Shifting Precipitation Patterns Drive Growth Variability and Drought Resilience of European Atlas Cedar Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites: Atlas Cedar Plantations in South-Western Europe
2.2. Climate Data, Climate and Atmospheric Circulation Indices, and Drought Index
2.3. Field Sampling and Tree-Ring Width Data
2.4. Processing Tree-Ring Data: Ring-Width Indices and Climate–Growth Relationships
2.5. Negative Pointer Years and Resilience Indices
2.6. Linear Mixed-Effects Models of Growth Resilience
3. Results
3.1. Growth Patterns and Negative Pointer Years at Site Level
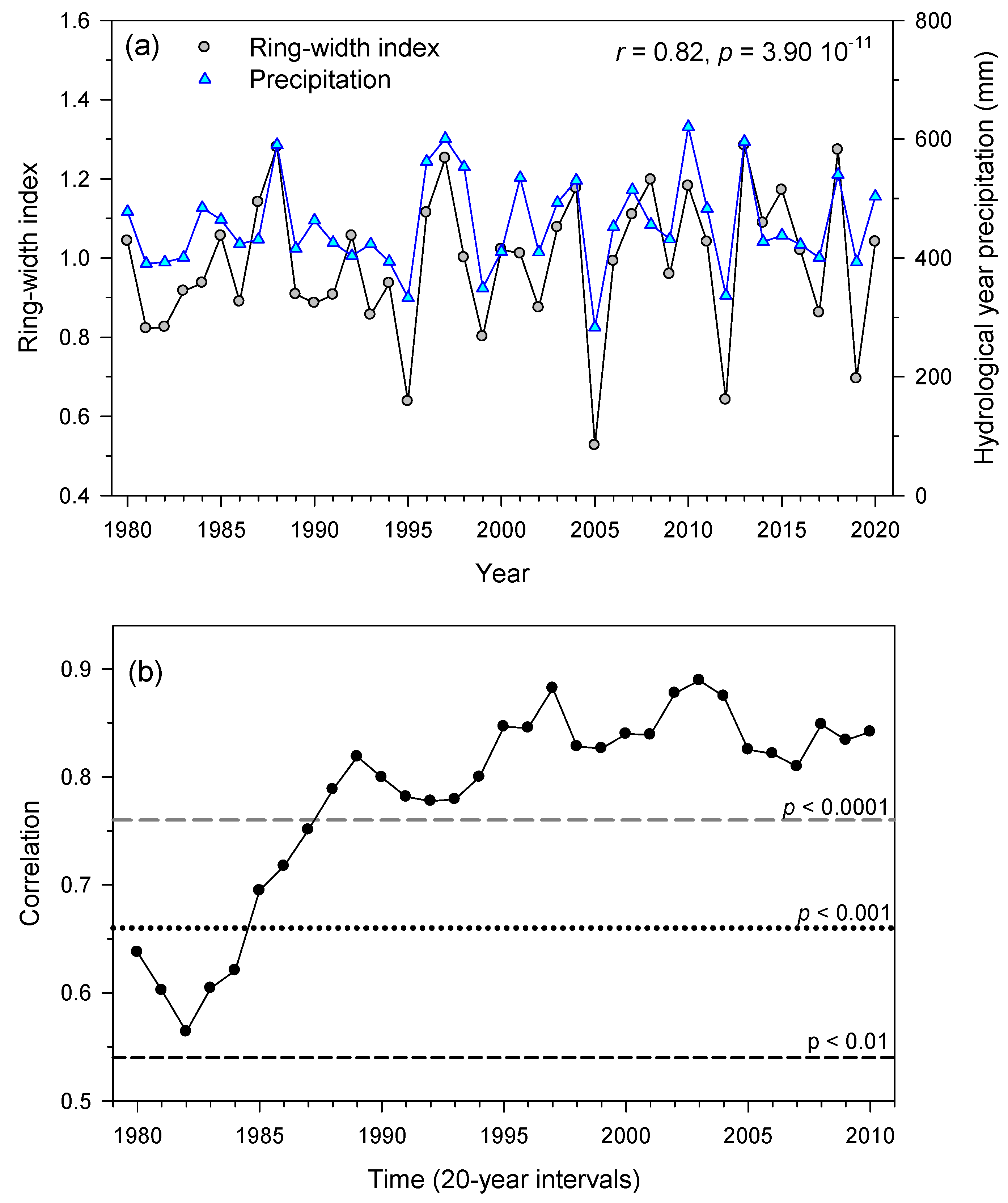
3.2. Links of Growth with Climate Variability and Drought Severity at Site Level
3.3. Models of Resilience Indices at Individual Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Chang. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Granda, E.; Camarero, J.J.; Vilà-Cabrera, A.; Jump, A.S.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Imbert, J.B.; Gazol, A. Impacts of recurrent dry and wet years alter long-term tree growth trajectories. J. Ecol. 2021, 109, 1561–1574. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Schwalm, C.R.; Biondi, F.; Camarero, J.J.; Koch, G.W.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef] [PubMed]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Domke, G.M.; Oswalt, S.N.; Walters, B.F.; Morin, R.S. Tree planting has the potential to increase carbon sequestration capacity of forests in the United States. Proc. Nat. Acad. Sci. USA 2020, 117, 24649–24651. [Google Scholar] [CrossRef]
- Isaac-Renton, M.G.; Roberts, D.R.; Hamann, A.; Spiecker, H. Douglas-fir plantations in Europe: A retrospective test of assisted migration to address climate change. Glob Chang. Biol. 2014, 20, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Regenerate natural forests to store carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Navarro-Cerrillo, R.M.; Camarero, J.J.; Fernández-Cancio, A. Selective drought-induced decline of pine species in southeastern Spain. Clim. Chang. 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodríguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative drought stress leads to a loss of growth resilience and explains higher mortality in planted than in naturally regenerated Pinus pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Courbet, F.; Lagacherie, M.; Marty, P.; Ladier, J.; Ripert, C.; Amandier, L.; Paillassa, E.; Guillemot, J. Le cèdre en France face au changement climatique: Un projet pour un bilan et un transfert des connaissances. Forêt Entrep. 2012, 204, 41–45. [Google Scholar]
- Messinger, J.; Güney, A.; Zimmermann, R.; Ganser, B.; Bachmann, M.; Remmele, S.; Aas, G. Cedrus libani: A promising tree species for Central European forestry facing climate change? Eur. J. For. Res. 2015, 134, 1005–1017. [Google Scholar] [CrossRef]
