Intra- and Inter-Annual Growth Patterns of a Mixed Pine-Oak Forest under Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Species
2.2. Climate Data Processing
2.3. Intra-Annual Observations: Stem Girth Measurements
2.4. Inter-Annual Observations: Tree-Ring Chronologies
2.5. Statistical Analyses
3. Results
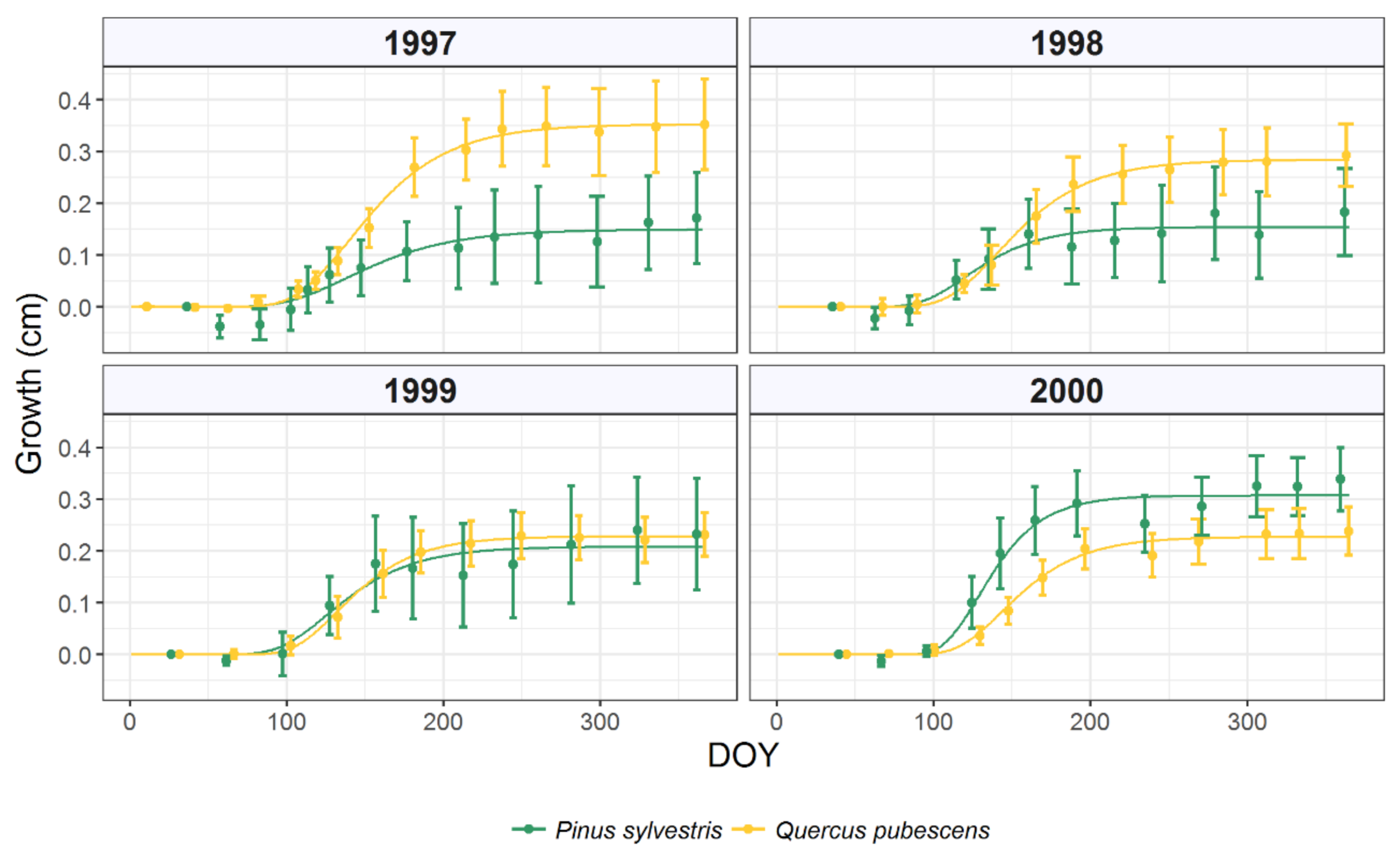
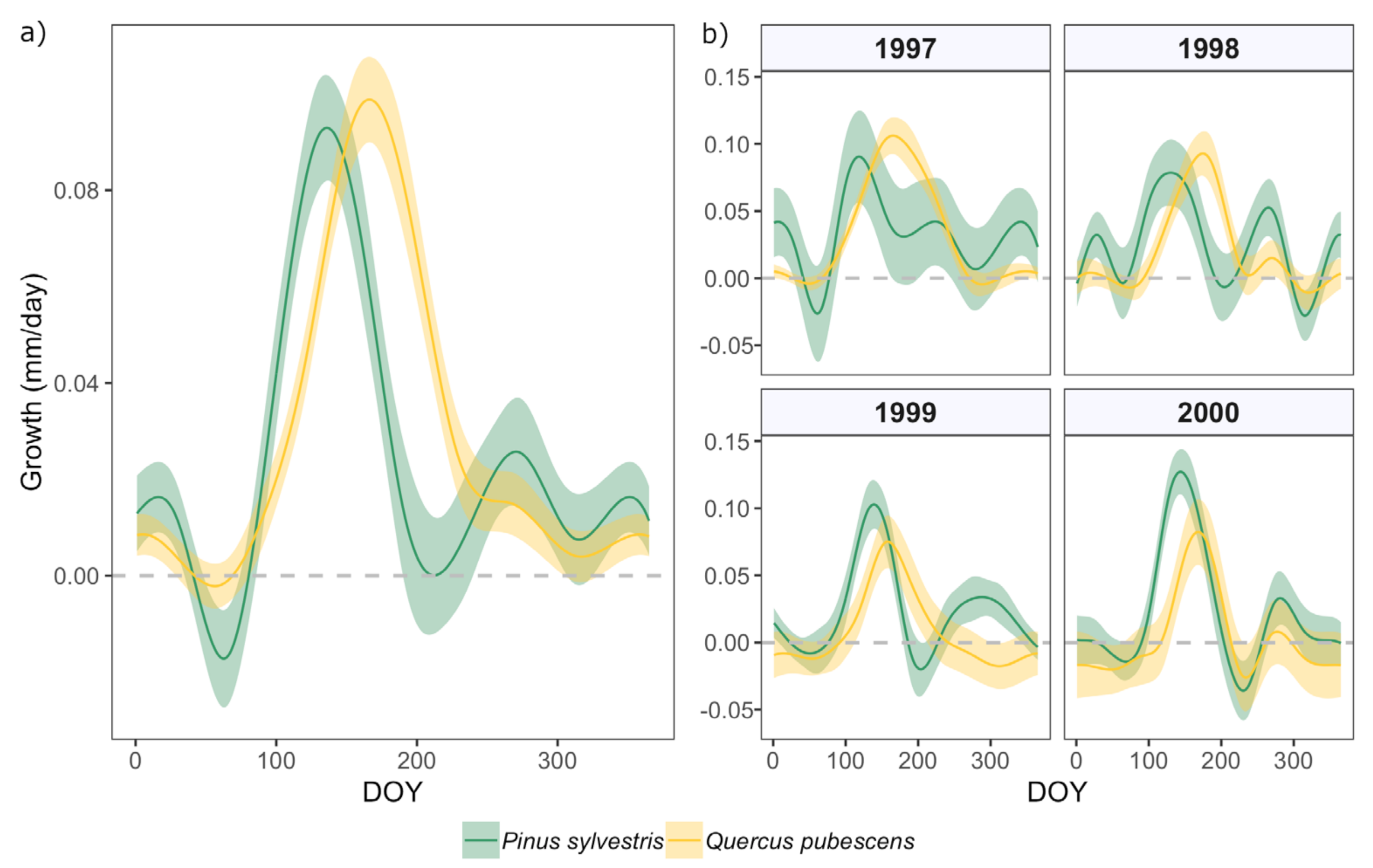
3.1. Intra-Annual Growth Patterns and Climatic Drivers
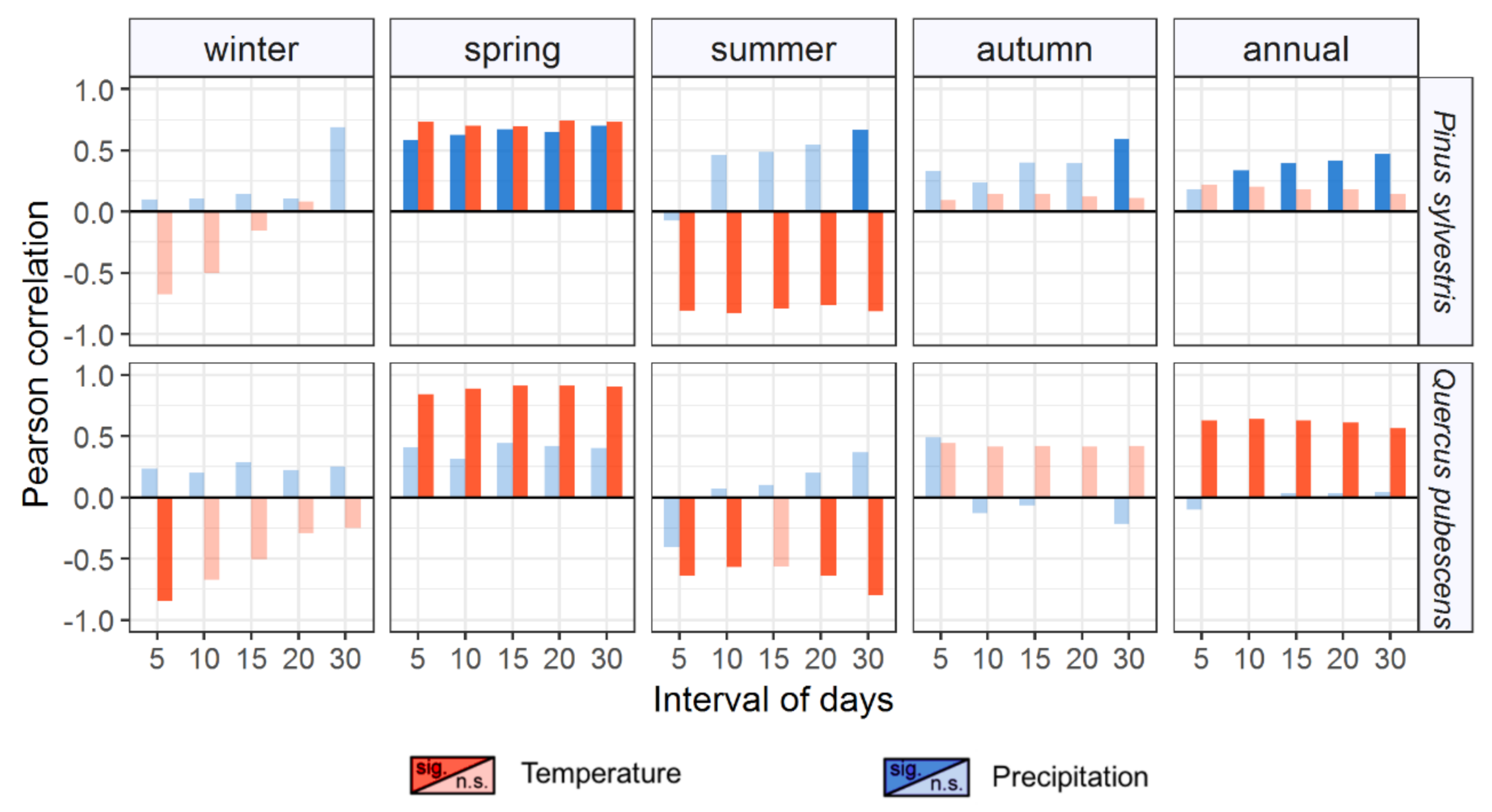
3.2. Long-Term Growth Patterns and Climatic Drivers
4. Discussion
4.1. Niche Complementarity: Phenology and Growth Patterns
4.2. Differential Sensitivity to Climate
4.3. Long-Term Tree Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Mitrakos, K.A. Theory for mediterranean plant life. Acta Oecol. 1980, 1, 245–252. [Google Scholar]
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Chang. 2008, 62, 195–209. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Martínez-Vilalta, J.; Piñol, J. Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. For. Ecol. Manag. 2002, 161, 247–256. [Google Scholar] [CrossRef]
- Bigler, C.; Bräker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an Inciting Mortality Factor in Scots Pine Stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Boada, M. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Chang. Biol. 2003, 9, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Drought-Induced Multifactor Decline of Scots Pine in the Pyrenees and Potential Vegetation Change by the Expansion of Co-occurring Oak Species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Martínez-Vilalta, J.; Galiano, L.; Retana, J. Patterns of Forest Decline and Regeneration across Scots Pine Populations. Ecosystems 2012, 16, 323–335. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Matesanz, S.; Valladares, F. Ecological and evolutionary responses of Mediterranean plants to global change. Environ. Exp. Bot. 2014, 103, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Gulías, J.; Flexas, J.; Abadía, A.; Medrano, H. Photosynthetic responses to water deficit in six Mediterranean sclerophyll species: Possible factors explaining the declining distribution of Rhamnus ludovici-salvatoris, an endemic Balearic species. Tree Physiol. 2002, 22, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D. Competition and biodiversity in spatially structured habitats. Ecology 1994, 75, 2–16. [Google Scholar] [CrossRef]
