Highlighting Complex Long-Term Succession Pathways in Mixed Forests of the Pacific Northwest: A Markov Chain Modelling Approach
Abstract
:1. Introduction
2. Materials and Methods
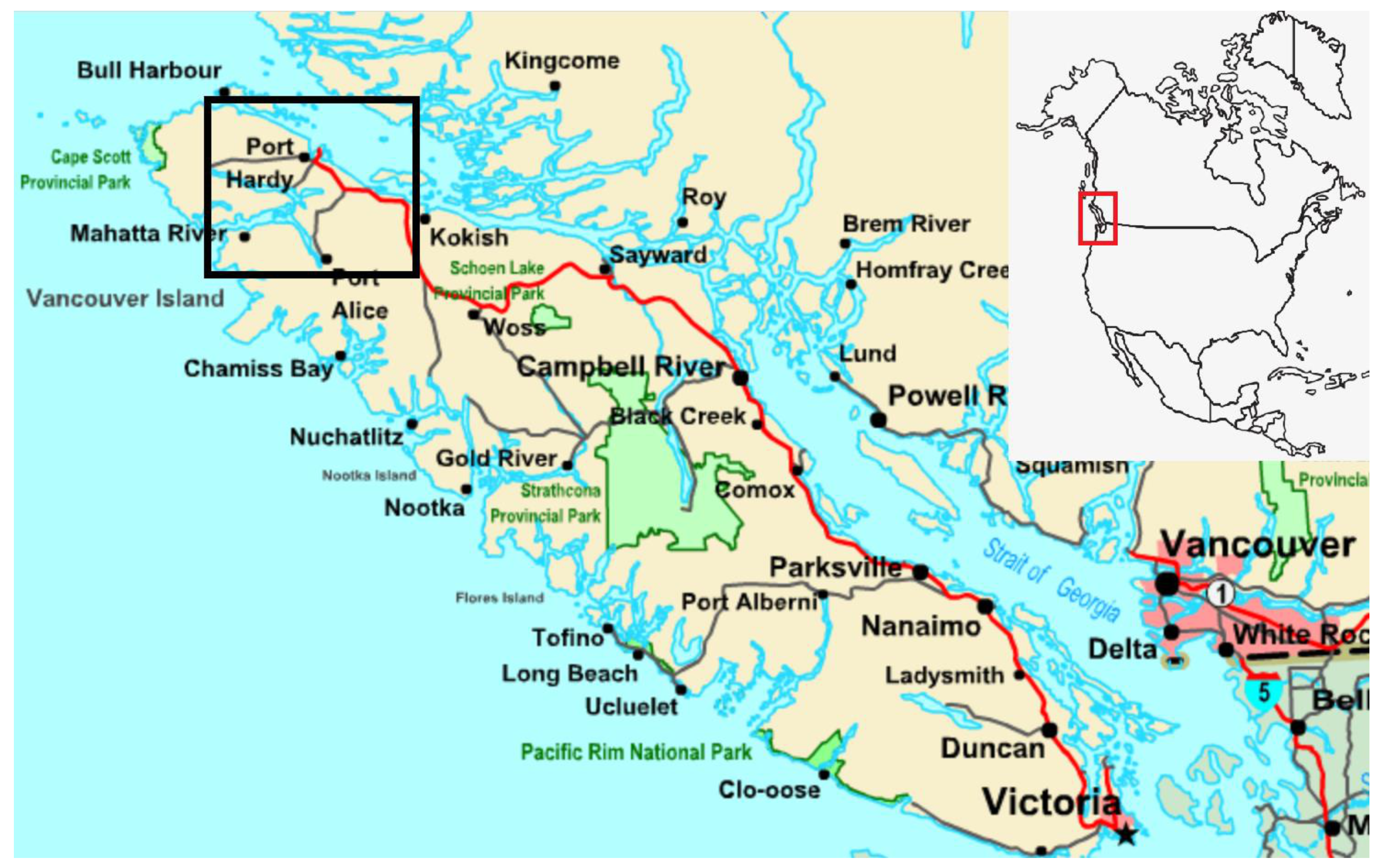
2.1. Region Description
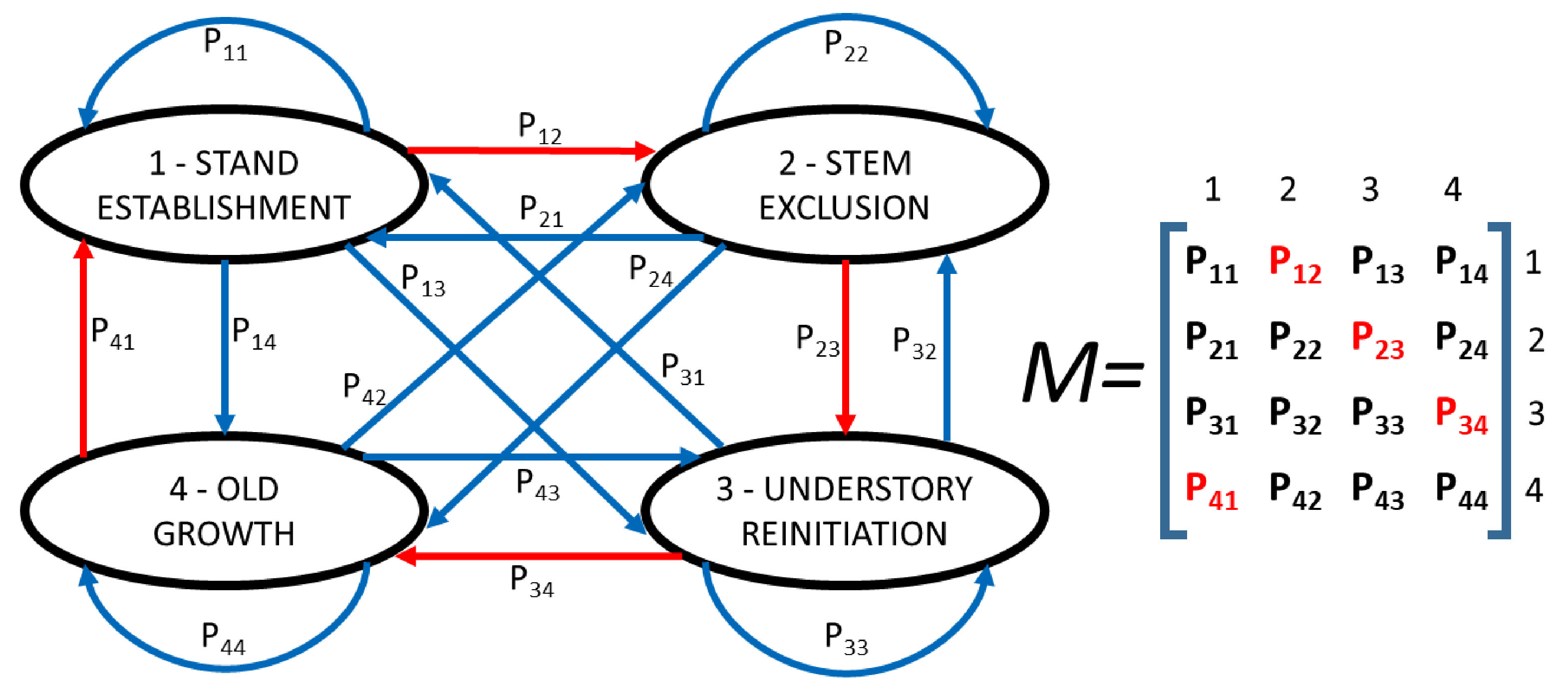
2.2. Succesion Models
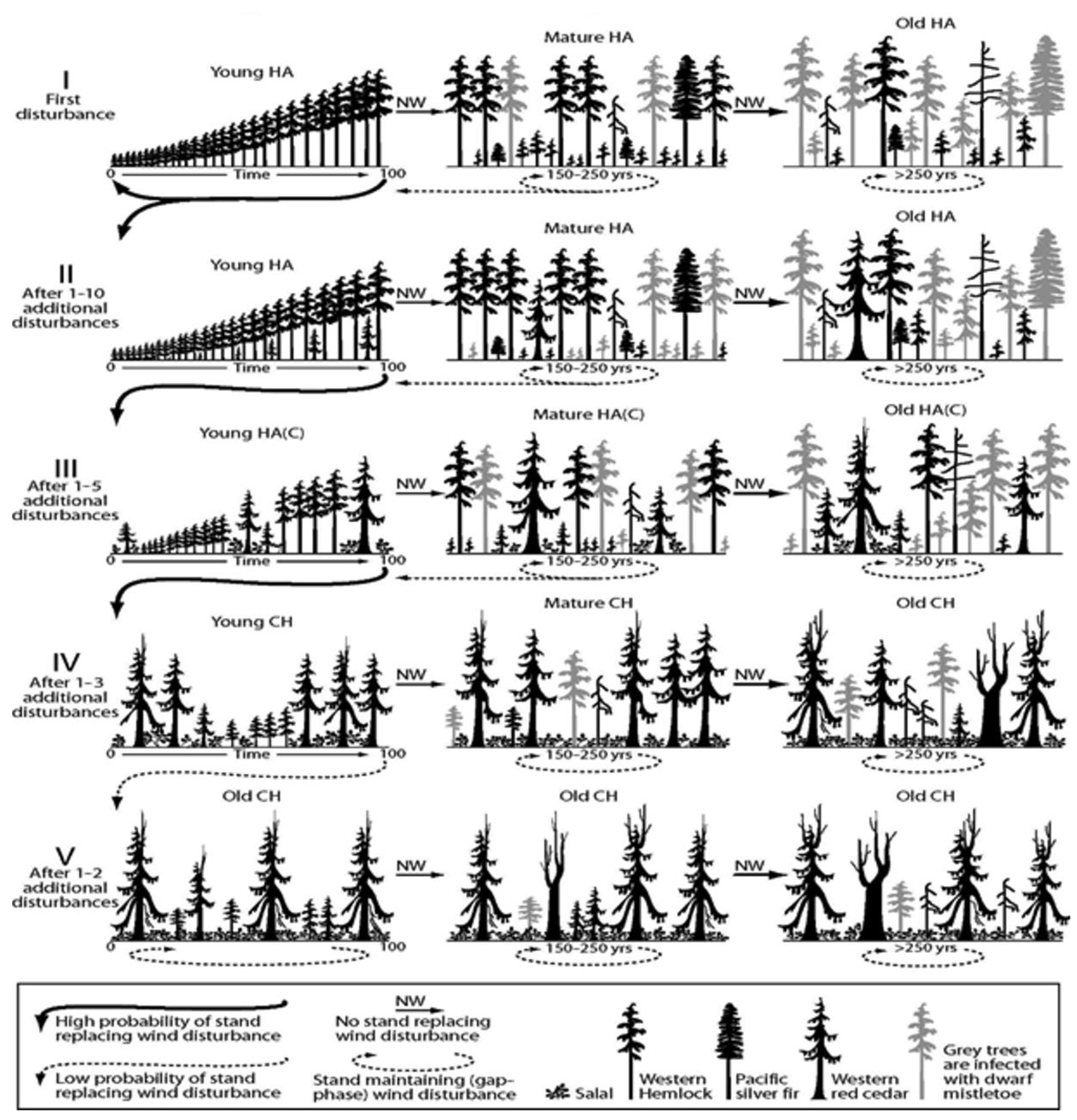
| Acronym | Ecosystem Stage (Stand Type) |
|---|---|
| HAy | Western hemlock—Pacific silver fir young stands (stand age < 100 years) |
| HAm | Western hemlock—Pacific silver fir mature stands (stand age 100–200 years) |
| HAo | Western hemlock—Pacific silver fir old-growth stands (stand age > 200 years) |
| HA(C)y | Western hemlock—Pacific silver fir young stand (stand age < 100 years) with ≤ 15% basal area as red cedar regeneration |
| HA(C)m | Western hemlock—Pacific silver fir mature stands (stand age 100–200 years) with ≤ 15% basal area as red cedar regeneration |
| HA(C)o | Western hemlock—Pacific silver fir old growth stands (stand age > 200 years) with ≤ 15% basal area as red cedar regeneration |
| CHy | Red cedar—Western hemlock young stands (stand age < 100 years) |
| CHm | Red cedar—Western hemlock mature stands (stand age 100–200 years) |
| CHo | Red cedar—Western hemlock old growth stands (stand age > 200 years) |
| Transition | Event |
|---|---|
| P1 | HAy will blow down to become HAy; otherwise becomes HAm |
| P2 | HAy and HAm will have no red cedar regeneration after windthrow, therefore they become HAy |
| P3 | HAy will have >0 but ≤15% stand basal area of red cedar regeneration windthrow and become HA(C)y |
| P4 | HAm will blow down to become HAy; otherwise become HAo |
| P5 | HAm will undergo gap formation |
| P6 | HAm will have >0 but ≤15% stand basal area red cedar regeneration windthrow and become HA(C)y |
| P7 | HAo will undergo gap formation |
| P8 | HAo gaps will have no red cedar regeneration and remain HAo |
| P9 | HAo gaps will recruit red cedar and become HA(C)m. |
| P10 | HAo gaps will recruit red cedar and become HA(C)o |
| P11 | HAo will blow down and become HAy |
| P12 | HA(C)y will blow down and remain HA(C)y |
| P13 | HA(C)y will recruit > 15% red cedar after blow down and become CHy |
| P14 | HA(C)m will blow down |
| P15 | HA(C)m undergoes gap formation |
| P16 | HA(C)m will blow down and become HA(C)y |
| P17 | HA(C)m will blow down, recruit > 15% basal area of red cedar and become CHy |
| P18 | HAo will undergo gap formation |
| P19 | HA(C)o remains HA(C)o after gap formation |
| P20 | HA(C)o recruits > 15% red cedar after gap formation and becomes CHo |
| P21 | HA(C)o will blow down and become HA(C)y |
| P22 | HA(C)y, HA(C)m and HA(C)o all lose their red cedar regeneration and become HAy after windthrow |
| P23 | HAy and HAm recruit > 15% basal area of red cedar after windthrow and become CHy |
| P24 | CHy will blow down |
| P25 | CHm will blow down |
