Estimating the Potential Impacts of Climate Change on the Spatial Distribution of Garuga forrestii, an Endemic Species in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species Occurrence Records
2.2. Climate Data Acquisition and Processing
2.3. Modeling Procedure and Performance Evaluation
2.4. Distribution Changes under Different Climatic Scenarios
3. Results
3.1. Evaluation of Model Performance and Variable Contribution
3.2. Current and Conditions of the Potential Distribution of G. forrestii
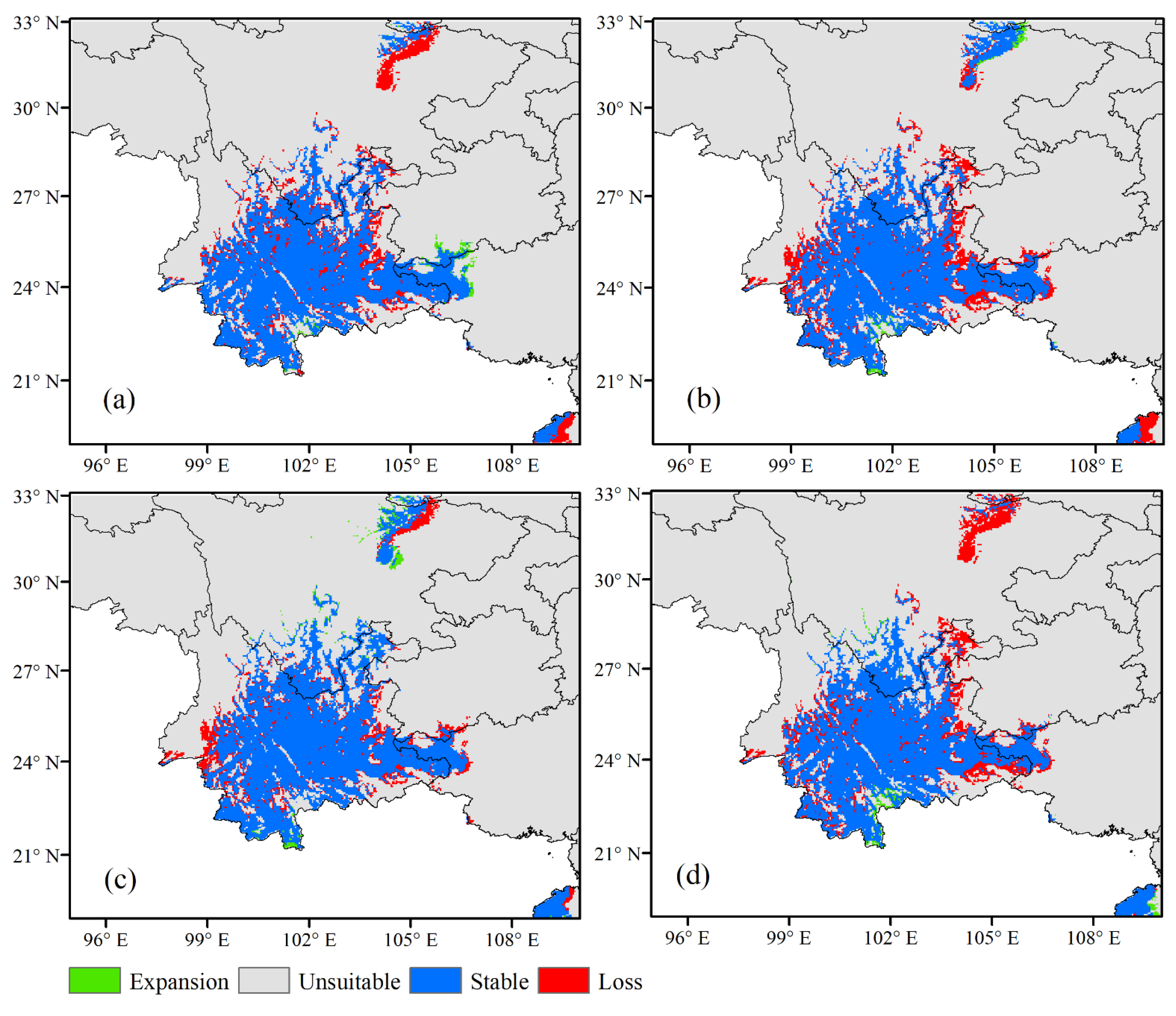
3.3. Future Climate Conditions of the Potential Distribution of G. forrestii
4. Discussion
Insights into the Conservation of G. forrestii
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Shukla, S.; Singh, A. The principal factors responsible for biodiversity loss. Open J. Plant Sci. 2021, 6, 011–014. [Google Scholar]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2021, 6, 011–014. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Mantyka-Pringle, C.S.; Martin, T.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Change Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B 2018, 285, 20180792. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Behroozian, M.; Ejtehadi, H.; Peterson, A.T.; Memariani, F.; Mesdaghi, M. Climate change influences on the potential distribution of Dianthus polylepis Bien. ex Boiss. (Caryophyllaceae), an endemic species in the Irano-Turanian region. PLoS ONE 2020, 15, e0237527. [Google Scholar] [CrossRef] [PubMed]
- Wagensommer, R.P.; Perrino, E.V.; Silletti, G.N. Carex phyllostachys CA Mey. (Cyperaceae) new for Italy and phytogeographical considerations. Phyton 2014, 54, 215–222. [Google Scholar]
- Garza, G.; Rivera, A.; Barrera, C.S.V.; Martinez-Ávalos, J.G.; Dale, J.; Arroyo, T.P.F. Potential Effects of Climate Change on the Geographic Distribution of the Endangered Plant Species Manihot walkerae. Forests 2020, 11, 689. [Google Scholar] [CrossRef]
- Ngarega, B.K.; Masocha, V.F.; Schneider, H. Forecasting the effects of bioclimatic characteristics and climate change on the potential distribution of Colophospermum mopane in southern Africa using Maximum Entropy (Maxent). Ecol. Inform. 2021, 65, 101419. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Chapter Seven. Modeling Ecological Niches. In Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; pp. 97–137. [Google Scholar]