- Benabid, A. Biogéographie phytosociologie et phytodynamique des cédraies de l’Atlas à Cedrus atlantica (Manetti). Ann. Rech. For. Maroc 1994, 27, 62–76. [Google Scholar]
- Linares, J.C.; Taïqui, L.; Sangüesa-Barreda, G.; Seco, J.I.; Camarero, J.J. Age-related drought sensitivity of Atlas cedar (Cedrus atlantica) in the Moroccan Middle Atlas forests. Dendrochronologia 2013, 31, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Bentouati, A. La situation du cèdre de l’Atlas en Algerie. Méditerranéenne 2008, 29, 203–209. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Linares, J.C.; Taïqui, L.; Camarero, J.J. Increasing drought sensitivity and decline of Atlas cedar (Cedrus atlantica) in the Moroccan Middle Atlas forests. Forests 2011, 2, 777–796. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Cerrillo, R.M.; Sarmoum, M.; Gazol, A.; Abdoun, F.; Camarero, J.J. The decline of Algerian Cedrus atlantica forests is driven by a climate shift towards drier conditions. Dendrochronologia 2019, 55, 60–70. [Google Scholar] [CrossRef]
- Camarero, J.J.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Lechuga, V.; Viñegla, B.; Seco, J.I.; Taïqui, L.; Carreira, J.A.; Linares, J.C. Drought, axe and goats. More variable and synchronized growth forecasts worsening dieback in Moroccan Atlas cedar forests. Sci. Tot. Env. 2021, 765, 142752. [Google Scholar] [CrossRef] [PubMed]
- Castagneri, D.; Vacchiano, G.; Hacket-Pain, A.; DeRose, R.J.; Klein, T.; Bottero, A. Meta-analysis reveals different competition effects on tree growth resistance and resilience to drought. Ecosystems 2021, 1–14. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Ryan, M.G.; Phillips, N.; Bond, B.J. The hydraulic limitation hypothesis revisited. Plant, Cell Environ. 2006, 29, 367–381. [Google Scholar] [CrossRef]
- M’Hirit, O.; Benzyane, M. Le Cèdre de l’Atlas. Mémoire du Temps; Éditions La croisée des chemins: Casablanca, Morocco, 2006. [Google Scholar]
- Aussenac, G.; Finkelstein, D. Influence de la sécheresse sur la croissance et la photosynthèse du cèdre. Ann. Sci. For. 1983, 40, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Ducrey, M. Adaptation du Cèdre de l’Atlas (Cedrus atlantica Manetti) au climat méditerranéen; aspects écophysiologiques de sa réaction à la sécheresse. Ann. Rech. For. Maroc. 1994, 27, 140–153. [Google Scholar]
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.J.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation datasets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, N.L. Climatic control of vegetation distribution: The role of the water balance. Am. Nat. 1990, 13, 649–670. [Google Scholar] [CrossRef]
- Willmott, C.J.; Rowe, C.M.; Mintz, Y. Climatology of the terrestrial seasonal water cycle. J. Clim. 1985, 5, 589–606. [Google Scholar] [CrossRef]