| P26 | CHy and CHm will become CHy after windthrow |
| P27 | CHo undergoes gap formation and remains CHo |
| P28 | CHo will blow down |

- WindSusc: Probability that a stand will suffer stand-replacing wind disturbance during a major wind event.
- Cinv≤15: Probability that red cedar regeneration will account for ≤15% basal area of the stand following stand-replacing wind disturbance.
- Cinv>15: Probability that red cedar regeneration will account for >15% basal area of the stand following stand-replacing wind disturbance.
- Csuc≤15: Probability that red cedar will regenerate ≤15% basal area of the stand during post wind disturbance succession.
- Csuc>15: Probability that red cedar will regenerate >15% basal area of the stand during post wind disturbance succession.
- Gap: Probability that major wind disturbance will result in gap formation rather than stand replacement, mostly due to mistletoe weakening tree stems.
- CinvGap≤15: Probability that red cedar will regenerate ≤15% basal area of the gap area following gap creation.
- CinvGap>15: Probability that red cedar will regenerate >15% basal area of the gap area following gap creation.
3. Results
3.1. Testing the Clementsian Model
3.2. Base Case of the Multiple Pathway Model
| Stand Type | Init | WindSusc | Cinv≤15 | Cinv>15 | Csuc≤15 | Csuc>15 | Gap | CinvGap≤15 | CinvGap>15 |
|---|---|---|---|---|---|---|---|---|---|
| HAy | 0.33 | 0.5 | 0.08 | 0.0 | 0 | 0 | 0 | 0 | 0 |
| HA(C)y | 0.00 | 0.5 | 0.20 | 0.8 | 1 | 0 | 0 | 0 | 0 |
| CHy | 0.00 | 0.0 | 0.00 | 1.0 | 0 | 1 | 0 | 0 | 0 |
| HAm | 0.33 | 1.0 | 0.08 | 0.0 | 0 | 0 | 1 | 0 | 0 |
| HA(C)m | 0.00 | 1.0 | 0.20 | 0.8 | 0 | 0 | 1 | 1 | 0 |
| CHm | 0.00 | 0.0 | 0.00 | 1.0 | 0 | 0 | 1 | 0 | 1 |
| HAo | 0.33 | 0.6 | 0.08 | 0.0 | 0 | 0 | 1 | 0 | 0 |
| HA(C)o | 0.00 | 0.6 | 0.20 | 0.8 | 0 | 0 | 1 | 1 | 0 |
| CHo | 0.00 | 0.0 | 0.00 | 1.0 | 0 | 0 | 1 | 0 | 1 |
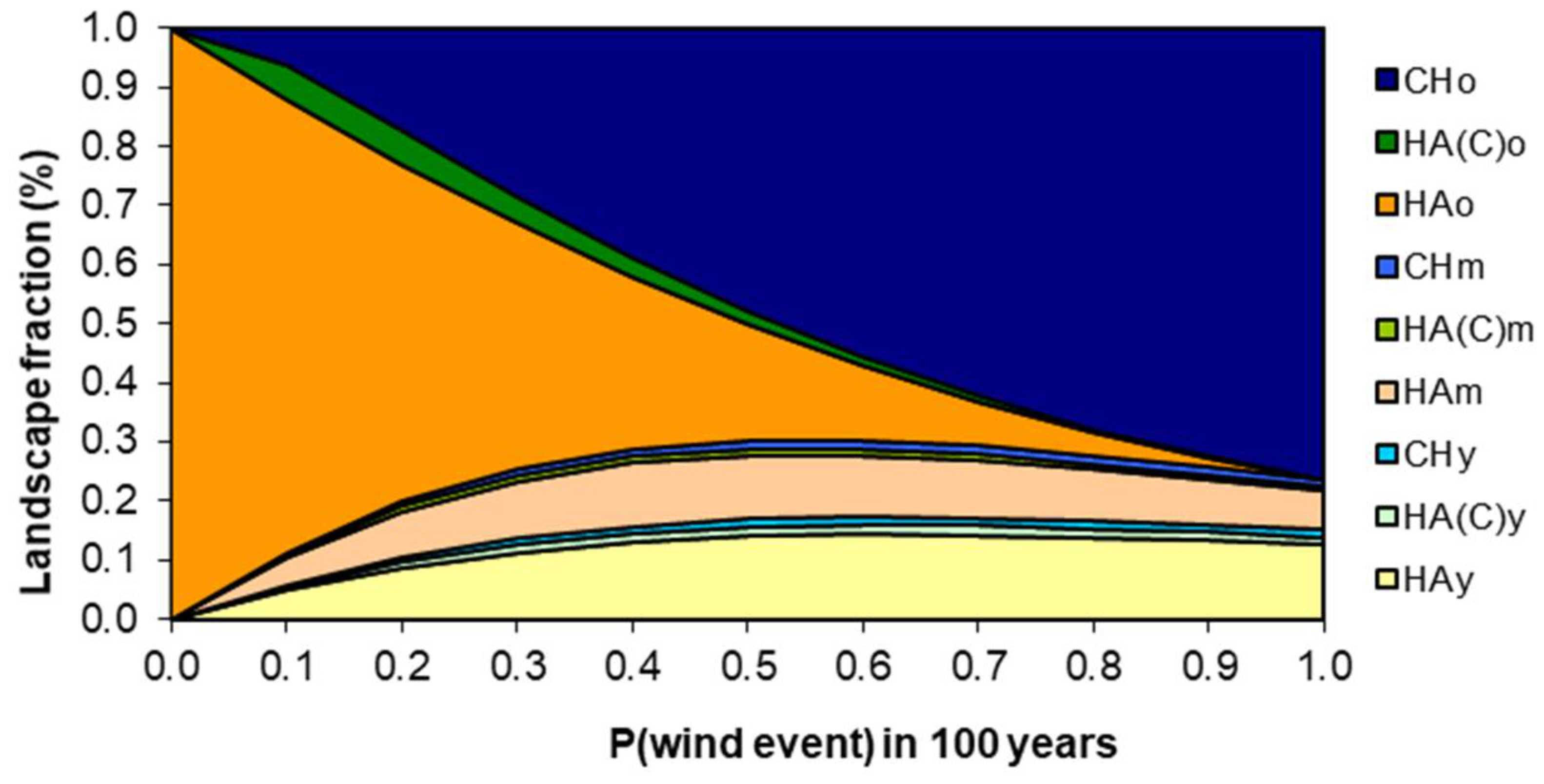
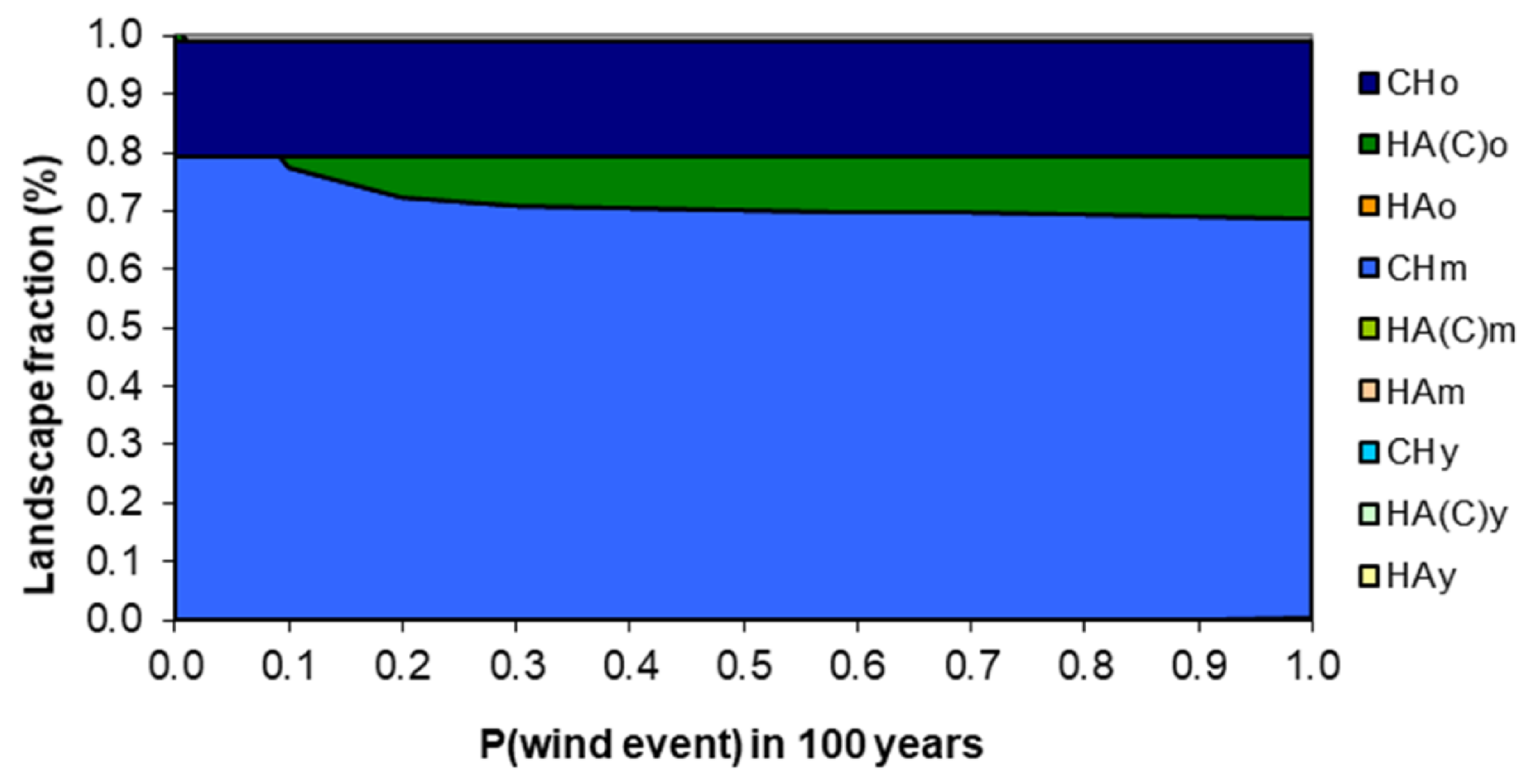
3.3. Model Sensitivity to Susceptibility to Stand-Replacing Events
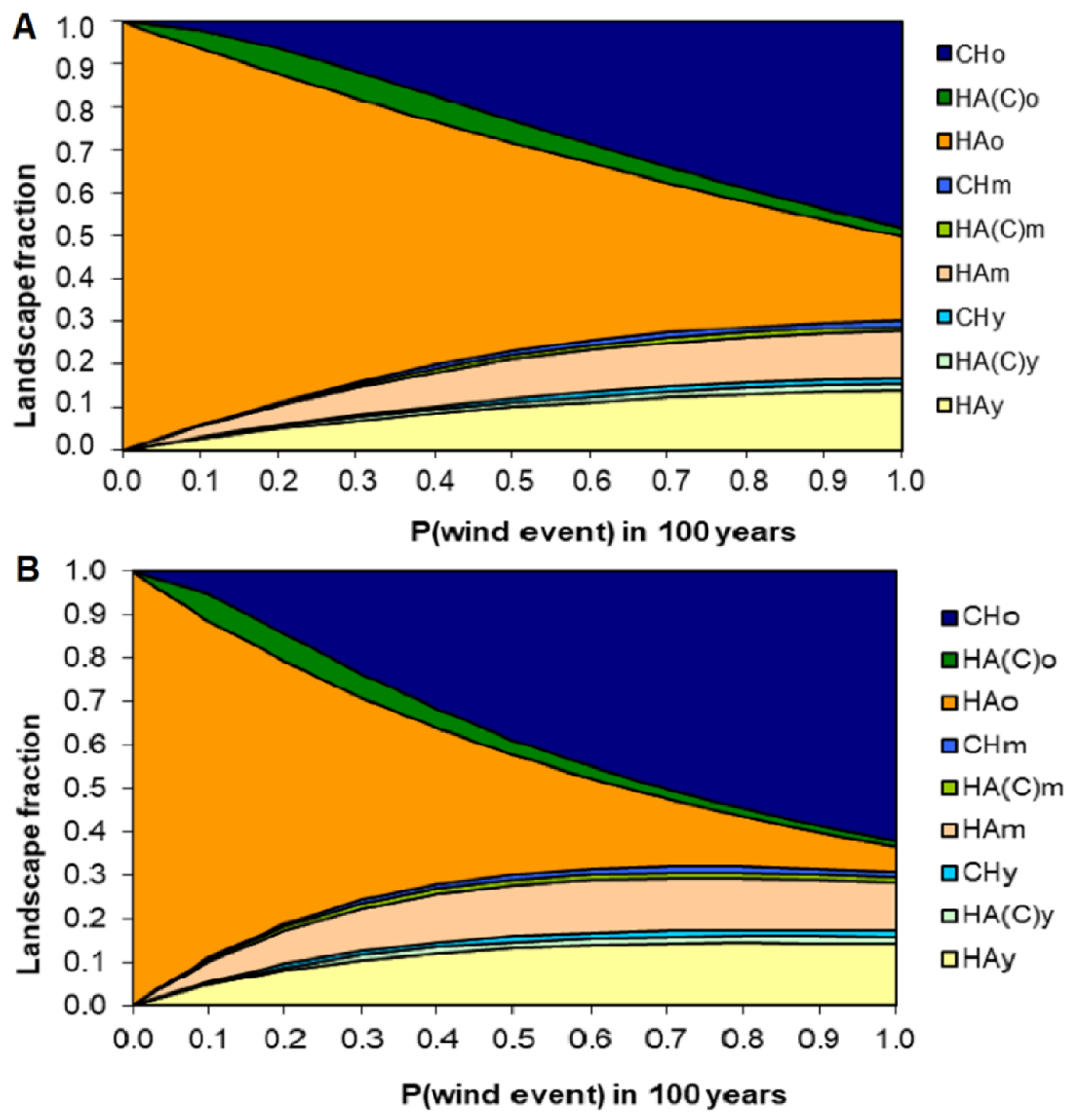
3.4. A Plausible Set of Probabilities
4. Discussion
4.1. The Clementsian Model
4.2. Plausibility of the Multiple Pathway Succession Model
4.3. Evaluation of the Model
4.4. Management Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spies, T.A.; Hessburg, P.F.; Skinner, C.N.; Puettmann, K.J.; Reilly Matthew, J.; Davis, R.J.; Kertis, J.A.; Long, J.W.; Shaw, D.C. Chapter 3: Old growth, disturbance, forest succession, and management in the area of the Northwest Forest Plan. In Synthesis of Science to Inform Land Management within the Northwest Forest Plan Area; General Technical Report PNW-GTR-966; Spies, T.A., Stine, P.A., Gravenmier, R., Long, J.W., Reilly, M.J., Eds.; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2018; pp. 95–243. [Google Scholar]