- Thuiller, W.; Albert, C.; Araújo, M.B.; Berry, P.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.; Paterson, J.; Schurr, F.M.; et al. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Salako, G.; Oyebanji, O.O.; Olagunju, T.E.; Howe, G.T. Potential impact of climate change on the distribution of some selected legumes in Cameroon and adjoining Nigeria border. Afr. J. Ecol. 2021, 59, 959–975. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Thomas, C. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Robinson, N.M.; Nelson, W.A.; Costello, M.J.; Sutherland, J.E.; Lundquist, C.J. A Systematic Review of Marine-Based Species Distribution Models (SDMs) with Recommendations for Best Practice. Front. Mar. Sci. 2017, 4, 194. [Google Scholar] [CrossRef]
- Oyebanji, O.O.; Salako, G.; Nneji, L.; Oladipo, S.; Bolarinwa, K.; Chukwuma, E.; Ayoola, A.; Olagunju, T.; Ighodalo, D.; Nneji, I. Impact of climate change on the spatial distribution of endemic legume species of the Guineo-Congolian forest, Africa. Ecol. Indic. 2021, 122, 107282. [Google Scholar] [CrossRef]
- Mohn, R.A.; Oleas, N.H.; Smith, A.B.; Swift, J.F.; Yatskievych, G.A.; Edwards, C.E. The phylogeographic history of a range disjunction in eastern North America: The role of post-glacial expansion into newly suitable habitat. Am. J. Bot. 2021, 108, 1042–1057. [Google Scholar] [CrossRef]
- Nzei, J.M.; Ngarega, B.K.; Mwanzia, V.M.; Musili, P.M.; Wang, Q.-F.; Chen, J.-M. The past, current, and future distribution modeling of four water lilies (Nymphaea) in Africa indicates varying suitable habitats and distribution in climate change. Aquat. Bot. 2021, 173, 103416. [Google Scholar] [CrossRef]
- Ahmed, N.; Atzberger, C.; Zewdie, W. Species Distribution Modelling performance and its implication for Sentinel-2-based prediction of invasive Prosopis juliflora in lower Awash River basin, Ethiopia. Ecol. Process. 2021, 10, 8. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Fois, M.; Fenu, G.; Cuena-Lombraña, A.; Cogoni, D.; Bacchetta, G. A practical method to speed up the discovery of unknown populations using Species Distribution Models. J. Nat. Conserv. 2015, 24, 42–48. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Erfanian, M.B.; Sagharyan, M.; Memariani, F.; Ejtehadi, H. Predicting range shifts of three endangered endemic plants of the Khorassan-Kopet Dagh floristic province under global change. Sci. Rep. 2021, 11, 9159. [Google Scholar] [CrossRef]