- Camarero, J.J. Direct and indirect effects of the North Atlantic Oscillation on tree growth and forest decline in northeastern Spain. In Hydrological, Socioeconomic and Ecological Impacts of the North Atlantic Oscillation in the Mediterranean Region; Vicente-Serrano, S.M., Trigo, R.M., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 129–152. [Google Scholar]
- Rodó, X.; Baert, E.; Comin, F.A. Variations in seasonal rainfall in southern Europe during the present century: Relationships with the North Atlantic Oscillation and the El Niño Southern Oscillation. Clim. Dyn. 1997, 13, 275–284. [Google Scholar] [CrossRef]
- Martín-Vide, J.; López-Bustins, J.-A. The Western Mediterranean Oscillation and rainfall in the Iberian Peninsula. Int. J. Clim. 2006, 26, 1455–1475. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, D.K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 21, 414–416. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Biondi, F.; Qeadan, F. A theory-driven approach to tree-ring standardization: Defining the biological trend from expected basal area increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Fritts, H. Tree-Rings and Climate; Academic Press: Lonndon, UK, 1976. [Google Scholar]
- Cook, E.R.; Krusic, P. A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics. Available online: https://ininet.org/program-arstan-a-tree-ring-standardization-program-based-on-de.html (accessed on 6 October 2021).
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; KAP: New York, NY, USA, 1990; pp. 137–152. [Google Scholar]
- Schweingruber, F.H.; Eckstein, D.; Serre-Bachet, F.; Bräker, O.U. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 1990, 8, 9–38. [Google Scholar]
- Van der Maaten-Theunissen, M.; van der Maaten, E.; Bouriaud, O. pointRes: An R package to analyze pointer years and components of resilience. Dendrochronologia 2015, 35, 34–38. [Google Scholar] [CrossRef]
- Hoffmann, N.; Schall, P.; Ammer, C.; Leder, B.; Vor, T. Drought sensitivity and stem growth variation of nine alien and native tree species on a productive forest site in Germany. Agric. For. Meteorol. 2018, 256–257, 431–444. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying growth responses of trees to drought—A critique of commonly used resilience indices and recommendations for future studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Zheng, T.; Martínez-Vilalta, J.; García-Valdés, R.; Gazol, A.; Camarero, J.J.; Mencuccini, M. Disentangling biology from mathematical necessity in twentieth-century gymnosperm resilience trends. Nat. Ecol. Evol. 2021, 5, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Linear and nonlinear mixed effects models. R Package Vers. 3 2007, 57, 1–89. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, K. MuMIn: Multi-Model Inference. Available online: http://CRAN.R-project.org/package=MuMIn (accessed on 18 October 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 6 October 2021).
