Characterizing the Utility of the Root-to-Shoot Ratio in Douglas-Fir Seedling Production
Abstract
:1. Introduction
- Is there an inherent R:S value for Douglas-fir seedlings?
- Is there a consistent R:S to which Douglas-fir seedlings are grown in bareroot and container systems?
- Do Douglas-fir seedlings align with a consistent R:S after transplanting?
- Does the starting R:S value correlate with survival after transplanting?
2. Materials and Methods
3. Results
4. Discussion
4.1. Is There an Inherent R:S Ratio for Douglas-Fir Seedlings?
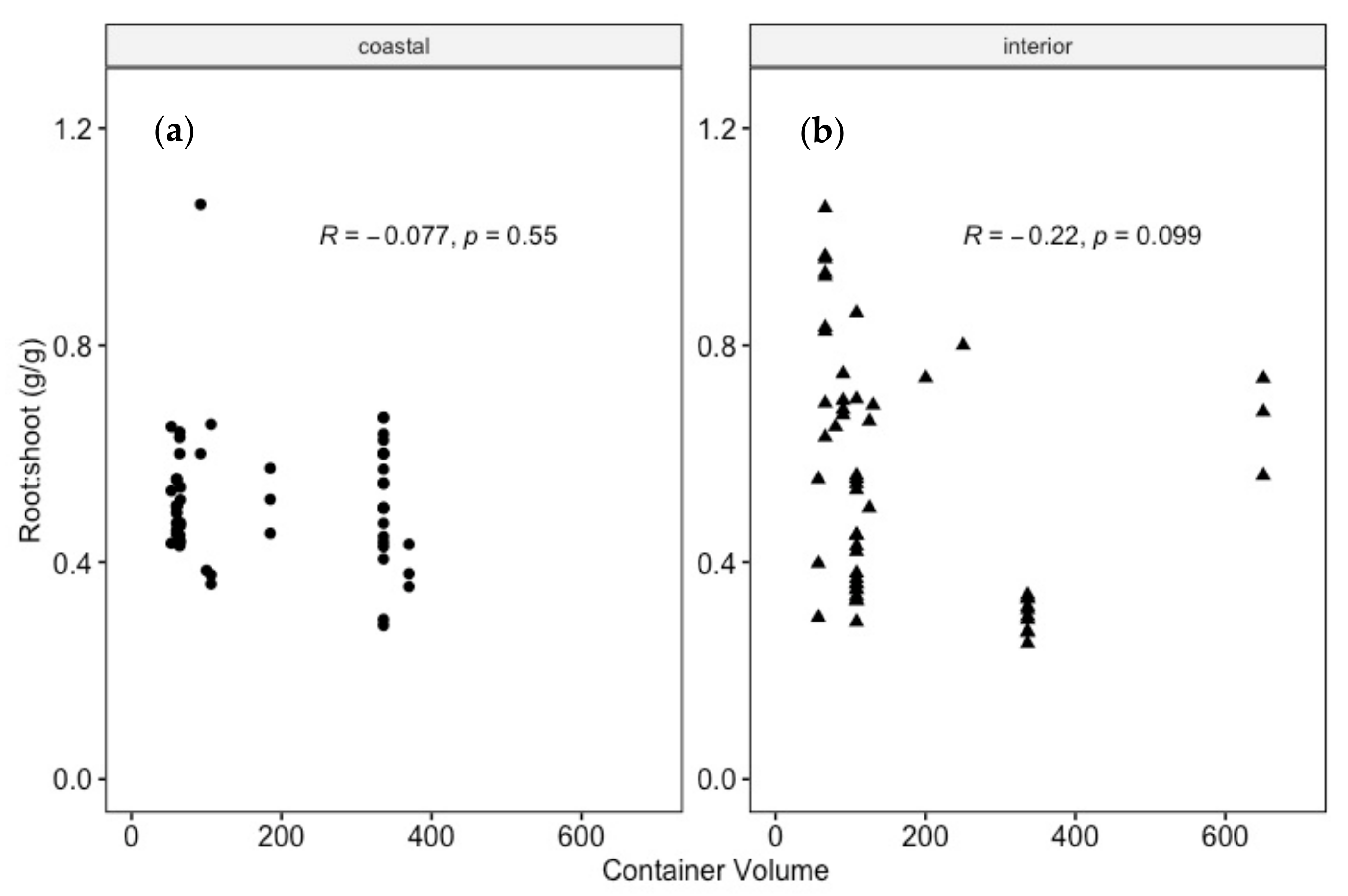
4.2. Is There a Consistent R:S to Which Douglas-Fir Seedlings Are Grown in Bareroot and Container Systems?
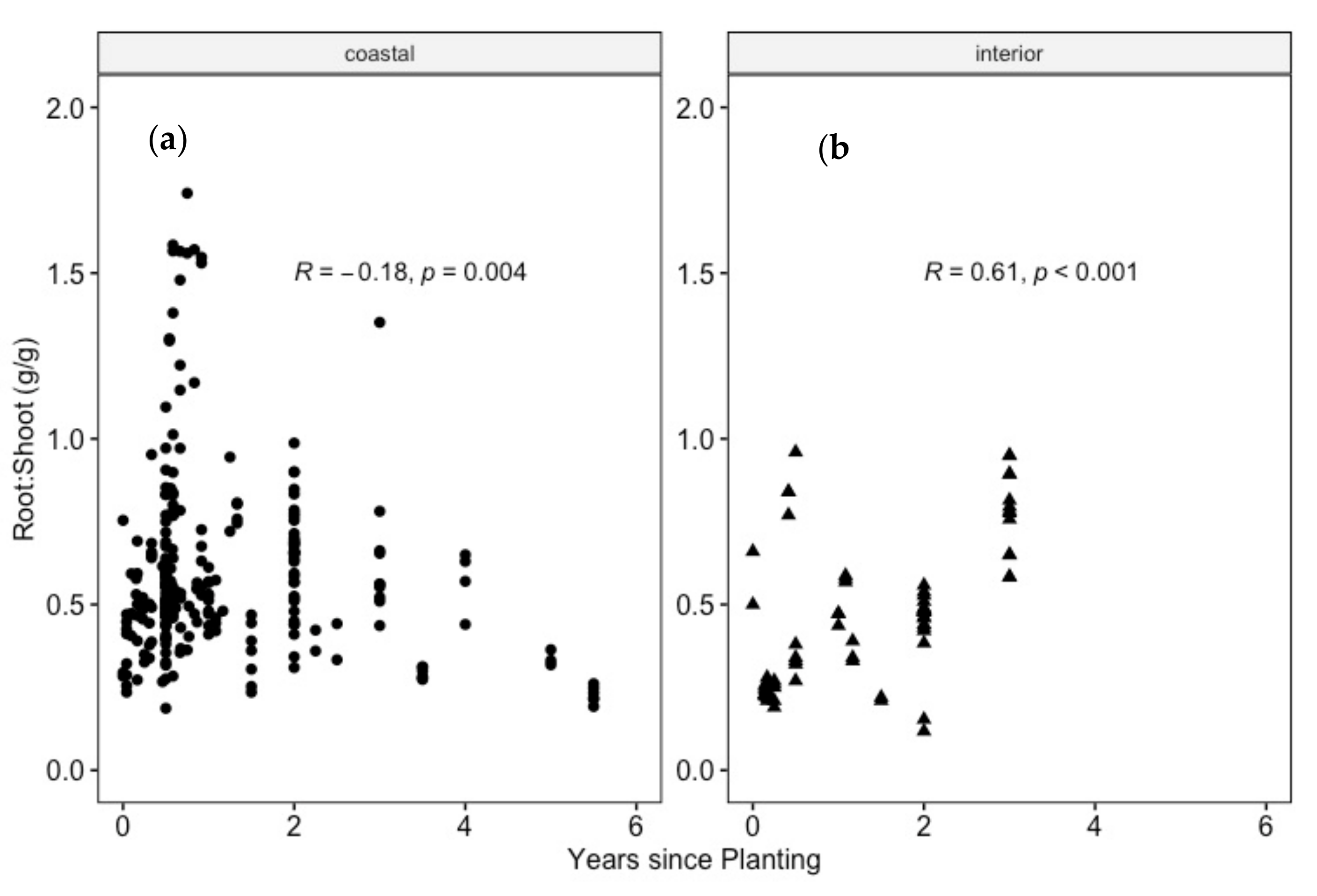
4.3. Do Douglas-Fir Seedlings Align with a Consistent R:S after Transplanting?
4.4. Does Starting R:S Correlate with Survival after Transplanting?
- Measure R:S at the onset of the project to establish a baseline, and after the implementation of experimental treatments,
- Include survival data, whether or not that was the primary focus of the research project, to help with the interpretation of R:S,
- Report the timeframe in which changes in R:S are considered, and
- Clearly communicate the methods used to measure and calculate R:S, and the total size of the seedlings measured.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fargione, J.; Haase, D.L.; Burney, O.T.; Kildisheva, O.A.; Edge, G.; Cook-Patton, S.C.; Chapman, T.; Rempel, A.; Hurteau, M.D.; Davis, K.T.; et al. Challenges to the Reforestation Pipeline in the United States. Front. For. Glob. Chang. 2021, 4, 629198. [Google Scholar] [CrossRef]
- Stone, E.C. Poor survival and the physiological condition of planting stock. For. Sci. 1955, 1, 90–94. [Google Scholar]
- Haase, D.L.; Pike, C.; Enebak, S.; Mackey, L.; Ma, Z.; Silva, C. Forest Nursery Seedling Production in the United States—Fiscal Year 2019. Tree Plant. Notes 2020, 63, 26–31. [Google Scholar]
