal proteins from Bacillus thuringiensis and of bacterial DNA bound on clays and humic acids. J. Environ. Qual. 2000, 29, 691–705. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Dick, R.P.; Burns, R.G. A Brief History of Soil Enzymology Research. In Methods of Soil Enzymology; Soil Science Society of America: Fitchburg, WI, USA, 2011. [Google Scholar]
- Zhu, F.; Zhou, Y.K.; Ji, Z.L.; Chen, X.R. The Plant Ribosome-Inactivating Proteins Play Important Roles in Defense against Pathogens and Insect Pest Attacks. Front. Plant Sci. 2018, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Saxena, D.; Stotzky, G.; Flores, S. Insecticidal toxin in root exudates from Bt corn. Nature 1999, 402, 480. [Google Scholar] [CrossRef] [PubMed]
- Stotzky, G. Persistence and biological activity in soil of the insecticidal proteins from Bacillus thuringiensis, especially from transgenic plants. Plant Soil 2005, 266, 77–89. [Google Scholar] [CrossRef]
- Hung, T.P.; Truong, L.V.; Binh, N.D.; Frutos, R.; Quiquampoix, H.; Staunton, S. Persistence of detectable insecticidal proteins from Bacillus thuringiensis (Cry) and toxicity after adsorption on contrasting soils. Environ. Pollut. 2016, 208, 318–325. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Liu, D.; Hao, J.; Liu, H.; Lu, X. Effects of toxin from Bacillus thuringiensis (Bt) on sorption of Pb (II) in red and black soils: Equilibrium and kinetics aspects. J. Hazard. Mater. 2018, 360, 172–181. [Google Scholar] [CrossRef]
- Helassa, N.; M’Charek, A.; Quiquampoix, H.; Noinville, S.; Déjardin, P.; Frutos, R.; Staunton, S. Effects of physicochemical interactions and microbial activity on the persistence of Cry1Aa Bt (Bacillus thuringiensis) toxin in soil. Soil Biol. Biochem. 2011, 43, 1089–1097. [Google Scholar] [CrossRef]
- Liu, J.; Liang, Y.-S.; Hu, T.; Zeng, H.; Gao, R.; Wang, L.; Xiao, Y.-H. Environmental fate of Bt proteins in soil: Transport, adsorption/desorption and degradation. Ecotoxicol. Environ. Saf. 2021, 226, 112805. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Yang, C.; Shen, Q.; Zhou, J.; Yang, L. Soil enzymatic activity and growth of rice and barley as influenced by organic manure in an anthropogenic soil. Geoderma 2003, 115, 149–160. [Google Scholar] [CrossRef]
- Zhang, H.S.; Zai, X.M.; Wu, X.H.; Qin, P.; Zhang, W.M. An ecological technology of coastal saline soil amelioration. Ecol. Eng. 2014, 67, 80–88. [Google Scholar] [CrossRef]
- Woese, C.R. Bacterial evolution. Microbiol. Rev. 1987, 51, 221–271. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Xu, Y.; Xiao, R. Lipases from the genus Rhizopus: Characteristics, expression, protein engineering and application. Prog. Lipid Res. 2016, 64, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Helal, S.E.; Abdelhady, H.M.; Abou-Taleb, K.A.; Hassan, M.G.; Amer, M.M. Lipase from Rhizopus Oryzae R1: In-depth characterization, immobilization, and evaluation in biodiesel production. J. Genet. Eng. Biotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Chen, X.; Guo, S.; Li, X.; Wang, H. New Endophytic Fungal Strain, i.e., Rhizopus fungi Dhs96 Useful for Preventing and Treating Soft Rot Diseases of Dendrobium, Seedling Nursery Beds or Seedling of Dendrobium Plant, and Planting Dendrobium Plant. CN102776127-A.; CN102776127-B, CN102776127-A 14 Nov 2012 C12N-001/14 201324 Pages: 7 Chinese CN102776127-B 10 Jul 2013 C12N-001/14 201371 Chinese. 2013. Available online: https://www.webofscience.com/wos/diidw/full-record/DIIDW:2013C38135 (accessed on 15 October 2023).