- Pandolfi, J.M. Succession. In Encyclopedia of Ecology; Jorgensen, S.E., Fath, B., Eds.; Academic Press: Oxford, UK, 2008; pp. 3416–3424. [Google Scholar]
- Finegan, B. Forest succession. Nature 1984, 312, 109–114. [Google Scholar] [CrossRef]
- Hall, F.G.; Botkin, D.B.; Strebel, D.E.; Woods, K.D.; Goetz, S.J. Large-scale patterns of forest succession as determined by remote sensing. Ecology 1991, 72, 628–640. [Google Scholar] [CrossRef]
- Prach, K.; Ujházy, K.; Knopp, V.; Fanta, J. Two centuries of forest succession, and 30 years of vegetation changes in permanent plots in an inland sand dune area, The Netherlands. PLoS ONE 2021, 16, e0250003. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Saunders, S.C.; Coates, K.D.; Meidinger, D.V.; MacKinnon, A.; O’Neill, G.A.; MacKillop, D.J.; DeLong, S.C.; Morgan, D.G. Ecological Resilience and Complexity: A Theoretical Framework for Understanding and Managing British Columbia’s Forest Ecosystems in a Changing Climate; Forest Science Program Technical Report 055; BC Ministry of Forests and Range: Victoria, BC, Canada, 2009. [Google Scholar]
- Connell, J.H.; Slatyer, R.O. Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Fresco, L.O.; Kroonenberg, S.B. Time and spatial scales in ecological sustainability. Land Use Policy 1992, 9, 155–168. [Google Scholar] [CrossRef]
- Turner, M.G.; Romme, W.H.; Gardner, R.H.; O’Neill, R.V.; Kratz, T.K. A revised concept of landscape equilibrium: Disturbance and stability on scaled landscapes. Landsc. Ecol. 1993, 8, 213–227. [Google Scholar] [CrossRef]
- Perera, A.H.; Buse, L.J. Emulating Natural Disturbance in Forest Management: An Overview. In Emulating Natural Forest Landscape Disturbances: Concepts and Applications; Perera, A.H., Buse, L.J., Webe, M.G., Eds.; Columbia University Press: New York, NY, USA, 2004; pp. 1–7. [Google Scholar]
- Keane, R.E.; Hessburg, P.F.; Landres, P.B.; Swanson, F.J. The use of historical range and variability (HRV) in landscape management. For. Ecol. Manag. 2009, 258, 1025–1037. [Google Scholar] [CrossRef]
- Blanco, J.A.; González de Andrés, E.; San Emeterio, L.; Lo, Y.H. Modelling mixed forest stands: Methodological challenges and approaches. In Advanced Modelling Techniques Studying Global Changes in Environmental Sciences; Park, Y.S., Lek, S., Baehr, C., Jorgensen, S.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 186–223. ISBN 978-0-444-63-536-5. [Google Scholar]
- Van Hulst, R. On the dynamics of vegetation: Markov chains as models of succession. Vegetatio 1979, 40, 3–14. [Google Scholar] [CrossRef]
- Usher, M.B. Modelling ecological succession, with particular reference to Markovian models. Vegetatio 1981, 46, 11–18. [Google Scholar] [CrossRef]
- Baltzer, H. Markov Chain models for vegetation dynamics. Ecol. Model. 2000, 126, 139–154. [Google Scholar]
