Changes in Soil Organic Carbon Concentration and Stock after Forest Regeneration of Agricultural Fields in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Soil Sampling and Measurements
2.3. Statistical Analysis
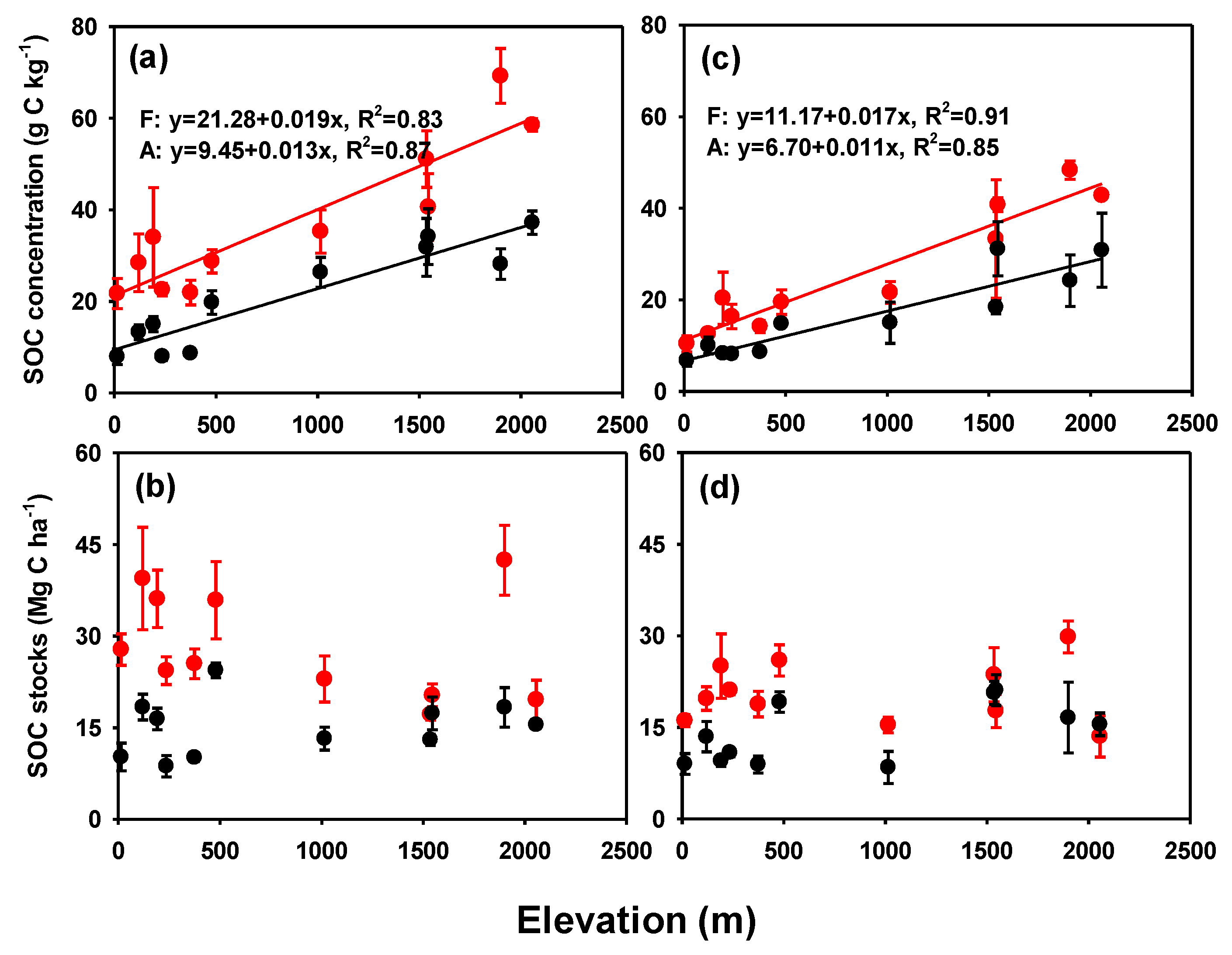
3. Results
4. Discussion
4.1. Forest Regeneration of Agricultural Fields
4.2. Elevation and Agricultural Practice Effects on SOC Stock Sequestration
4.3. Implications of Afforestation/Abandonment of Agricultural Fields
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.H.; Verardo, D.J.; Dokken, D.J. Land Use, Land-Use Change and Forestry: A Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2000; p. 388. [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Silver, W.L.; Ostertag, R.; Lugo, A.E. The potential for carbon sequestration through reforestation of abandoned tropical agricultural and pasture lands. Restor. Ecol. 2000, 8, 394–407. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef] [Green Version]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone–carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of soil organic carbon stocks and fractions to different land-use changes across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- Barcena, T.G.; Kiar, L.P.; Vesterdal, L.; Stefansdottir, H.M.; Gundersen, P.; Sigudsson, B.D. Soil carbon stock change following afforestation in Northern Europe: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2393–2405. [Google Scholar] [CrossRef]
- Kämpf, I.; Hölzel, N.; Störrle, M.; Broll, G.; Kiehl, K. Potential of temperate agricultural soils for carbon sequestration: A meta-analysis of land-use effects. Sci. Total Environ. 2016, 566–567, 428–435. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef]
- Vesterdal, L.; Ritter, E.; Gundersen, P. Change in soil organic carbon following afforestation of former arable land. For. Ecol. Manag. 2002, 169, 137–147. [Google Scholar] [CrossRef]
- Richter, D.D.; Markewitz, D.; Trumbore, S.E.; Wells, C.G. Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 1999, 400, 56–58. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthrong, S.T.; Jobbagy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.R.; Cheng, C.H.; Tseng, T.Y. Carbon sequestration potential of afforestation in the plain areas in Taiwan: Examples from long-term abandoned orchards and afforested sites. Quart. J. Chin. For. 2011, 44, 567–588. (In Chinese) [Google Scholar]