- Pretzsch, H.; Dieler, J. Evidence of variant intra- and interspecific scaling of tree crown structure and relevance for allometric theory. Oecologia 2012, 169, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Pacala, S.W. Limiting Similarity in Mechanistic and Spatial Models of Plant Competition in Heterogeneous Environments. Am. Nat. 1994, 143, 222–257. [Google Scholar]
- Terradas, J.; Peñuelas, J.; Lloret, F. The Fluctuation Niche in Plants. Int. J. Ecol. 2009, 2009, 959702. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Terradas, J.; Lloret, F. Solving the conundrum of plant species coexistence: Water in space and time matters most. New Phytol. 2011, 189, 5–8. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Benavides, R.; Scherer-Lorenzen, M.; Valladares, F. The functional trait space of tree species is influenced by the species richness of the canopy and the type of forest. Oikos 2019, 128, 1435–1445. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Lebourgeois, F.; Fortin, M.; Fournier, M. Life strategies in intra-annual dynamics of wood formation: Example of three conifer species in a temperate forest in north-east France. Tree Physiol. 2012, 32, 612–625. [Google Scholar] [CrossRef] [Green Version]
- Delpierre, N.; Vitasse, Y.; Chuine, I.; Guillemot, J.; Bazot, S.; Rutishauser, T.; Rathgeber, C.B.K. Temperate and boreal forest tree phenology: From organ-scale processes to terrestrial ecosystem models. Ann. For. Sci. 2016, 73, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees-Struct. Funct. 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Campelo, F.; Gutiérrez, E.; Sánchez-salguero, R.; Nabais, C.; Camarero, J.J. The facultative bimodal growth pattern in Quercus ilex–A simple model to predict sub-seasonal and inter-annual growth. Dendrochronologia 2018, 49, 77–88. [Google Scholar] [CrossRef]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Campelo, F.; Camarero, J.J.; Ribas, M.; Muntán, E.; Nabais, C.; Freitas, H. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees 2011, 25, 637–6463. [Google Scholar] [CrossRef]