- Weber, A.; Leckie, S.; Kimmins, J.P.; Gilbert, B.; Blanco, J.A.; Lo, Y.H. Survival and growth as measures of shade tolerance of planted western redcedar, western hemlock and amabilis fir seedlings in hemlock-fir forests of northern Vancouver Island. For. Ecol. Manag. 2017, 386, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Liénard, J.; Florescu, I.; Strigul, N. An appraisal of the classic forest succession paradigm with the Shade Tolerance Index. PLoS ONE 2015, 10, e01117138. [Google Scholar] [CrossRef] [Green Version]
- Koven, C.D.; Knox, R.G.; Fisher, R.A.; Chambers, J.Q.; Christoffersen, B.O.; Davies, S.J.; Detto, M.; Dietze, M.C.; Faybishenko, B.; Holm, J.; et al. Benchmarking and parameter sensitivity of physiological and vegetation dynamics using the Functionally Assembled Terrestrial Ecosystem Simulator (FATES) at Barro Colorado Island, Panama. Biogeosciences 2020, 17, 3017–3044. [Google Scholar] [CrossRef]
- Rödig, E.; Cuntz, M.; Heinke, J.; Rammig, A.; Huth, A. Spatial heterogeneity of biomass and forest structure of the Amazon rain forest: Linking remote sensing, forest modelling and field inventory. Glob. Ecol. Biogeogr. 2017, 26, 1292–1302. [Google Scholar] [CrossRef]
- Wei, X.; Larsen, C.P.S. Assessing the minimum number of time since last fire sample-points required to estimate the fire cycle: Influences of fire rotation length and study area scale. Forests 2018, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Dixon, G.E. Essential FVS: A User’s Guide to the Forest Vegetation Simulator; Internal Rep.; U.S. Department of Agriculture, Forest Service, Forest Management Service Center: Fort Collins, CO, USA, 2002; 226p. [Google Scholar]
- Blanco, J.A.; Ameztegui, A.; Rodríguez, F. Modelling Forest Ecosystems: A crossroad between scales, techniques and applications. Ecol. Model. 2020, 425, 109030. [Google Scholar] [CrossRef]
- Kimmins, J.P.; Blanco, J.A.; Seely, B.; Welham, C.; Scoullar, K. Forecasting Forest Futures: A Hybrid Modelling Approach to the Assessment of Sustainability of Forest Ecosystems and Their Values; Earthscan Ltd.: London, UK, 2010; 281p, ISBN 978-1-84407-922-3. [Google Scholar]
- Delsole, T. A Fundamental Limitation of Markov Models. J. Atmos. Sci. 2000, 57, 2158–2168. [Google Scholar] [CrossRef]
- Prescott, C.E.; Weetman, G. Salal Cedar Hemlock Integrated Research Program: A Synthesis; Faculty of Forestry, University of British Columbia: Vancouver, BC, Canada, 1994; 85p. [Google Scholar]
- Weber, A.; Kimmins, J.H.; Gilbert, B.; Lo, Y.-H.; Blanco, J.A. Multiple-pathway succession in coastal Tsuga heterophylla, Thuja plicata, and Abies amabilis forests on northeastern Vancouver Island, British Columbia. Can. J. For. Res. 2014, 44, 1145–1155. [Google Scholar] [CrossRef]
- Lewis, T. Ecosystems of the Port McNeill Block (Block 4) of Tree Farm License 25; Western Forest Products: Vancouver, BC, Canada, 1982. [Google Scholar]