- Rubio-Cuadrado, A.; Camarero, J.J.; Gómez, C.; Cañellas, I.; Aulló-Maestro, I.; Gil, L.; Montes, F. Scots pine plantations growth adaptation to climate warming in locations at the southernmost distribution limit of the species. Dendrochronologia 2020, 63, 125745. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Abrupt growth changes in conifers. IAWA J. 1986, 7, 277–283. [Google Scholar] [CrossRef]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef]
- Güney, A.; Kerr, D.; Sökücü, A.; Zimmermann, R.; Küppers, M. Cambial activity and xylogenesis in stems of Cedrus libani A. Rich at different altitudes. Bot. Stud. 2015, 56, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Till, C. The summary response function of Cedrus atlantica (Endl.) Carrière in Morocco. Tree-Ring Bull. 1987, 47, 23–36. [Google Scholar]
- Akkemik, U. Tree rings of Cedrus libani at the northern boundary of its natural distribution. IAWA J. 2003, 24, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Preisler, Y.; Tatarinov, F.; Grünzweig, J.M.; Bert, D.; Ogée, J.; Wingate, L.; Rotenberg, E.; Rohatyn, S.; Her, N.; Moshe, I.; et al. Mortality versus survival in drought-affected Aleppo pine forest depends on the extent of rock cover and soil stoniness. Funct. Ecol. 2019, 33, 901–912. [Google Scholar] [CrossRef]
- Hurrell, J.W. Decadal trends in the North Atlantic Oscillation: Regional temperatures and precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Esper, J.; Frank, D.; Büntgen, U.; Verstege, A.; Luterbacher, J.; Xoplaki, E. Long-term drought severity variations in Morocco. Geophys. Res. Lett. 2007, 34, L17702. [Google Scholar] [CrossRef] [Green Version]
- Trigo, R.M.; Pozo-Vázquez, D.; Osborn, T.J.; Castro-Díez, Y.; Gámiz-Fortis, S.; Esteban-Parra, M.J. North Atlantic Oscillation influence on precipitation, river flow and water resources in the Iberian Peninsula. Int. J. Climatol. 2004, 24, 925–944. [Google Scholar] [CrossRef]