- Zizka, A.; Azevedo, J.A.R.; Leme, E.; Neves, B.; Da Costa, A.F.; Caceres, D.; Zizka, G. Biogeography and conservation status of the pineapple family (Bromeliaceae). Divers. Distrib. 2020, 26, 183–195. [Google Scholar] [CrossRef]
- Rej, J.E.; Joyner, T.A. Niche modeling for the genus Pogona (Squamata: Agamidae) in Australia: Predicting past (late Quaternary) and future (2070) areas of suitable habitat. PeerJ 2018, 6, e6128. [Google Scholar] [CrossRef]
- Smith, W.W. eFloras. Garuga forrestii. Notes Roy. Bot. Gard. Edinburgh. 1921, 13, 162. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=242323081 (accessed on 14 October 2021).
- Smith, W.W. EncyclopediaofLife. Garuga forrestii. Available online: https://eol.org/pages/2906865 (accessed on 17 October 2021).
- Shirwaikar, A.; Rajendran, K.; Barik, R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozoto-cin-nicotinamide induced type-II diabetes mellitus. J. Ethnopharmacol. 2006, 107, 285–290. [Google Scholar] [CrossRef]
- Achmad, F.H.; Aty, W.; Arannya, P.D.; Nike, F.; Lidya, T.; Indah, S.T. In Vitro Antimalarial Activity Screening of Several Indonesian Plants Using HRP2 Assay. Int. J. Pharm. Pharm. Sci. 2014, 6, 125–128. [Google Scholar]
- Yin, S.W.; He, X.M.; Wang, B.G.; Xiao, Y.A. Chemical constituents from the twigs of Garuga forrestii. J. Trop. Subtrop. Bot. 2008, 16, 46–50. [Google Scholar]
- Tang, C.Q. Semi-Savannas in Hot-Dry Valleys. In The Subtropical Vegetation of Southwestern China: Plant Distribution, Diversity and Ecology; Springer: Dordrecht, The Netherlands, 2015; pp. 165–184. [Google Scholar]
- Hon, Y.; Botanic Gardens Conservation International (BGCI) & IUCN SSC Global Tree Specialist Group. Garuga forrestii. The IUCN Red List of Threatened Species 2019. Available online: https://www.iucnredlist.org/species/152839038/152839040 (accessed on 14 October 2021).
- GBIF. Occurrence Download. 2021, The Global Biodiversity Information Facility. Available online: https://doi.org/10.15468/dl.qfaum6 (accessed on 16 September 2021).