- DeMontigny, L.E. An Investigation into the Factors Contributing to the Growth Check of Conifer Regeneration on Northern Vancouver Island. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 1992. [Google Scholar]
- Prescott, C.E.; Sajedi, T. The role of salal in forest regeneration problems in coastal British Columbia: Problem or symptom? For. Chron. 2008, 84, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Sajedi, T.; Prescott, C.E.; Seely, B.; Lavkulich, L.M. Relationships among soil moisture, aeration and plant communities in natural and harvested coniferous forests in coastal British Columbia, Canada. J. Ecol. 2012, 100, 605–618. [Google Scholar] [CrossRef]
- Prescott, C.E.; Nery, V.; Van Niejenhuis, A.; Sajedi, T.; Marshall, P. Nutrition management of cedar and hemlock plantations in coastal British Columbia. New For. 2013, 44, 769–784. [Google Scholar] [CrossRef]
- Keenan, R.J.; Prescott, C.E.; Kimmins, J.H. Mass and nutrient content of woody debris and forest floor in western red cedar and western hemlock forests on northern Vancouver Island. Can. J. For. Res. 1993, 23, 1052–1059. [Google Scholar] [CrossRef]
- Weber, A.; Gilbert, B.; Kimmins, J.P.; Prescott, C.E. Factors limiting the early survivorship of Thuja plicata on northern Vancouver Island, British Columbia. Can. J. For. Res. 2003, 33, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.; Karst, J.; Gilbert, B.; Kimmins, J.P. Thuja plicata exclusion in ectomycorrhiza-dominated forests: Testing the role of inoculum potential of arbuscular mycorrhizal fungi. Oecologia 2004, 143, 148–156. [Google Scholar] [CrossRef]
- Hebda, R.J. British Columbia vegetation and climate history with focur on 6 ka BP. Géograp. Phys. Quatern. 1995, 49, 55–79. [Google Scholar] [CrossRef] [Green Version]
- Pojar, J.; Klinka, K.; Meidinger, D. Biogeoclimatic ecosystem classification in British Columbia. For. Ecol. Manag. 1987, 22, 119–154. [Google Scholar] [CrossRef]
- Gavin, D.G.; Brubaker, L.B.; Lertzman, K.P. An 1800-year record of the spatial and temporal distribution of fire from the west coast of Vancouver Island, Canada. Can. J. For. Res. 2003, 33, 573–586. [Google Scholar] [CrossRef]
- Gavin, D.G.; Hallett, D.J.; Hu, F.S.; Lertzman, K.P.; Prichard, S.J.; Brown, K.J.; Lynch, J.A.; Bartlein, P.; Peterson, D.L. Forest fire and climate change in western North America: Insights from sediment charcoal records. Front. Ecol. Environ. 2007, 5, 499–506. [Google Scholar] [CrossRef]
- Oliver, C.; Larson, B. Forest Stand Dynamics; Wiley: New York, NY, USA, 1996; p. 544. [Google Scholar]
- Usher, M.B. Markovian Approaches to Ecological Succession. J. Anim. Ecol. 1979, 48, 413. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecology, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004; p. 611. [Google Scholar]
- Clements, F.E. Plant Succession: Analysis of the Development of Vegetation; Carnegie Institution of Washington Publication Sciences: Washington, DC, USA, 1916; Volume 242, pp. 1–512. [Google Scholar]