- Haase, D.L.; Davis, A.S. Developing and supporting quality nursery facilities and staff are necessary to meet gl obal forest and landscape restoration needs. Reforesta 2017, 4, 69–93. [Google Scholar]
- Dumroese, R.K.; Landis, T.D.; Pinto, J.R.; Haase, D.L.; Wilkinson, K.W.; Davis, A.S. Meeting forest restoration challenges: Using the Target Plant Concept. Reforesta 2016, 1, 37–52. [Google Scholar]
- Folk, R.; Grossnickle, S.C. Determining field performance potential with the use of limiting environmental conditions. New For. 1997, 13, 121–138. [Google Scholar]
- Ritchie, G.A. Assessing Seedling Quality. In Forest Nursery Manual: Production of Bareroot Seedlings; Duryea, M.L., Landis, T.L., Eds.; Martinus Nijhoff/Dr W. Junk Publishers: The Hague, The Netherlands; Boston, MA, USA; Lancaster, UK; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1984; pp. 243–259. [Google Scholar]
- Duryea, M.L. Evaluating seedling quality: Importance to reforestation. In Evaluating Seedling Quality: Principles, Procedures, and Predictive Abilities of Major Tests, Proceedings of the Workshop, Corvallis, OR, USA, 16–18 October 1984; Duryea, M.L., Ed.; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1985; pp. 1–4. [Google Scholar]
- Dumroese, R.K.; James, R.L.; Wenny, D.L. Hot Water and Copper Coating in Reused Containers Decrease Inoculum of Fusarium and Cylindrocarpon and Increase Douglas Fir Seedling Growth. HortScience 2002, 37, 943–947. [Google Scholar]
- Jacobs, D.F.; Rose, R.; Haase, D.L.; Morgan, P.D. Influence of nursery soil amendments on water relations, root architectural development, and field performance of Douglas-fir transplants. New For. 2003, 26, 263–277. [Google Scholar]
- Jacobs, D.F.; Davis, A.S.; Wilson, B.C.; Dumroese, R.K.; Goodman, R.C.; Salifu, K.F. Short-day treatment alters Douglas-fir seedling dehardening and transplant root proliferation at varying rhizosphere temperatures. Can. J. For. Res. 2008, 38, 1526–1535. [Google Scholar]