- Jackson, M.M.; Gergel, S.E.; Martin, K. Citizen science and field survey observations provide comparable results for map-ping Vancouver Island White-tailed Ptarmigan (Lagopus leucura saxatilis) distributions. Biol. Conserv. 2015, 181, 162–172. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Gomes, V.H.; Ijff, S.D.; Raes, N.; Amaral, I.L.; Salomão, R.; Coelho, L.D.S.; Matos, F.D.D.A.; Castilho, C.V.; Filho, D.D.A.L.; López, D.C.; et al. Species Distribution Modelling: Contrasting presence-only models with plot abundance data. Sci. Rep. 2018, 8, 1003. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Quan, C.; Han, S.; Utescher, T.; Zhang, C.; Liu, Y.-S. (Validation of temperature–precipitation based aridity index: Paleoclimatic implications. Palaeogeogr. Palaeoclim. Palaeoecol. 2013, 386, 86–95. [Google Scholar] [CrossRef]
- Ashraf, U.; Peterson, A.T.; Chaudhry, M.N.; Ashraf, I.; Saqib, Z.; Rashid Ahmad, S.; Ali, H. Ecological niche model comparison under different climate scenarios: A case study of Olea spp. in Asia. Eco-sphere 2017, 8, e01825. [Google Scholar] [CrossRef]
- Bede-Fazekas, Á.; Somodi, I. The way bioclimatic variables are calculated has impact on potential distribution models. Methods Ecol. Evol. 2020, 11, 1559–1570. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting Multicollinearity in Ecological Multiple Regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberon, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.; Lawrence, D.