- Pacheco, A.; Camarero, J.J.; Ribas, M.; Gazol, A.; Gutierrez, E.; Carrer, M. Disentangling the climate-driven bimodal growth pattern in coastal and continental Mediterranean pine stands. Sci. Total Environ. 2018, 615, 1518–1526. [Google Scholar] [CrossRef]
- Aguadé, D.; Poyatos, R.; Rosas, T.; Martínez-Vilalta, J. Comparative drought responses of Quercus ilex L. and Pinus sylvestris L. In a montane forest undergoing a vegetation shift. Forests 2015, 6, 2505–2529. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sancho, E.; Dorado-Liñán, I.; Gutiérrez Merino, E.; Matiu, M.; Helle, G.; Heinrich, I.; Menzel, A. Increased water-use efficiency translates into contrasting growth patterns of Scots pine and sessile oak at their southern distribution limits. Glob. Chang. Biol. 2017, 24, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Uña, L.; Rossi, S.; Aranda, I.; Fonti, P.; González-González, B.D.; Cañellas, I.; Gea-Izquierdo, G. Xylem and leaf functional adjustments to drought in Pinus sylvestris and Quercus pyrenaica at their elevational boundary. Front. Plant Sci. 2017, 8, 1200. [Google Scholar] [CrossRef]
- Fritts, H. Tree Rings and Climate; Blackburn Press: Caldwell, NJ, USA, 1976; ISBN 0-12-268450-8. [Google Scholar]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The response of Pinus sylvestris to drought: Stomatal control of transpiration and hydraulic conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef]
- Hereş, A.M.; Voltas, J.; López, B.C.; Martínez-Vilalta, J. Drought-induced mortality selectively affects Scots pine trees that show limited intrinsic water-use efficiency responsiveness to raising atmospheric CO2. Funct. Plant Biol. 2014, 41, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Steppe, K.; Sterck, F.J. Stomatal regulation by microclimate and tree water relations: Interpreting ecophysiological field data with a hydraulic plant model. J. Exp. Bot. 2007, 58, 2113–2131. [Google Scholar] [CrossRef]
- Poyatos, R.; Llorens, P.; Piñol, J.; Rubio, C. Response of Scots pine (Pinus sylvestris L.) and pubescent oak (Quercus pubescens Willd.) to soil and atmospheric water deficits under Mediterranean mountain climate. Ann. For. Sci. 2008, 65, 306. [Google Scholar] [CrossRef] [Green Version]