- Rudnicki, M.; Mitchell, S.J.; Novak, M.D. Wind tunnel measurements of crown streamlining and drag relationships for three conifer species. Can. J. For. Res. 2004, 34, 666–676. [Google Scholar] [CrossRef]
- Klinka, K.; Brisco, D. Silvics and Silviculture of Coastal Western Redcedar: A Literature Review. B.C. Min. For. Range, For. Sci. Prog., Victoria, B.C. Spec. Rep. Ser. 11. 2009. Available online: www.for.gov.bc.ca/hfd/pubs/Docs/Srs/Srs11.htm (accessed on 11 December 2021).
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Int. J. For. Res. 2012, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Franklin, J.F.; Spies, T.A.; Swanson, F.J. Setting the Stage: Vegetation Ecology and Dynamics. In People, Forests, and Change; Olson, D.H., Van Horne, B., Eds.; Island Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Beese, W.J.; Rollerson, T.P.; Peters, C.M. Quantifying wind damage associated with variable retention harvesting in coastal British Columbia. For. Ecol. Manag. 2019, 443, 117–131. [Google Scholar] [CrossRef]
- Feller, M.; Klinka, K. Seedfall, seed germination, and initial survival and growth of seedlings of Thuja Plicata in southwestern British Columbia. Northwest Sci. 1998, 72, 157–169. [Google Scholar]
- Daniels, L.; Marshall, P.; Carter, R.; Klinka, K. Age structure of Thuja plicata in the tree layer of old-growth stands near Vancouver, BC. Northwest Sci. 1995, 69, 175–183. [Google Scholar]
- Daniels, L.D. Western redcedar population dynamics in old-growth forests: Contrasting ecological paradigms using tree rings. For. Chron. 2003, 79, 517–530. [Google Scholar] [CrossRef] [Green Version]
- Messier, C.; Doucet, R.; Ruel, J.C.; Claveau, Y.; Kelly, C.; Lechowicz, M. Functional ecology of advance regeneration in relation to light in boreal forests. Can. J. For. Res. 1999, 29, 812–823. [Google Scholar] [CrossRef]
- Parish, R.; Antos, J.A. Structure and dynamics of an ancient montane forest in coastal British Columbia. Oecologia 2004, 141, 562–576. [Google Scholar] [CrossRef]
- Antos, J.A.; Filipescu, C.N.; Negrave, R.W. Ecology of western redcedar (Thuja plicata): Implications for management of a high-value multiple-use resource. For. Ecol. Manag. 2016, 375, 211–222. [Google Scholar] [CrossRef]
- Carter, R.; Klinka, K. Variation in shade tolerance of Douglas fir, western hemlock, and western red cedar in coastal British Columbia. For. Ecol. Manag. 1992, 55, 87–105. [Google Scholar] [CrossRef]
- Kobe, R.; Coates, K.D. Models of sapling mortality as a function of growth to characterize interspecific variation in shade tolerance of eight tree species of northwestern British Columbia. Can. J. For. Res. 1997, 27, 227–236. [Google Scholar] [CrossRef]
- Khan, S.; Rose, R.; Haase, D.; Sabin, T. Effects of shading on morphology, chlorophyll concentration, and chlorophyll flourescence of four Pacific Northwest conifer species. New Forest. 2000, 19, 171–186. [Google Scholar] [CrossRef]