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The Community Climate System Model Version. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-Term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013-The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Owens, H.; Campbell, L.P.; Dornak, L.L.; Saupe, E.; Barve, N.; Soberon, J.; Ingenloff, K.; Lira-Noriega, A.; Hensz, C.M.; Myers, C.E.; et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Ellis, C.J. Predicting the biodiversity response to climate change: Challenges and advances. Syst. Biodivers. 2011, 9, 307–317. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Pinto-Ledezma, J.N.; Cavender-Bares, J. Predicting species distributions and community composition using satellite remote sensing predictors. Sci. Rep. 2021, 11, 16448. [Google Scholar] [CrossRef]
- Bosso, L.; Luchi, N.; Maresi, G.; Cristinzio, G.; Smeraldo, S.; Russo, D. Predicting current and future disease outbreaks of Diplodia sapinea shoot blight in Italy: Species distribution models as a tool for forest management planning. For. Ecol. Manag. 2017, 400, 655–664. [Google Scholar] [CrossRef]
- Kafash, A.; Ashrafi, S.; Ohler, A.; Yousefi, M.; Malakoutikhah, S.; Koehler, G.; Schmidt, B.R. Climate change produces winners and losers: Differential responses of amphibians in mountain forests of the Near East. Glob. Ecol. Conserv. 2018, 16, e00471. [Google Scholar] [CrossRef]