- Conlin, T.S.S.; van den Driessche, R. Influence of nutrient supply and water vapour pressure on root architecture of Douglas-fir and western hemlock seedlings. Funct. Plant Biol. 2006, 33, 941–948. [Google Scholar]
- Everett, K.T.; Hawkins, B.J.; Kiiskila, S. Growth and nutrient dynamics of Douglas-fir seedlings raised with exponential or conventional fertilization and planted with or without fertilizer. Can. J. For. Res. 2007, 37, 2552–2562. [Google Scholar]
- Dučić, T.; Berthold, D.; Langenfeld-Heyser, R.; Beese, F.; Polle, A. Mycorrhizal communities in relation to biomass production and nutrient use efficiency in two varieties of Douglas fir (Pseudotsuga menziesii var. menziesii and var. glauca) in different forest soils. Soil Biol. Biochem. 2009, 41, 742–753. [Google Scholar]
- Fleming, R.L.; Black, T.A.; Adams, R.S.; Stathers, R.J. Silvicultural treatments, microclimatic conditions and seedling response in Southern Interior clearcuts. Can. J. Soil Sci. 1998, 78, 115–126. [Google Scholar]
- Schneider, W.G.; Knowe, S.A.; Harrington, T.B. Predicting survival of planted Douglas-fir and ponderosa pine seedlings on dry, low-elevation sites in southwestern Oregon. New For. 1998, 15, 139–159. [Google Scholar]
- Wahlenberg, W.G. Experiments with classes of stock suitable for forest planting in the Northern Rocky Mountains. J. Agric. Res. 1928, 36, 977–1000. [Google Scholar]
- Sutton, R.F.; Tinus, R.W. Root and Root System Terminology. For. Sci. 1983, 29 (Suppl. 1), a0001–z0001. [Google Scholar]
- Racey, G.D.; Glerum, C.; Hutchison, R.E. The Practicality of Top-root Ratio in Nursery Stock Characterization. For. Chron. 1983, 2, 240–243. [Google Scholar]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar]
- Bernier, P.Y.; Lamhamedi, M.S.; Simpson, D.G. Shoot: Root Ratio Is of Limited Use in Evaluating the Quality of Container Conifer Stock. Tree Plant. Notes 1995, 46, 102–106. [Google Scholar]