- Guillemot, J.; Klein, E.K.; Davi, H.; Courbet, F. The effects of thinning intensity and tree size on the growth response to annual climate in Cedrus atlantica: A linear mixed modeling approach. Ann. For. Sci. 2015, 72, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Paquette, A.; Messier, C. The role of plantations in managing the world’s forests in the Anthropocene. Front. Ecol. Env. 2010, 8, 27–34. [Google Scholar] [CrossRef] [Green Version]



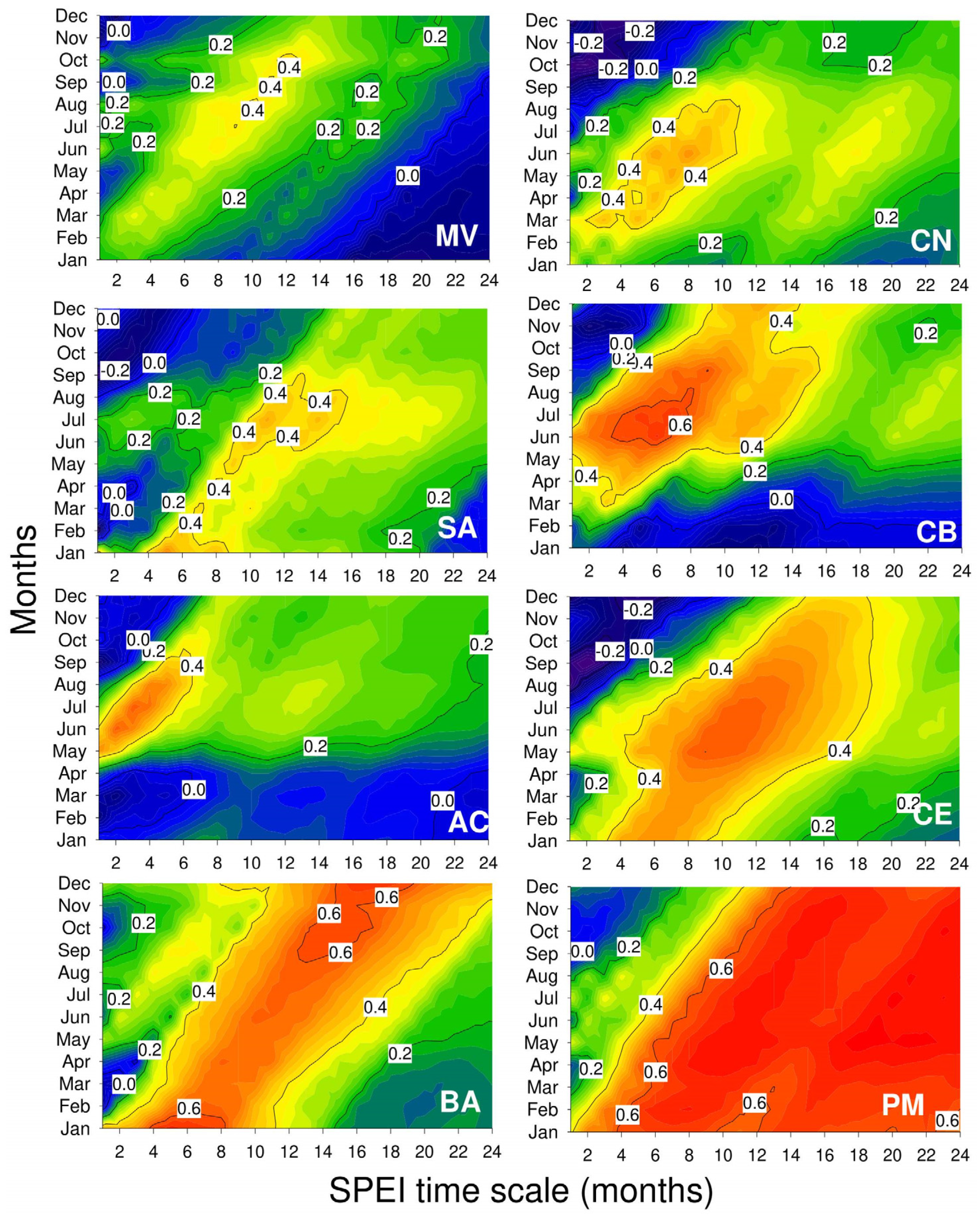

| Site (Code) | Latitude N | Longitude (−W, +E) | Elevation (m a.s.l.) | Mean Annual Temperature (Mean Minimum–Maximum Temperatures) (°C) | Annual Precipitation (mm) | Annual Water Balance (mm) |
|---|---|---|---|---|---|---|
| Valliguières (MV) | 44.019 | 4.624 | 256 | 14.0 (−1.8–26.5) | 699 | −66 |
| Berriozar (CN) | 42.844 | −1.664 | 450 | 12.6 (−0.1–24.7) | 829 | 156 |
| Sariñena (SA) | 41.783 | −0.222 | 295 | 14.4 (−0.5–30.5) | 390 | −620 |
| Bañón (CB) | 40.800 | −1.179 | 1358 | 13.0 (−1.0–26.5) | 423 | −386 |
| Monte Abantos (AC) | 40.609 | −4.146 | 1325 | 12.0 (−0.1–28.0) | 526 | −158 |
| Monte Mario-Béjar (CE) | 40.384 | −5.749 | 1010 | 11.0 (0.8–29.5) | 507 | −144 |
| Baza (BA) | 37.305 | −2.908 | 1780 | 10.5 (0.1–26.4) | 470 | −295 |
| Puerto de la Mora (PM) | 37.286 | −3.458 | 1380 | 13.8 (0.5–28.6) | 411 | −470 |
| Site | Dbh (cm) | Age at 1.3 m (Years) | No. Trees (No. Radii) | Tree-Ring Width (mm) | First-Order Autocorrelation (AC1) | Mean Sensitivity (MSx) | Correlation with Master | Best Replicated Period | Frequency of Negative Pointer Years (%) | R2prec (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| MV | 32.1 ± 1.5 | 39 ± 3 | 8 (16) | 2.30 ± 0.07 | 0.79 ± 0.01 | 0.28 ± 0.01 | 0.69 ± 0.01 | 1977–2017 | 6.8 ± 1.9 | 27.3 |
| CN | 39.0 ± 2.1 | 69 ± 2 | 12 (24) | 2.20 ± 0.08 | 0.73 ± 0.02 | 0.37 ± 0.01 | 0.81 ± 0.02 | 1957–2020 | 17.0 ± 3.8 | 32.4 |
| SA | 36.8 ± 3.0 | 36 ± 1 | 12 (24) | 4.00 ± 0.25 | 0.69 ± 0.04 | 0.26 ± 0.02 | 0.60 ± 0.02 | 1981–2020 | 11.5 ± 1.8 | 15.0 |
| CB | 28.3 ± 0.9 | 53 ± 1 | 19 (38) | 2.22 ± 0.06 | 0.59 ± 0.02 | 0.49 ± 0.01 | 0.88 ± 0.01 | 1967–2020 | 21.0 ± 4.9 | 45.1 |
| AC | 43.4 ± 2.3 | 65 ± 2 | 17 (34) | 2.53 ± 0.15 | 0.65 ± 0.09 | 0.31 ± 0.01 | 0.62 ± 0.02 | 1957–2020 | 13.6 ± 2.2 | 22.5 |
| CE | 41.6 ± 1.0 | 58 ± 1 | 16 (32) | 2.91 ± 0.09 | 0.65 ± 0.03 | 0.41 ± 0.01 | 0.80 ± 0.01 | 1965–2020 | 15.0 ± 3.3 | 47.7 |