- Grubb, P.J. The Maintenance of Species-Richness in Plant Communities: The Importance of the Regeneration Niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Gashwiler, J.S. Seed fall of three conifers in west central Oregon. For. Sci. 1969, 15, 290–295. [Google Scholar]
- Blanco, J.A.; Welham, C.; Kimmins, J.P.; Seely, B.; Mailly, D. Guidelines for modeling natural regeneration in boreal forests. For. Chron. 2009, 85, 427–439. [Google Scholar] [CrossRef]
- Kimmins, J.P.; Blanco, J.A.; Seely, B.; Welham, C.; Scoullar, K. Complexity in Modeling Forest Ecosystems; How Much is Enough? For. Ecol. Manag. 2008, 256, 1646–1658. [Google Scholar] [CrossRef]
- Janda, P.; Bače, R.; Trotsiuk, V.; Mikoláš, M.; Nagel, T.; Seidel, R.; Mrhalová, H.; Morrisey, R.C.; Matějů, L.; Lábusová, J.; et al. The historical disturbance regime of mountain Norway spruce forests in the Western Carpathians and its influence on current forest structure and composition. For. Ecol. Manag. 2017, 388, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Johnston, J.D.; Dunn, C.J.; Vernon, M.J.; Bailey, J.D.; Morrissette, B.A.; Morici, K.E. Restoring historical forest conditions in a diverse inland Pacific Northwest landscape. Ecosphere 2018, 9, e02400. [Google Scholar] [CrossRef]
- Messier, C. Effects of neutral shade and growing media on growth, biomass and competitive ability of Gaultheria shallon. Can. J. Bot. 1992, 70, 2271–2276. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Ritland, K.; Thompson, S.L. Patterns of post-glacial colonization by western redcedar (Thuja plicata, Cupressaceae) as revealed by microsatellite markers. Botany 2008, 86, 194–203. [Google Scholar] [CrossRef]
- Donato, D.C.; Halofsky, J.S.; Reilly, M.J. Corralling a black swan: Natural range of variation in a forest landscape driven by rare, extreme events. Ecol. Appl. 2020, 30, e02013. [Google Scholar] [CrossRef]
- Shugart, H.H.; West, D.C. Development of an Appalachian Deciduous Forest Succession Model and its Application to Assessment of the Impact of the Chestnut Blight. J. Environ. Manag. 1977, 5, 161–179. [Google Scholar]
- Horn, H.S. Succession. In Theoretical Ecology: Principles and Applications; May, R.M., Ed.; Blackwell Scientific Publishers: Oxford, UK, 1976; pp. 187–204. [Google Scholar]
- Hebda, R.J. Late-glacial and postglacial vegetation history at Bear Cove Bog, northeast Vancouver Island, British Columbia. Can. J. Bot. 1983, 61, 3172–3192. [Google Scholar] [CrossRef]
- Blanco, J.A. Forests may need centuries to recover their original productivity after continuous intensive management: An example from Douglas-fir. Sci. Total. Environ. 2012, 437, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Haeussler, S. Rethinking biogeoclimatic ecosystem classification for a changing world. Environ. Rev. 2011, 19, 254–277. [Google Scholar] [CrossRef]