- Chen, Y.Y.; Huang, W.; Wang, W.H.; Juang, J.Y.; Hong, J.S.; Kato, T.; Luyssaert, S. Reconstructing Taiwan’s land cover changes between 1904 and 2015 from historical maps and satellite images. Sci. Rep. 2019, 9, 3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.L.; Kuan, W.H.; Lui, P.S.; Hu, C.Y. Relationship between landscape characteristics and surface water quality. Environ. Monit. Assess. 2008, 147, 57–64. [Google Scholar] [CrossRef]
- Thomas, G.D. pH. In Encyclopedia of Soil Science; Lal, R., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 1270–1274. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Ledo, A.; Smith, P.; Zerihun, A.; Whitaker, J.; Vicente-Vicente, J.L.; Qin, Z.; McNamara, N.P.; Zinn, Y.L.; Llorente, M.; Liebig, M.; et al. Changes in soil organic carbon under perennial crops. Glob. Chang. Biol. 2020, 26, 4158–4168. [Google Scholar] [CrossRef]
- Del Galdo, I.; Six, J.; Peressotti, A.; Cotrufo, M.F. Assessing the impact of land-use change on soil C sequestration in agricultural soils by means of organic matter fractionation and stable C isotops. Glob. Chang. Biol. 2009, 9, 1204–1213. [Google Scholar] [CrossRef]
- Garten, C.T., Jr.; Hanson, P.J. Measured forest soil C stocks and estimated turnover times along an elevation gradient. Geoderma 2006, 136, 342–352. [Google Scholar] [CrossRef]
- Tsui, C.C.; Tsai, C.C.; Chen, Z.S. Soil organic carbon stocks in relation to elevation gradients in volcanic ash soils of Taiwan. Geoderma 2013, 209–210, 119–127. [Google Scholar] [CrossRef]
- Huang, Y.H.; Hung, C.Y.; Lin, I.R.; Kume, T.; Menyailo, O.V.; Cheng, C.H. Soil respiration patterns and rates at three Taiwanese forest plantations: Dependence on elevation, temperature, precipitation, and litterfall. Bot. Stud. 2017, 58, 1–12. [Google Scholar]
- Smith, P.; Powlson, D.S.; Glendining, M.J.; Smith, J. Potential for carbon sequestration in European soils: Preliminary estimates for five scenarios using results from long-term experiments. Glob. Chang. Biol. 1997, 3, 67–79. [Google Scholar] [CrossRef]
- Amelung, W.; Bossio, D.; de Vries, D.; Kogel-Knabner, I.; Lehmann, J.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 11, 5427. [Google Scholar] [CrossRef]