- Morán-López, T.; Poyatos, R.; Llorens, P.; Sabate, S. Effects of past growth trends and current water use strategies on scots pine and pubescent oak drought sensitivity. Eur. J. For. Res. 2014, 133, 369–382. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 68–78. [Google Scholar]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree -ring width series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic responses of Abies pinsapo xylogenesis to drought and competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Wood, S. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006; ISBN 9781420010404. [Google Scholar]
- Wood, S. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometereology. J. Clim. Appl. Metereol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- R Core Team. R Core Team 2014 R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Rossi, S.; Rathgeber, C.B.K.; Deslauriers, A.; Rossi, S. Comparing needle and shoot phenology with xylem development on three conifer species in Italy. Ann. For. Sci. 2009, 66, 606. [Google Scholar] [CrossRef] [Green Version]
- Gričar, J.; Lavrič, M.; Ferlan, M.; Vodnik, D.; Eler, K. Intra-annual leaf phenology, radial growth and structure of xylem and phloem in different tree parts of Quercus pubescens. Eur. J. For. Res. 2017, 136, 625–637. [Google Scholar] [CrossRef]
- Lavrič, M.; Eler, K.; Ferlan, M.; Vodnik, D.; Gričar, J. Chronological Sequence of Leaf Phenology, Xylem and Phloem Formation and Sap Flow of Quercus pubescens from Abandoned Karst Grasslands. Front. Plant Sci. 2017, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Pérez-de-Lis, G.; Olano, J.M.; Rozas, V.; Rossi, S.; Vázquez-Ruiz, R.A.; García-González, I. Environmental conditions and vascular cambium regulate carbon allocation to xylem growth in deciduous oaks. Funct. Ecol. 2017, 31, 592–603. [Google Scholar] [CrossRef] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Martínez-Sancho, E.; Navas, L.K.V.; Seidel, H.; Dorado-Liñán, I.; Menzel, A. Responses of contrasting tree functional types to air warming and drought. Forests 2017, 8, 450. [Google Scholar] [CrossRef] [Green Version]