- Liu, R.; Bao, Z.X.; Li, G.H.; Li, C.Q.; Wang, S.L.; Pan, X.R.; Zhang, K.Q.; Zhao, P.J. Identification of Nematicidal Metabolites from Purpureocillium lavendulum. Microorganisms 2022, 1, 1343. [Google Scholar] [CrossRef]
- Li, Z.; Bu, N.; Chen, X.; Cui, J.; Xiao, M.; Song, Z.; Nie, M.; Fang, C. Soil incubation studies with Cry1Ac protein indicate no adverse effect of Bt crops on soil microbial communities. Ecotoxicol. Environ. Saf. 2018, 152, 33–41. [Google Scholar] [CrossRef]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversty. Appl. Environ. Microbiol. 2008, 74, 738–744. [Google Scholar] [CrossRef]
- Kumar, U.; Saqib, H.S.A.; Islam, W.; Prashant, P.; Patel, N.; Chen, W.; Yang, F.; You, M.; He, W. Landscape Composition and Soil Physical-Chemical Properties Drive the Assemblages of Bacteria and Fungi in Conventional Vegetable Fields. Microorganisms 2022, 10, 1202. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Ge, L.; Hu, C.; Wu, G.; Sun, Y.; Song, L.; Wu, X.; Pan, A.; Xu, Q.; et al. Environmental Behaviors of Bacillus thuringiensis (Bt) Insecticidal Proteins and Their Effects on Microbial Ecology. Plants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Lorenz, N.; Dick, L.K.; Dick, R.P. FAME Profiling and Activity of Microbial Communities During Jatropha curcas L. Residue Decomposition in Semiarid Soils. Soil Sci. 2011, 176, 625–633. [Google Scholar] [CrossRef]
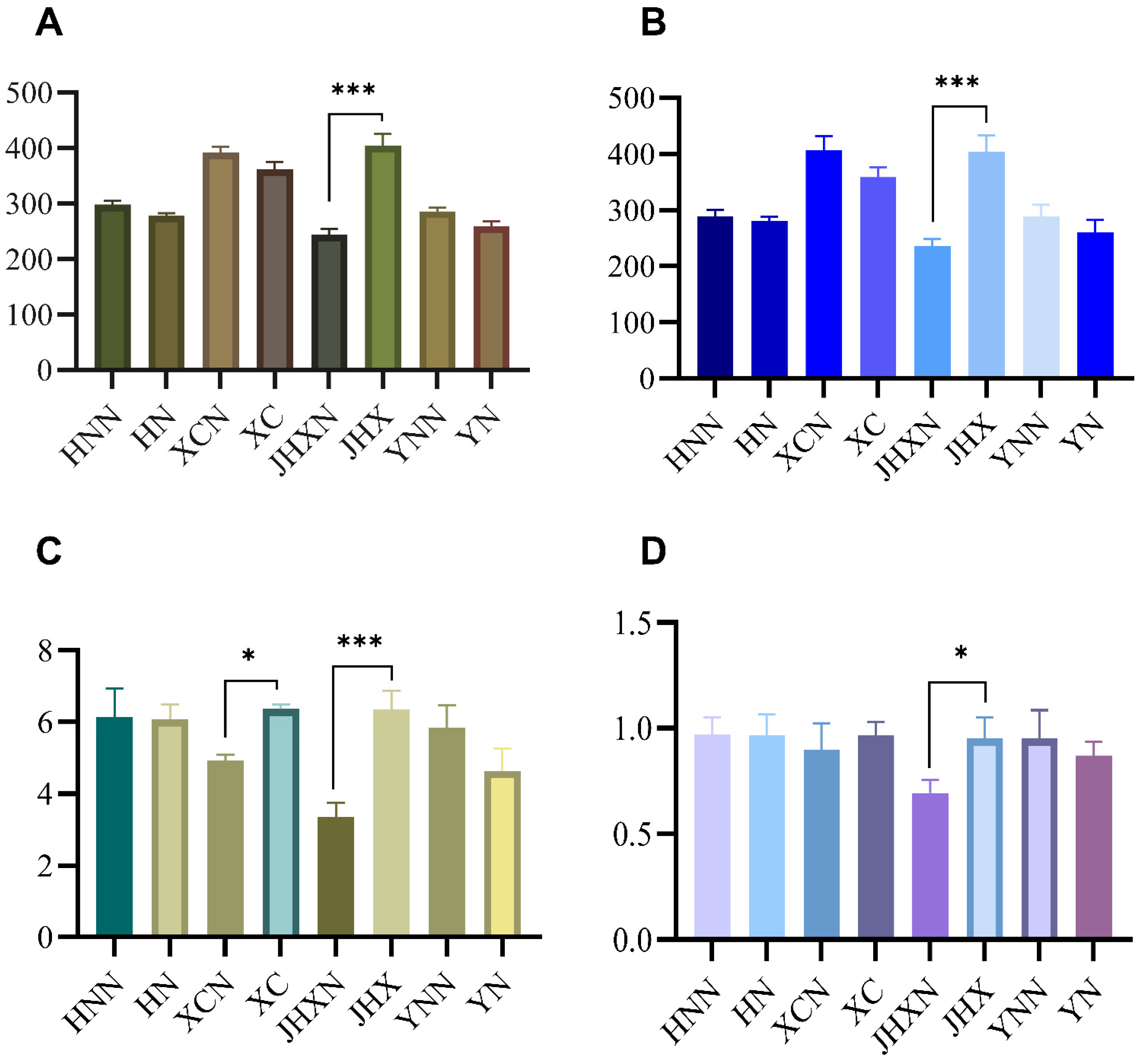
| Day | Treatment | OTU | Chao1 | ACE | Shannon | Simpson |
|---|---|---|---|---|---|---|
| 1 | CK | 183.5 ± 0.5 f | 221.1 ± 15.4 d | 284.8 ± 2.6 c | 4.21 ± 0.01 bc | 0.031 ± 0.000 b |
| C0.5 | 277.0 ± 3.07 bc | 303.7 ± 11.3 b | 298.0 ± 13.7 b | 4.28 ± 0.05 bc | 0.030 ± 0.004 b | |
| C5 | 300.0 ± 0.1 b | 326.2 ± 4.2 b | 321.1 ± 6.4 b | 4.47 ± 0.01 ab | 0.028 ± 0.00 b | |
| C50 | 275.5 ± 3.5 bc | 302.7 ± 0.3 b | 289.8 ± 4.0 bc | 3.57 ± 0.01 d | 0.08 ± 0.01 a | |
| 3 | CK | 229.5 ± 3.5 de | 268.5 ± 2.5 c | 284.4 ± 10.0 c | 4.32 ± 0.06 b | 0.021 ± 0.003 b |