- Warren, D.L.; Wright, A.N.; Seifert, S.N.; Shaffer, H.B. Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Divers. Distrib. 2014, 20, 334–343. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.; Townsend Peterson, A.; Soberon, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, Y.; Jiang, H. The Vegetation of Yunnan; Science Press: Beijing, China, 1987; p. 781. [Google Scholar]
- Zhu, J.-J.; Zhang, J.-L.; Liu, H.-C.; Cao, K.-F. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiol. Plant. 2009, 135, 62–72. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Sujakhu, N.M.; Lu, Y.; Wang, Q.; Wang, M.; He, J.; Mortimer, P.E.; Xu, J.; Kindt, R.; Zomer, R. Climate modelling for agroforestry species selection in Yunnan Province, China. Environ. Model. Softw. 2016, 75, 263–272. [Google Scholar] [CrossRef]
- Keppel, G.; Robinson, T.P.; Wardell-Johnson, G.W.; Yates, C.J.; Van Niel, K.P.; Byrne, M.; Schut, A.G. A low-altitude mountain range as an important refugium for two narrow endemics in the Southwest Australian Floristic Region biodiversity hotspot. Ann. Bot. 2016, 119, 289–300. [Google Scholar] [CrossRef][Green Version]
- Corlett, R.T. Impacts of warming on tropical lowland rainforests. Trends Ecol. Evol. 2011, 26, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Zhen-Feng, M.; Jia, L.; Shu-Qun, Y. Climate Change in Southwest China during 1961–2010: Impacts and Adaptation. Adv. Clim. Chang. Res. 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Gove, A.D.; Sanders, N.J.; Dunn, R.R. Climate change, plant migration, and range collapse in a global biodiversity hotspot: The Banksia (Proteaceae) of Western Australia. Glob. Chang. Biol. 2008, 14, 1337–1352. [Google Scholar] [CrossRef]