- Page, K.P.; Dang, Y.P.; Dalal, R.C. The ability of conservation agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Poulton, P.; Johnston, J.; Macdonald, A.; White, R.; Powlson, D. Major limitations to achieving “4 per 1000” increases in soil organic carbon stock in temperate regions: Evidence from long-term experiments at Rothamsted Research, United Kingdom. Glob. Chang. Biol. 2018, 24, 2563–2584. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.J.; Bohm, B.; Haile-Mariam, S.; Paul, E.A. Evaluation of carbon accrual in afforested agricultural soils. Glob. Chang. Biol. 2007, 13, 1145–1156. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.H.; Huang, Y.H.; Menyailo, O.V.; Chen, C.T. Stand development and aboveground biomass carbon accumulation with cropland afforestation in Taiwan. Taiwan J. For. Sci. 2016, 31, 105–118. [Google Scholar]
- Cheng, C.H.; Hung, C.Y.; Chen, C.P.; Pei, C.W. 2013. Biomass carbon accumulation in aging Japanese cedar plantations in Xitou, central Taiwan. Bot. Stud. 2013, 54, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Site | Elevation | Precipitation | Temperature | Regeneration Age | Vegetation/Crop Types | |
|---|---|---|---|---|---|---|
| (m) | (mm) | (°C) | (Years) | Forest Regenerated (F) Fields | Agricultural (A) Fields | |
| Meifeng (MF) | 2056 | 3200 | 12.3 | 19 | Metasequoia glyptostroboides | High altitude cabbage/flower garden |
| Wuling (WL) | 1840 | 3271 | 13.1 | 20 | Chamaecyparis formosensis | High altitude cabbage |
| Chinjing (CJ) | 1545 | 2857 | 13.5 | 19 | Calocedrus formosana | High altitude cabbage |
| Lishan (LS) | 1535 | 2564 | 12.5 | 18 | Chamaecyparis formosensis | Orchard (persimmon) |
| Xibao (XB) | 1015 | 2100 | 16.5 | 18 | Secondary broadleaf forest with bamboo (Phyllostachys makinoi) | High altitude vegetables |
| Hsinshe (HS) | 480 | 2250 | 20.9 | 17 | Secondary broadleaf forest (Scattered trees and dense bushes) | Orchard (loquat) |
| Mingjian (MJ) | 375 | 2043 | 21.5 | 27 | Fraxinus formosana Cinnamomum camphora | Pineapple/Ginger/Yam |
| Rueisui (RS) | 236 | 1725 | 22.3 | 19 | Cinnamomum camphora | Miscellaneous vegetables |
| Guanxi (GX) | 192 | 2263 | 23.0 | 22 | Secondary broadleaf forest | Tea/Miscellaneous vegetables |
| Ershui (ES) | 120 | 2055 | 23.7 | 21 | Heritiera littoralis Dillenia indica | Miscellaneous vegetables |
| Tainan (TN) | 14 | 1698 | 24.3 | 33 | Secondary broadleaf forest | Sugarcane |
| SOC Concentration (g C kg−1) | SOC Stocks (Mg C ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |||||
| F | A | F | A | F | A | F | A | |
| MF | 58.5 ± 13.6 1 a 2 | 37.2 ± 2.6 b | 42.8 ± 0.4 | 30.8 ± 8.1 | 19.6 ± 3.4 | 15.5 ± 0.6 | 13.5 ± 3.6 | 15.5 ± 1.9 |
| WL | 69.2 ± 6.0 a | 28.2 ± 3.3 b | 48.3 ± 2.0 a | 24.2 ± 5.6 b | 42.4 ± 7.3 a | 18.3 ± 3.2 b | 29.8 ± 3.3 | 16.6 ± 5.8 |