| BA | 26.3 ± 1.4 | 36 ± 1 | 16 (32) | 2.94 ± 0.16 | 0.54 ± 0.04 | 0.34 ± 0.02 | 0.75 ± 0.02 | 1981–2020 | 12.2 ± 2.6 | 61.3 |
| PM | 35.5 ± 1.1 | 45 ± 1 | 15 (30) | 2.69 ± 0.06 | 0.81 ± 0.01 | 0.32 ± 0.01 | 0.80 ± 0.02 | 1974–2020 | 19.1 ± 3.6 | 38.6 |
| Coefficient | SE | df | t | p | |
|---|---|---|---|---|---|
| Rt | |||||
| Intercept | 0.983 | 0.010 | 120 | 101.168 | <0.001 |
| Age | 0.002 | 0.001 | 120 | 2.989 | 0.003 |
| MSx | 0.103 | 0.025 | 120 | 4.135 | <0.001 |
| Rc | |||||
| Intercept | 0.263 | 0.005 | 120 | 52.287 | <0.001 |
| MSx | 0.186 | 0.009 | 120 | 20.451 | <0.001 |
| TRW | 0.003 | 0.001 | 120 | 3.027 | 0.002 |
| Rs | |||||
| Intercept | 0.990 | 0.011 | 121 | 86.948 | <0.001 |
| Age | 0.002 | 0.000 | 121 | 7.451 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero, J.J.; Gazol, A.; Colangelo, M.; Linares, J.C.; Navarro-Cerrillo, R.M.; Rubio-Cuadrado, Á.; Silla, F.; Dumas, P.-J.; Courbet, F. Shifting Precipitation Patterns Drive Growth Variability and Drought Resilience of European Atlas Cedar Plantations. Forests 2021, 12, 1751. https://doi.org/10.3390/f12121751
Camarero JJ, Gazol A, Colangelo M, Linares JC, Navarro-Cerrillo RM, Rubio-Cuadrado Á, Silla F, Dumas P-J, Courbet F. Shifting Precipitation Patterns Drive Growth Variability and Drought Resilience of European Atlas Cedar Plantations. Forests. 2021; 12(12):1751. https://doi.org/10.3390/f12121751
Chicago/Turabian StyleCamarero, Jesús Julio, Antonio Gazol, Michele Colangelo, Juan Carlos Linares, Rafael M. Navarro-Cerrillo, Álvaro Rubio-Cuadrado, Fernando Silla, Pierre-Jean Dumas, and François Courbet. 2021. "Shifting Precipitation Patterns Drive Growth Variability and Drought Resilience of European Atlas Cedar Plantations" Forests 12, no. 12: 1751. https://doi.org/10.3390/f12121751
APA StyleCamarero, J. J., Gazol, A., Colangelo, M., Linares, J. C., Navarro-Cerrillo, R. M., Rubio-Cuadrado, Á., Silla, F., Dumas, P.-J., & Courbet, F. (2021). Shifting Precipitation Patterns Drive Growth Variability and Drought Resilience of European Atlas Cedar Plantations. Forests, 12(12), 1751. https://doi.org/10.3390/f12121751