- Delpierre, N.; Berveiller, D.; Granda, E.; Dufrêne, E. Wood phenology, not carbon input, controls the interannual variability of wood growth in a temperate oak forest. New Phytol. 2016, 210, 459–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, J.; Campelo, F.; Rossi, S.; Carvalho, A.; Freitas, H.; Nabais, C. Adjustment capacity of maritime pine cambial activity in drought-prone environments. PLoS ONE 2015, 10, e0126223. [Google Scholar] [CrossRef] [Green Version]
- De Micco, V.; Balzano, A.; Čufar, K.; Aronne, G.; Gričar, J.; Merela, M.; Battipaglia, G. Timing of false ring formation in Pinus halepensis and arbutus Unedo in southern Italy: Outlook from an analysis of xylogenesis and tree-ring chronologies. Front. Plant Sci. 2016, 7, 705. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Xylogenesis of Pinus pinaster under a Mediterranean climate. Ann. For. Sci. 2014, 71, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Mäkinen, H.; Seo, J.W.; Nöjd, P.; Schmitt, U.; Jalkanen, R. Seasonal dynamics of wood formation: A comparison between pinning, microcoring and dendrometer measurements. Eur. J. For. Res. 2008, 127, 235–245. [Google Scholar] [CrossRef]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Bräker, O.U.; Schoch, W.; Innes, J.L. Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol. Rev. Camb. Philos. Soc. 2003, 78, 119–148. [Google Scholar] [CrossRef] [Green Version]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Fonti, P.; Rigling, A. Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol. 2009, 29, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Graf Pannatier, E.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- García González, I.; Eckstein, D. Climatic signal of earlywood vessels of oak on a maritime site. Tree Physiol. 2003, 23, 497–504. [Google Scholar] [CrossRef]
- Hereş, A.-M.; Martínez-Vilalta, J.; Claramunt López, B. Growth patterns in relation to drought-induced mortality at two Scots pine (Pinus sylvestris L.) sites in NE Iberian Peninsula. Trees 2011, 26, 621–630. [Google Scholar] [CrossRef]
- Dorado-Liñán, I.; Piovesan, G.; Martínez-Sancho, E.; Gea-Izquierdo, G.; Zang, C.; Cañellas, I.; Castagneri, D.; Di Filippo, A.; Gutiérrez, E.; Ewald, J.; et al. Geographical adaptation prevails over species-specific determinism in trees’ vulnerability to climate change at Mediterranean rear-edge forests. Glob. Chang. Biol. 2019, 25, 1296–1314. [Google Scholar] [CrossRef]