- Qian, L.-S.; Chen, J.-H.; Deng, T.; Sun, H. Plant diversity in Yunnan: Current status and future directions. Plant Divers. 2020, 42, 281–291. [Google Scholar] [CrossRef] [PubMed]


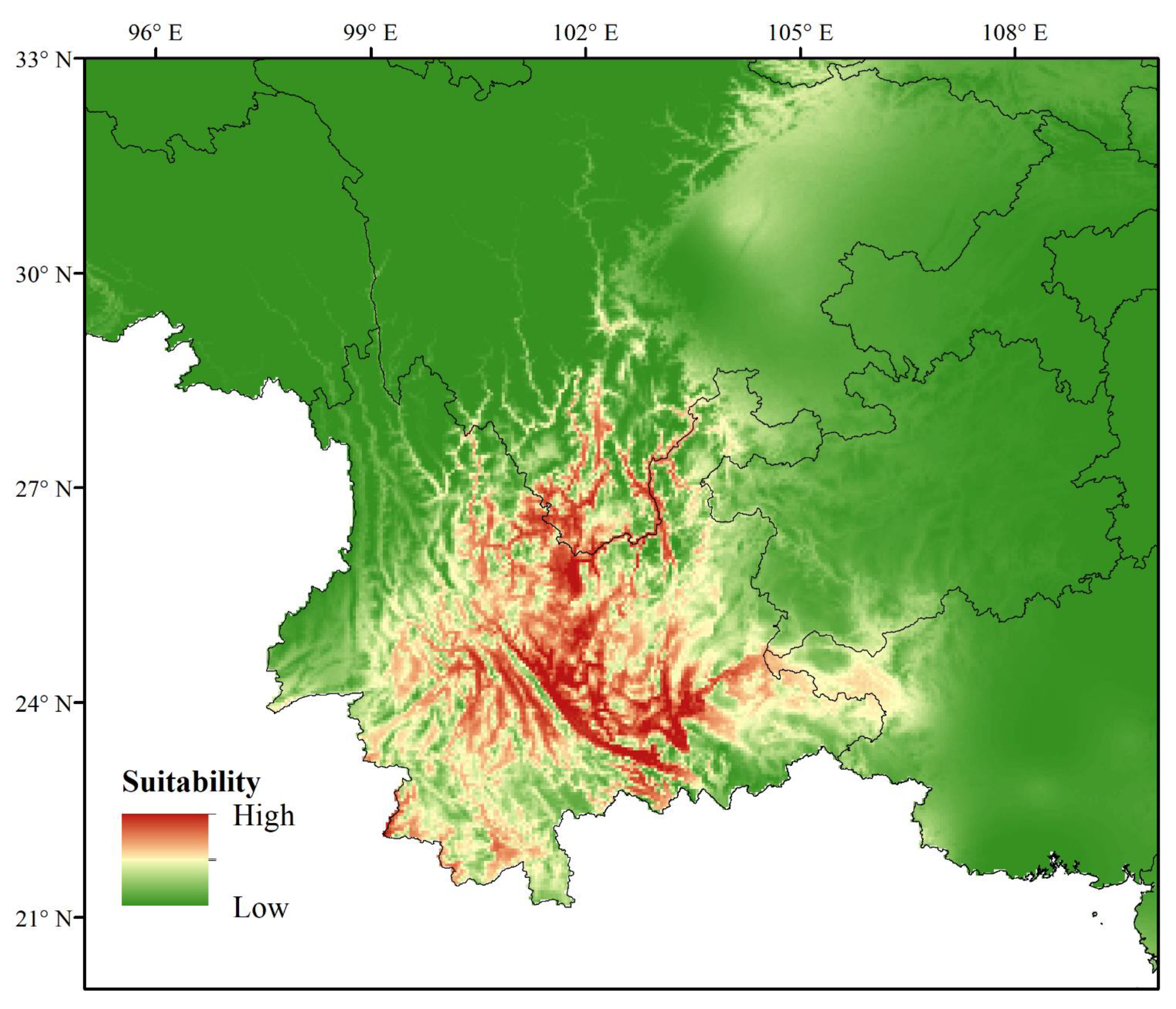
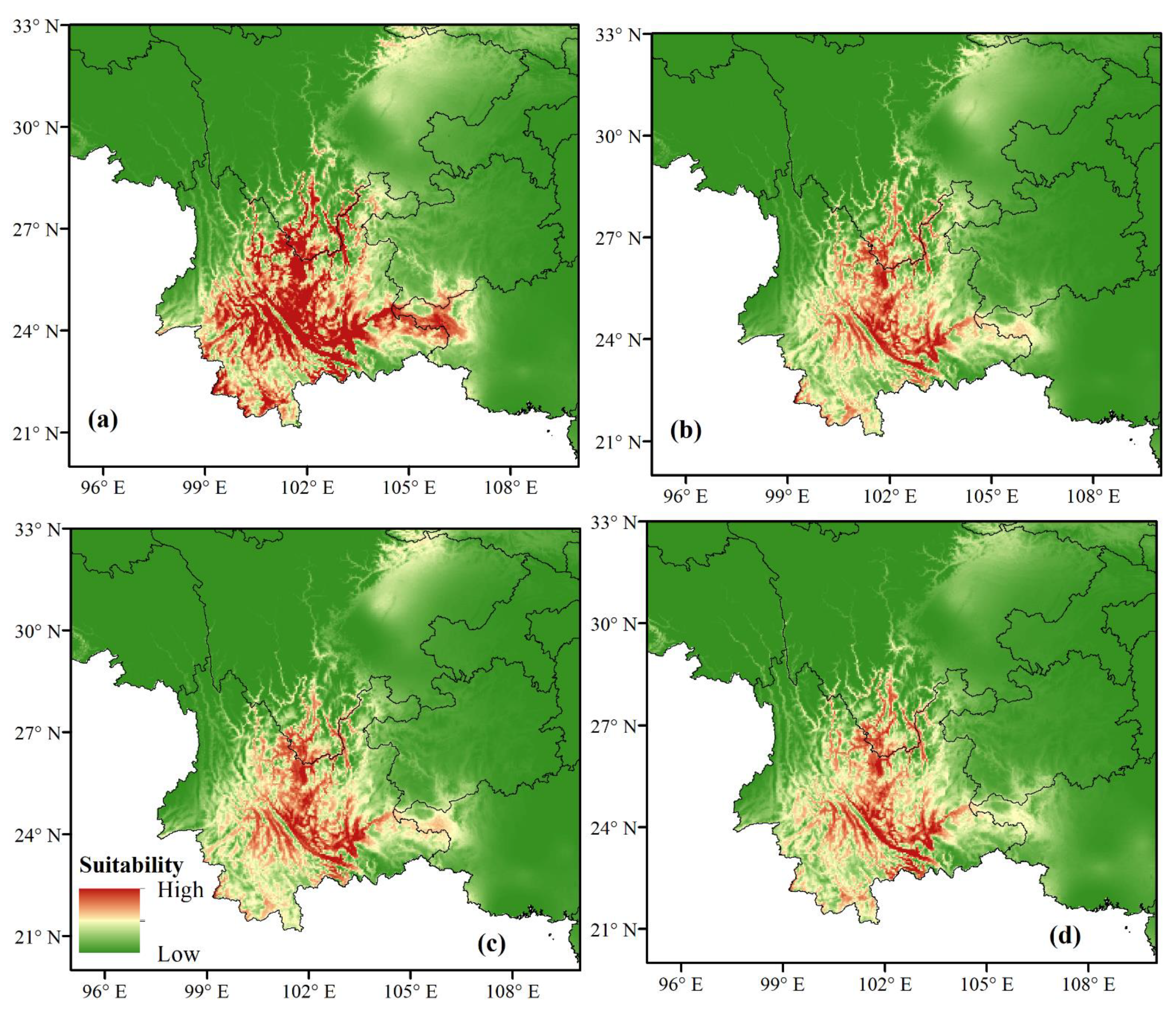
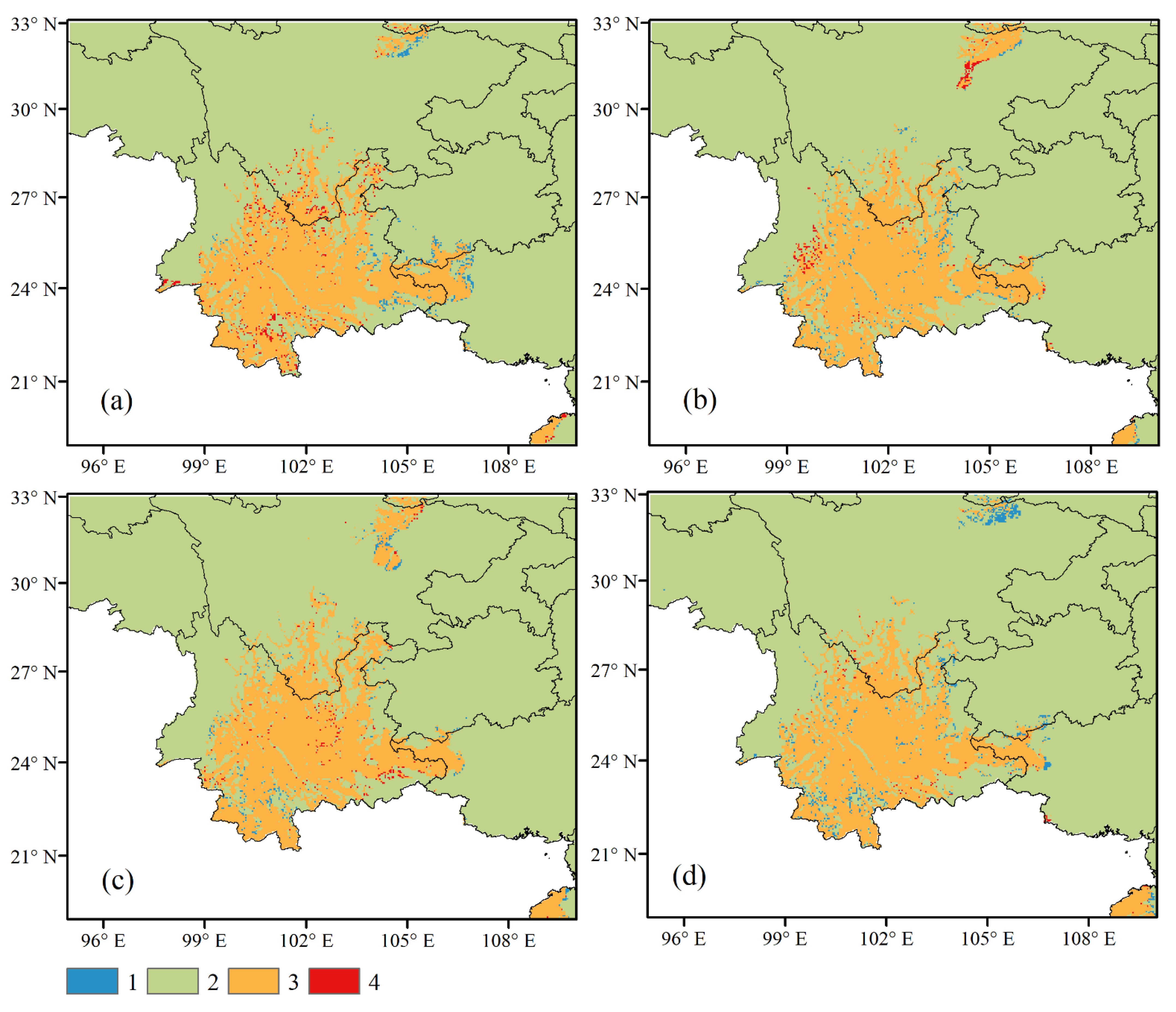
| Variable | Description | % Contribution | Permutation Importance |
|---|---|---|---|
| Bio3 | Isothermality (BIO2/BIO7) (×100) | 9.2 | 12.6 |
| Bio4 | Temperature Seasonality (standard deviation × 100) | 35.6 * | 16.7 * |
| Bio7 | Temperature Annual Range (BIO5-BIO) | 0.3 | 0.3 |
| Bio13 | Precipitation of Wettest Month | 12 * | 6.3 * |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | 1.5 | 2.9 |
| Bio17 | Precipitation of Driest Quarter | 10.2 | 9.1 |
| Elev | Altitude (m) | 27.5 * | 42.7 * |
| PET | Potential Evapotranspiration | 1.7 | 2.8 |
| AI | Aridity Index | 2 | 7 |
| Period | Current | RCP4.5 | RCP8.5 | ||
|---|---|---|---|---|---|
| 2050 | 2070 | 2050 | 2070 | ||
| AUC (SD) | 0.932 (0.016) | 0.939 (0.019) | 0.943 (0.012) | 0.941 (0.017) | 0.937 (0.019) |
| TSS | 0.835 | 0.796 | 0.842 | 0.881 | 0.857 |
| pROC | 1.620 (0.004) | 1.583 (0.005) | 1.534 (0.003) | 1.592 (0.005) | 1.609 (0.004) |
| 10 PTP | 0.2448 | 0.2371 | 0.2519 | 0.2824 | 0.2721 |
| Scenario | Stable | Range Expansion | Range Contraction | |||
|---|---|---|---|---|---|---|
| Area (km2) | % | Area (km2) | % | Area (km2) | % | |
| Current | 309,516.2 | |||||