| C0.5 | 211.5 ± 0.5 e | 313.8 ± 2.3 b | 479.7 ± 9.7 a | 4.44 ± 0.02 ab | 0.021 ± 0.001 b | |
| C5 | 263.0 ± 4.0 cd | 261.4 ± 12.6 c | 265.4 ± 14.5 c | 4.47 ± 0.01 ab | 0.023 ± 0.00 b | |
| C50 | 235.5 ± 4.5 de | 302.7 ± 0.3 b | 275.6 ± 8.3 c | 4.06 ± 0.08 c | 0.06 ± 0.02 ab | |
| 7 | CK | 236.0 ± 12 de | 248.1 ± 15.1 cd | 257.6 ± 18.8 c | 3.96 ± 0.08 c | 0.034 ± 0.001 b |
| C0.5 | 274.0 ± 10.0 c | 268.5 ± 1.5 c | 292.2 ± 13.2 bc | 4.37 ± 0.08 ab | 0.024 ± 0.001 b | |
| C5 | 293.5 ± 20.5 bc | 297.6 ± 23.6 bc | 254.8 ± 38.7 c | 4.56 ± 0.08 a | 0.024 ± 0.00 b | |
| C50 | 240.5 ± 4.5 d | 244.0 ± 6.0 cd | 241.4 ± 4.1 c | 4.07 ± 0.00 c | 0.039 ± 0.01 ab | |
| 12 | CK | 463.0 ± 1.0 a | 489.8 ± 1.9 a | 488.8 ± 1.9 a | 3.56 ± 0.005 de | 0.08 ± 0.002 a |
| C0.5 | 236.5 ± 3.5 de | 224.3 ± 13.8 d | 244.1 ± 7.9 c | 4.08 ± 0.005 c | 0.03 ± 0.001 b | |
| C5 | 475.5 ± 18.5 a | 508.0 ± 20.1 a | 501.8 ± 22.8 a | 3.60 ± 0.25 d | 0.077 ± 0.05 a | |
| C50 | 469.0 ± 3.0 a | 508.0 ± 5.1 a | 501.3 ± 0.9 a | 3.33 ± 0.01 e | 0.08 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Z.; Zhang, B.; Niu, X.; Ma, R.; Wang, T.; Cheng, C.; Ren, Y.; Wang, X.; Hu, N.; Jiang, N.; et al. The Effect of Curcin Protein and Jatropha Plantation on Soil Fungi. Forests 2023, 14, 2088. https://doi.org/10.3390/f14102088
Lai Z, Zhang B, Niu X, Ma R, Wang T, Cheng C, Ren Y, Wang X, Hu N, Jiang N, et al. The Effect of Curcin Protein and Jatropha Plantation on Soil Fungi. Forests. 2023; 14(10):2088. https://doi.org/10.3390/f14102088
Chicago/Turabian StyleLai, Zhiping, Bingbing Zhang, Xianfei Niu, Rui Ma, Ting Wang, Cheng Cheng, Yingying Ren, Xueying Wang, Na Hu, Nan Jiang, and et al. 2023. "The Effect of Curcin Protein and Jatropha Plantation on Soil Fungi" Forests 14, no. 10: 2088. https://doi.org/10.3390/f14102088
APA StyleLai, Z., Zhang, B., Niu, X., Ma, R., Wang, T., Cheng, C., Ren, Y., Wang, X., Hu, N., Jiang, N., & Xu, Y. (2023). The Effect of Curcin Protein and Jatropha Plantation on Soil Fungi. Forests, 14(10), 2088. https://doi.org/10.3390/f14102088