| Pinus Sylvestris L. | |||||
|---|---|---|---|---|---|
| Year | A | rmax | DOYrmax | DOYi | DOYe |
| 1997 | 0.149 | 0.055 | 137 | 95 | 251 |
| 1998 | 0.154 | 0.057 | 119 | 89 | 201 |
| 1999 | 0.208 | 0.077 | 126 | 92 | 217 |
| 2000 | 0.307 | 0.113 | 129 | 104 | 195 |
| mean ± SD | 0.205 ± 0.07 a | 0.076 ± 0.03 a | 127 ± 7 a | 95 ± 6 a | 216 ± 25 a |
| Quercus Pubescens Willd. | |||||
| Year | A | rmax | DOYrmax | DOYi | DOYe |
| 1997 | 0.353 | 0.130 | 140 | 102 | 242 |
| 1998 | 0.284 | 0.105 | 141 | 105 | 236 |
| 1999 | 0.228 | 0.084 | 132 | 102 | 215 |
| 2000 | 0.228 | 0.083 | 144 | 112 | 228 |
| mean ± SD | 0.273 ± 0.06 a | 0.101 ± 0.02 b | 139 ± 5 b | 105 ± 5 b | 230 ± 12 a |
| ID | Cores/Trees | Time Span | Mean Length (Years) | Corr | Ms | Growth (SD) mm/yr | EPS > 0.85 Since | |
|---|---|---|---|---|---|---|---|---|
| RW-Quercus | 17/9 | 1925 | 2017 | 70.9 | 0.557 | 0.267 | 0.93 (0.44) | 1943 |
| RW-Pinus | 31/15 | 1853 | 2014 | 120.3 | 0.647 | 0.362 | 1.31 (0.65) | 1865 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sancho, E.; Gutiérrez, E.; Valeriano, C.; Ribas, M.; Popkova, M.I.; Shishov, V.V.; Dorado-Liñán, I. Intra- and Inter-Annual Growth Patterns of a Mixed Pine-Oak Forest under Mediterranean Climate. Forests 2021, 12, 1746. https://doi.org/10.3390/f12121746
Martínez-Sancho E, Gutiérrez E, Valeriano C, Ribas M, Popkova MI, Shishov VV, Dorado-Liñán I. Intra- and Inter-Annual Growth Patterns of a Mixed Pine-Oak Forest under Mediterranean Climate. Forests. 2021; 12(12):1746. https://doi.org/10.3390/f12121746
Chicago/Turabian StyleMartínez-Sancho, Elisabet, Emilia Gutiérrez, Cristina Valeriano, Montse Ribas, Margarita I. Popkova, Vladimir V. Shishov, and Isabel Dorado-Liñán. 2021. "Intra- and Inter-Annual Growth Patterns of a Mixed Pine-Oak Forest under Mediterranean Climate" Forests 12, no. 12: 1746. https://doi.org/10.3390/f12121746
APA StyleMartínez-Sancho, E., Gutiérrez, E., Valeriano, C., Ribas, M., Popkova, M. I., Shishov, V. V., & Dorado-Liñán, I. (2021). Intra- and Inter-Annual Growth Patterns of a Mixed Pine-Oak Forest under Mediterranean Climate. Forests, 12(12), 1746. https://doi.org/10.3390/f12121746