| RCP4.5 2050 | 256,691.22 | 82.93 | 5013.57 | 1.62 | 52,825.03 | 17.07 |
| RCP4.5 2070 | 266,948.88 | 86.25 | 7337.88 | 2.37 | 42,567.36 | 13.76 |
| RCP8.5 2050 | 249,430.18 | 80.59 | 4168.38 | 1.35 | 60,086.07 | 19.41 |
| RCP8.5 2070 | 247,643.73 | 80.00 | 5032.78 | 1.62 | 61,872.52 | 19.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiamiyu, B.B.; Ngarega, B.K.; Zhang, X.; Zhang, H.; Kuang, T.; Huang, G.-Y.; Deng, T.; Wang, H. Estimating the Potential Impacts of Climate Change on the Spatial Distribution of Garuga forrestii, an Endemic Species in China. Forests 2021, 12, 1708. https://doi.org/10.3390/f12121708
Tiamiyu BB, Ngarega BK, Zhang X, Zhang H, Kuang T, Huang G-Y, Deng T, Wang H. Estimating the Potential Impacts of Climate Change on the Spatial Distribution of Garuga forrestii, an Endemic Species in China. Forests. 2021; 12(12):1708. https://doi.org/10.3390/f12121708
Chicago/Turabian StyleTiamiyu, Bashir B., Boniface K. Ngarega, Xu Zhang, Huajie Zhang, Tianhui Kuang, Gui-Yun Huang, Tao Deng, and Hengchang Wang. 2021. "Estimating the Potential Impacts of Climate Change on the Spatial Distribution of Garuga forrestii, an Endemic Species in China" Forests 12, no. 12: 1708. https://doi.org/10.3390/f12121708
APA StyleTiamiyu, B. B., Ngarega, B. K., Zhang, X., Zhang, H., Kuang, T., Huang, G.-Y., Deng, T., & Wang, H. (2021). Estimating the Potential Impacts of Climate Change on the Spatial Distribution of Garuga forrestii, an Endemic Species in China. Forests, 12(12), 1708. https://doi.org/10.3390/f12121708