| CJ | 40.6 ± 7.3 a | 34.2 ± 6.1 b | 40.8 ± 1.5 a | 31.1 ± 5.9 b | 20.3 ± 0.9 | 17.4 ± 2.7 | 17.7 ± 4.4 | 21.1 ± 2.5 |
| LS | 51.1 ± 6.2 | 31.6 ± 6.4 | 33.3 ± 12.9 | 18.4 ± 1.4 | 17.0 ± 1.4 | 12.6 ± 2.7 | 23.6 ± 5.1 | 7.8 ± 2.9 |
| XB | 35.3 ± 4.7 | 26.3 ± 3.3 | 21.6 ± 2.4 | 15.0 ± 4.5 | 22.7 ± 4.3 a | 13.2 ± 1.9 b | 15.4 ± 1.5 a | 8.4 ± 2.6 b |
| HS | 28.8 ± 2.6 a | 19.8 ± 2.6 b | 19.5 ± 2.7 a | 14.8 ± 0.4 b | 32.4 ± 7.2 | 24.4 ± 1.2 | 26.0 ± 2.9 | 19.1 ± 1.7 |
| MJ | 21.9 ± 2.7 a | 8.7 ± 0.5 b | 14.2 ± 1.3 a | 8.6 ± 0.6 b | 25.5 ± 2.9 a | 10.1 ± 0.7 b | 18.8 ± 2.5 a | 8.9 ± 1.4 b |
| RS | 22.6 ± 1.4 a | 8.0 ± 1.0 b | 16.4 ± 2.7 a | 8.2 ± 0.8 b | 24.4 ± 2.4 a | 8.7 ± 1.7 b | 21.1 ± 0.8 a | 10.9 ± 0.7 b |
| GX | 34.0 ± 10.8 a | 15.0 ± 1.7 b | 20.4 ± 5.7 a | 8.3 ± 0.9 b | 36.1 ± 5.6 a | 16.4 ± 1.8 b | 25.0 ± 6.3 a | 9.5 ± 0.9 b |
| ES | 28.4 ± 6.3 a | 13.3 ± 1.6 b | 12.6 ± 1.0 | 10.0 ± 1.8 | 40.3 ± 7.9 a | 18.4 ± 2.1 b | 10.7 ± 2.0 a | 13.5 ± 2.5 b |
| TN | 21.7 ± 3.3 a | 7.9 ± 1.7 b | 10.4 ± 1.8 a | 6.7 ± 1.2 b | 27.8 ± 3.2 a | 10.2 ± 2.3 b | 16.1 ± 1.3 a | 9.0 ± 1.7 b |
| Mean | 37.5 ± 15.9 | 20.9 ± 11.1 | 25.5 ± 13.4 | 16.0 ± 9.1 | 28.1 ± 8.6 | 15.0 ± 4.6 | 20.6 ± 5.0 | 12.8 ± 4.7 |
| Pair t test | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | ||||
| Absolute SOC Concentration Change | Relative SOC Concentration Change | Annual SOC Concentration Increasing Rate | Absolute SOC Stocks Change | Relative SOC Stocks Change | Annual SOC Stocks Accumulation Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g C kg−1) | (%) | (g C kg−1 Year−1) | (ton C ha−1) | (%) | (ton C ha−1 Year−1) | |||||||
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| MF | 21.3 | 12.0 | 57 | 39 | 1.1 | 0.6 | 4.1 | −1.9 | 26 | −13 | 0.22 | −0.10 |
| WL | 41.1 | 24.1 | 146 | 100 | 2.1 | 1.2 | 24.1 | 13.2 | 131 | 80 | 1.27 | 0.70 |
| CJ | 6.4 | 9.6 | 19 | 31 | 0.3 | 0.5 | 2.9 | −3.4 | 17 | −16 | 0.15 | −0.18 |
| LS | 19.3 | 14.9 | 61 | 81 | 1.1 | 0.8 | 4.1 | 2.9 | 32 | 14 | 0.22 | 0.15 |
| XB | 8.9 | 6.6 | 34 | 44 | 0.5 | 0.4 | 9.6 | 7.0 | 72 | 83 | 0.50 | 0.37 |
| HS | 9.0 | 4.7 | 46 | 32 | 0.5 | 0.3 | 8.0 | 6.8 | 33 | 36 | 0.42 | 0.36 |
| MJ | 13.3 | 5.6 | 153 | 64 | 0.5 | 0.2 | 15.4 | 9.9 | 152 | 111 | 0.81 | 0.52 |
| RS | 14.6 | 8.2 | 182 | 100 | 0.8 | 0.4 | 15.7 | 10.2 | 181 | 94 | 0.83 | 0.54 |
| GX | 19.0 | 12.1 | 127 | 145 | 0.9 | 0.5 | 19.7 | 15.6 | 120 | 164 | 1.04 | 0.82 |
| ES | 15.1 | 2.6 | 114 | 26 | 0.7 | 0.1 | 21.9 | 6.3 | 119 | 47 | 1.15 | 0.33 |
| TN | 13.8 | 3.7 | 175 | 54 | 0.4 | 0.1 | 17.6 | 7.1 | 173 | 79 | 0.93 | 0.38 |