- Roberts, R.; Struckmeyer, B. The effect of top environment and flowering upon top-root ratios. Plant Physiol. 1946, 21, 332–344. [Google Scholar]
- Lavender, D. Plant physiology and nursery environment: Interactions affecting seedling growth. In Forest Nursery Manual: Production of Bareroot Seedlings; Duryea, M.L., Landis, T.L., Eds.; Martinus Nijhoff/Dr W. Junk Publishers: The Hague, The Netherlands; Boston, MA, USA; Lancaster, UK; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1984; pp. 133–141. [Google Scholar]
- Thompson, B.E. Seedling morphological evaluation: What you can tell by looking. In Evaluating Seedling Quality: Principles, Procedures, and Predictive Abilities of Major Tests, Proceedings of the Workshop, Corvallis, OR, USA, 16–18 October 1984; Duryea, M.L., Ed.; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1985; pp. 59–71. [Google Scholar]
- Haase, D. Seedling Root Targets. In National Proceedings of the Forest and Conservation Nursery Associations—2010; Riley, L.E., Haase, D.L., Pinto, J.R., Eds.; Tech. Coords; Proc. RMRS-P-65; United States Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 80–82. [Google Scholar]
- Lopushinsky, W.; Beebe, T. Relationship of Shoot-Root Ratio to Survival and Growth of Outplanted Douglas-Fir and Ponderosa Pine Seedlings; Research Note PNW-274; United States Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1976; 8p. [Google Scholar]
- Strothmann, R.O.; Roy, D.F. Regeneration of Douglas-Fir in the Klamath Mountains Region, California and Oregon; General Technical Report PSW-81; United States Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1984; 35p. [Google Scholar]
- Hermann, R.K.; Lavender, D.P. Douglas-fir planted forests. New For. 1999, 17, 53–70. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer Version 4.2. Available online: https://automeris.io/WebPlotDigitizer (accessed on 16 April 2019).