- Logofet, D.O.; Lesnaya, E.V. The Mathematics of Markov Models: What Markov Chains Can Really Predict in Forest Successions. Ecol. Modell. 2000, 126, 285–298. [Google Scholar] [CrossRef]
- Kimmins, J.P. Emulation of natural forest disturbance. What does this mean. In Emulating Natural Forest Landscape Disturbances: Concepts and Applications; Perera, A.H., Buse, L.J., Webe, M.G., Eds.; Columbia University Press: New York, NY, USA, 2004; pp. 8–28. [Google Scholar]


| Stand Type | Init | WindSusc | Cinv≤15 | Cinv>15 | Csuc≤15 | Csuc>15 | Gap | CinvGap≤15 | CinvGap>15 |
|---|---|---|---|---|---|---|---|---|---|
| HAy | 0.33 | 0.8 | 0.05 | 0.1 | 0 | 0 | 0.0 | 0.0 | 0 |
| HA(C)y | 0.00 | 0.8 | 0.05 | 0.2 | 0 | 0 | 0.0 | 0.0 | 0 |
| CHy | 0.00 | 0.0 | 1.00 | 0.1 | 0 | 1 | 0.2 | 0.0 | 0 |
| HAm | 0.33 | 1.0 | 0.05 | 0.5 | 0 | 1 | 0.2 | 0.0 | 1 |
| HA(C)m | 0.00 | 1.0 | 0.05 | 0.1 | 0 | 1 | 0.2 | 0.1 | 1 |
| CHm | 0.00 | 0.0 | 1.00 | 0.1 | 0 | 1 | 0.2 | 0.8 | 1 |
| HAo | 0.33 | 0.5 | 0.05 | 0.8 | 0 | 1 | 1.0 | 1.0 | 1 |
| HA(C)o | 0.00 | 0.5 | 0.05 | 0.8 | 0 | 1 | 1.0 | 0.0 | 1 |
| CHo | 0.00 | 0.0 | 1.00 | 0.1 | 0 | 1 | 0.2 | 0.8 | 1 |
| Stand Type | Init | WindSusc | Cinv≤15 | Cinv>15 | Csuc≤15 | Csuc>15 | Gap | CinvGap≤15 | CinvGap>15 |
|---|---|---|---|---|---|---|---|---|---|
| HAy | 0.33 | 0.5 | 0.04 | 0.0 | 0 | 0 | 0 | 0 | 0 |
| HA(C)y | 0.00 | 0.5 | 0.10 | 0.8 | 1 | 0 | 0 | 0 | 0 |
| CHy | 0.00 | 0.0 | 0.00 | 1.0 | 0 | 1 | 0 | 0 | 0 |
| HAm | 0.33 | 1.0 | 0.04 | 0.0 | 0 | 0 | 1 | 0 | 0 |
| HA(C)m | 0.00 | 1.0 | 0.10 | 0.8 | 0 | 0 | 1 | 1 | 0 |
| CHm | 0.00 | 0.0 | 0.00 | 1.0 | 0 | 0 | 1 | 0 | 1 |
| HAo | 0.33 | 0.6 | 0.04 | 0.0 | 0 | 0 | 1 | 0 | 0 |
| HA(C)o | 0.00 | 0.6 | 0.10 | 0.0 | 0 | 0 | 1 | 1 | 0 |
| CHo | 0.00 | 0.0 | 0.00 | 1.0 | 0 | 0 | 1 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.A.; Lo, Y.-H.; Kimmins, J.P.; Weber, A. Highlighting Complex Long-Term Succession Pathways in Mixed Forests of the Pacific Northwest: A Markov Chain Modelling Approach. Forests 2021, 12, 1770. https://doi.org/10.3390/f12121770
Blanco JA, Lo Y-H, Kimmins JP, Weber A. Highlighting Complex Long-Term Succession Pathways in Mixed Forests of the Pacific Northwest: A Markov Chain Modelling Approach. Forests. 2021; 12(12):1770. https://doi.org/10.3390/f12121770
Chicago/Turabian StyleBlanco, Juan A., Yueh-Hsin Lo, James P. Kimmins, and Adrian Weber. 2021. "Highlighting Complex Long-Term Succession Pathways in Mixed Forests of the Pacific Northwest: A Markov Chain Modelling Approach" Forests 12, no. 12: 1770. https://doi.org/10.3390/f12121770
APA StyleBlanco, J. A., Lo, Y.-H., Kimmins, J. P., & Weber, A. (2021). Highlighting Complex Long-Term Succession Pathways in Mixed Forests of the Pacific Northwest: A Markov Chain Modelling Approach. Forests, 12(12), 1770. https://doi.org/10.3390/f12121770