| Mean | 16.5 | 9.5 | 101 | 65 | 0.81 | 0.48 | 13.0 | 6.7 | 96 | 62 | 0.68 | 0.35 |
| Absolute SOC Concentration Change | Relative SOC Concentration Change | Annual SOC Concentration Increasing Rate | Absolute SOC Stocks Change | Relative SOC Stocks Change | Annual SOC Stock Accumulation Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g C kg−1) | (%) | (g C kg−1 Year−1) | (ton C ha−1) | (%) | (ton C ha−1 Year−1) | |||||||
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| Middle-elevation | 20.0 | 13.4 | 66 | 62 | 1.04 | 0.71 | 9.0 | 3.6 | 56 | 30 | 0.47 | 0.19 |
| Low-elevation | 14.1 | 6.1 | 133 | 71 | 0.63 | 0.28 | 16.4 | 9.3 | 130 | 89 | 0.86 | 0.49 |
| p = 0.15 | p = 0.06 | p = 0.02 | p = 0.38 | p = 0.07 | p = 0.04 | p = 0.11 | p = 0.10 | p = 0.07 | p = 0.04 | p = 0.11 | p = 0.10 | |
| Middle-elevation 1 | 14.7 | 10.8 | 46 | 53 | 0.79 | 0.58 | 5.2 | 1.1 | 37 | 17 | 0.27 | 0.06 |
| (exclude WL site) | ||||||||||||
| Low-elevation | 14.1 | 6.1 | 133 | 71 | 0.63 | 0.28 | 16.4 | 9.3 | 130 | 89 | 0.86 | 0.49 |
| p = 0.41 | p = 0.13 | p = 0.01 | p = 0.28 | p = 0.14 | p = 0.09 | p < 0.01 | p = 0.01 | p < 0.01 | p = 0.04 | p < 0.01 | p = 0.01 | |
| Annual crops | 16.8 | 9.0 | 110 | 57 | 0.80 | 0.45 | 13.9 | 6.1 | 109 | 58 | 0.73 | 0.32 |
| Perennial crops | 15.7 | 1.1 | 78 | 86 | 0.82 | 0.55 | 10.6 | 8.4 | 61 | 71 | 0.56 | 0.44 |
| p = 0.88 | p = 0.74 | p = 0.45 | p = 0.28 | p = 0.95 | p = 0.67 | p = 0.54 | p = 0.57 | p = 0.27 | p = 0.74 | p = 0.55 | p = 0.57 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Lee, P.-C.; Menyailo, O.V.; Cheng, C.-H. Changes in Soil Organic Carbon Concentration and Stock after Forest Regeneration of Agricultural Fields in Taiwan. Forests 2021, 12, 1222. https://doi.org/10.3390/f12091222
Lin Y-H, Lee P-C, Menyailo OV, Cheng C-H. Changes in Soil Organic Carbon Concentration and Stock after Forest Regeneration of Agricultural Fields in Taiwan. Forests. 2021; 12(9):1222. https://doi.org/10.3390/f12091222
Chicago/Turabian StyleLin, Yi-Han, Pei-Chen Lee, Oleg V. Menyailo, and Chih-Hsin Cheng. 2021. "Changes in Soil Organic Carbon Concentration and Stock after Forest Regeneration of Agricultural Fields in Taiwan" Forests 12, no. 9: 1222. https://doi.org/10.3390/f12091222
APA StyleLin, Y.-H., Lee, P.-C., Menyailo, O. V., & Cheng, C.-H. (2021). Changes in Soil Organic Carbon Concentration and Stock after Forest Regeneration of Agricultural Fields in Taiwan. Forests, 12(9), 1222. https://doi.org/10.3390/f12091222