- Teste, F.P.; Simard, S.W.; Durall, D.M.; Guy, R.D.; Berch, S.M. Net carbon transfer between Pseudotsuga menziesii var. Glauca seedlings in the field is influenced by soil disturbance. J. Ecol. 2010, 98, 429–439. [Google Scholar] [CrossRef]
- Sheridan, R.A. Influence of Limited Water Access on Douglas-fir Seedling Root Systems, Physiology, and Growth. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2019. [Google Scholar]
- Barker, J.S.; Simard, S.W.; Jones, M.D. Clearcutting and high severity wildfire have comparable effects on growth of direct-seeded interior Douglas-fir. For. Ecol. Manag. 2014, 331, 188–195. [Google Scholar] [CrossRef]
- Strothmann, R.O. Douglas-fir in Northern California: Effects of Shade on Germination, Survival, and Growth; General Technical Report PSW-84; United States Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1972; 10p. [Google Scholar]
- Cannell, M.G.R.; Tabbush, P.M.; Deans, J.D.; Hollingsworth, M.K.; Sheppard, L.J.; Philipson, J.J.; Murray, M.B. Sitka spruce and douglas fir seedlings in the nursery and in cold storage: Root growth potential, carbohydrate content, dormancy, frost hardiness and mitotic index. Forestry 1990, 63, 9–27. [Google Scholar] [CrossRef]
- Minore, D. Germination, Survival and Early Growth of Conifer Seedlings in Two Habitat Types; Research Paper PNW-348; United States Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 1986; 25p. [Google Scholar]
- Chan, S.S.; Radosevich, S.R.; Grotta, A.T. Effects of contrasting light and soil moisture availability on the growth and biomass allocation of Douglas-fir and red alder. Can. J. For. Res. 2003, 33, 106–117. [Google Scholar] [CrossRef]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.K.; Davis, A.S.; Cobos, D.R. Photosynthetic response, carbon isotopic composition, survival, and growth of three stock types under water stress enhanced by vegetative competition. Can. J. For. Res. 2012, 42, 333–344. [Google Scholar] [CrossRef]
- Joly, R.J.; Adams, W.T.; Stafford, S.G. Phenological and Morphological Responses of Mesic and Dry Site Sources of Coastal Douglas-fir to Water Deficit. For. Sci. 1989, 35, 987–1005. [Google Scholar]
- Danjon, F.; Stokes, A.; Bakker, M.R. Root Systems of Woody Plants. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Baton Rouge, LA, USA, 2013; pp. 29-1–29-26. ISBN 9781439846483. [Google Scholar]
- McCulloh, K.A.; Domec, J.; Johnson, D.M.; Smith, D.D.; Meinzer, F.C. A dynamic yet vulnerable pipeline: Integration and coordination of hydraulic traits across whole plants. Plant Cell Environ. 2019, 42, 2789–2807. [Google Scholar] [CrossRef] [Green Version]
- Tyree, M.T.; Patiño, S.; Bennink, J.; Alexander, J. Dynamic Measurements of Root hydraulic conductance using a high-pressure flowmeter in the laboratory and field. J. Exp. Bot. 1995, 46, 83–94. [Google Scholar]
- Meinzer, F.C.; Bond, B.J.; Warren, J.M.; Woodruff, D.R. Does water transport scale universally with tree size? Funct. Ecol. 2005, 19, 558–565. [Google Scholar] [CrossRef]
- Tyree, M.T.; Velez, V.; Dalling, J.W. Growth conductance dynamics of root and shoot hydraulic in seedlings of five neotropical tree species: To differng light regimes scaling to show possible adaptation. Oecologia 1998, 114, 293–298. [Google Scholar]
- Sperry, J.S.; Love, D.M. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 2015, 207, 14–27. [Google Scholar]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Downie, H.; Holden, N.; Otten, W.; Spiers, A.J.; Valentine, T.A.; Dupuy, L.X. Transparent Soil for Imaging the Rhizosphere. PLoS ONE 2012, 7, e44276. [Google Scholar] [CrossRef]
- Lobet, G.; Koevoets, I.T.; Noll, M.; Meyer, P.E.; Tocquin, P.; Pagès, L.; Périlleux, C. Using a structural root system model to evaluate and improve the accuracy of root image analysis pipelines. Front. Plant Sci. 2017, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- McCormack, M.L.; Guo, D.; Iversen, C.M.; Chen, W.; Eissenstat, D.M.; Fernandez, C.W.; Li, L.; Ma, C.; Ma, Z.; Poorter, H.; et al. Building a better foundation: Improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 2017, 215, 27–37. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheridan, R.A.; Davis, A.S. Characterizing the Utility of the Root-to-Shoot Ratio in Douglas-Fir Seedling Production. Forests 2021, 12, 1745. https://doi.org/10.3390/f12121745
Sheridan RA, Davis AS. Characterizing the Utility of the Root-to-Shoot Ratio in Douglas-Fir Seedling Production. Forests. 2021; 12(12):1745. https://doi.org/10.3390/f12121745
Chicago/Turabian StyleSheridan, Rebecca A., and Anthony S. Davis. 2021. "Characterizing the Utility of the Root-to-Shoot Ratio in Douglas-Fir Seedling Production" Forests 12, no. 12: 1745. https://doi.org/10.3390/f12121745
APA StyleSheridan, R. A., & Davis, A. S. (2021). Characterizing the Utility of the Root-to-Shoot Ratio in Douglas-Fir Seedling Production. Forests, 12(12), 1745. https://doi.org/10.3390/f12121745





