Corrosiveness of Thermally Modified Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermally Modified Wood
2.2. Fasteners
2.3. Exposure
2.4. Post-Test Cleaning Procedure
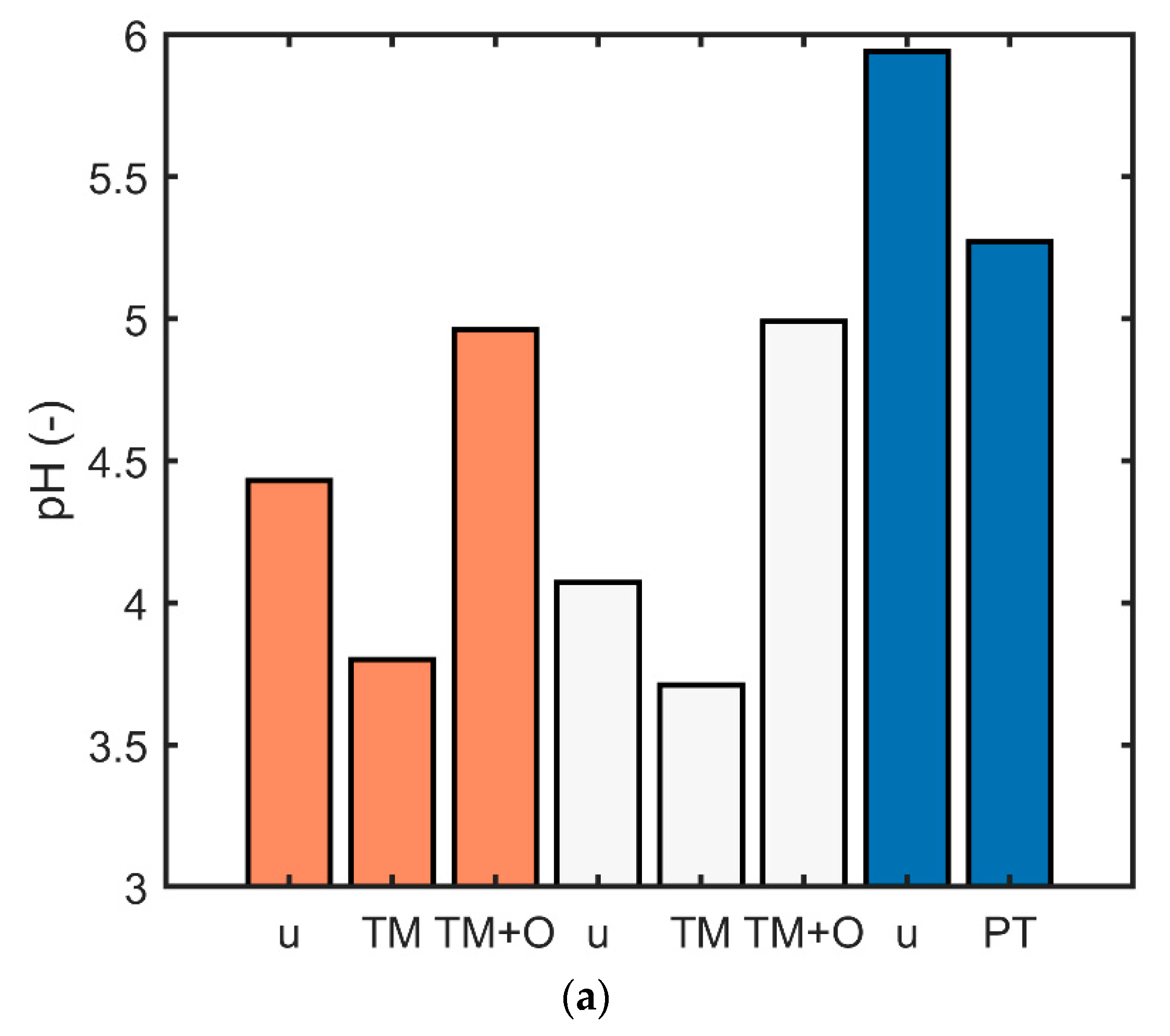
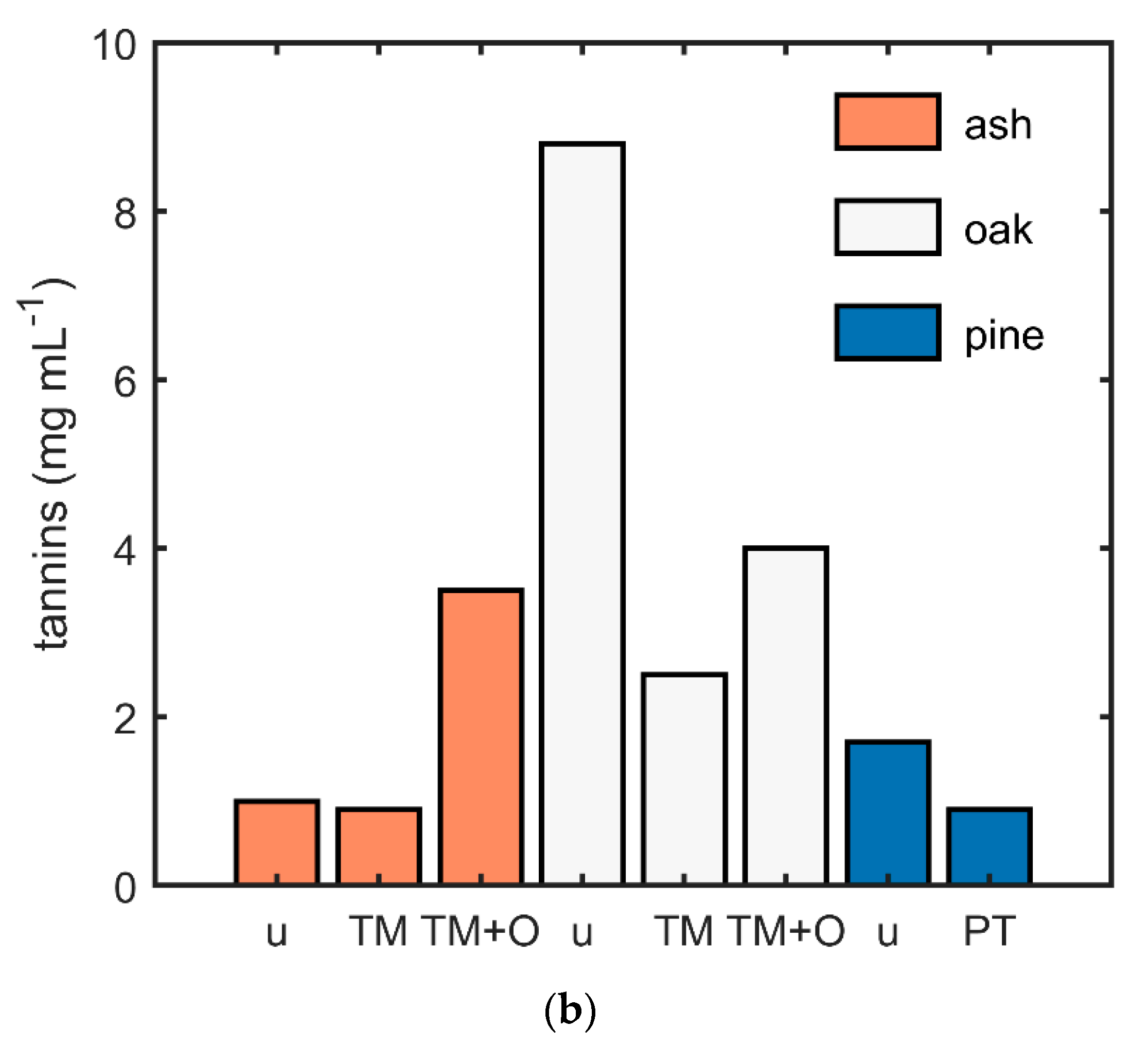
2.5. Determination of the pH and Tannin Content
3. Results and Discussion
3.1. Stainless Steel Fasteners
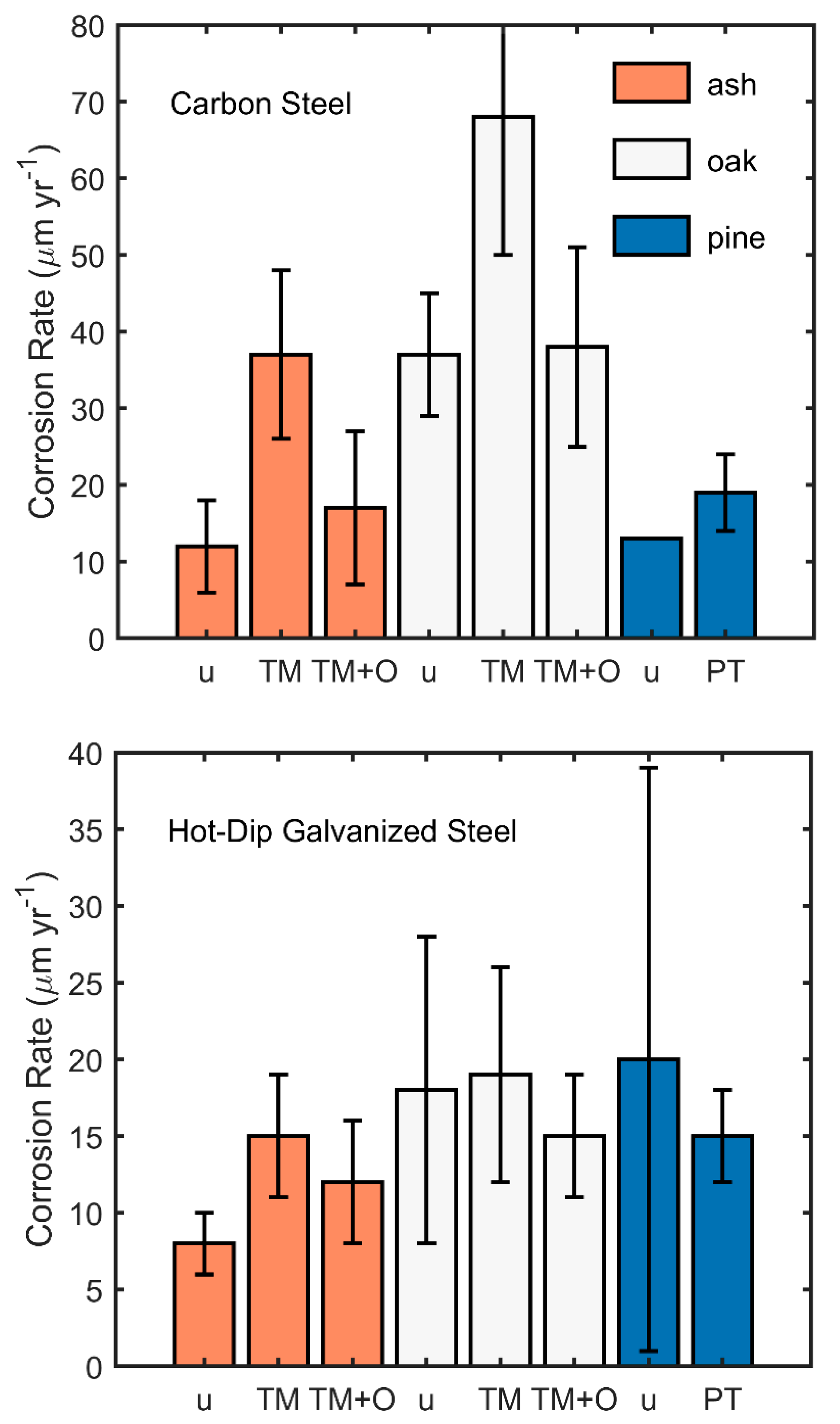
3.2. Carbon Steel Fasteners
3.3. Hot-Dip Galvanized Fasteners
3.4. Comparison of the Corrosion Rates of Hot-Dip Galvanized Fasteners and Carbon Steel Fasteners
3.5. Comparison of the Wood Species and the Effect of pH and Tannins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savchenko, S.; Lillie, M.C.; Zhilin, M.G.; Budd, C.E. New AMS dating of bone and antler weapons from the shigir collections housed in the sverdlovsk regional museum, Urals, Russia. In Proceedings of the Prehistoric Society; Cambridge University Press: Cambridge, UK, 2015; pp. 265–281. [Google Scholar]
- Rifai, M.M.; El Hadidi, N.M. Investigation and analysis of three gilded wood samples from the tomb of Tutankhamun. In Decorated Surfaces on Ancient Egyptian Objects, Technology, Deterioration and Conservation; Dawson, J., Rozeik, C., Wright, M.M., Eds.; Cairo University: Giza, Egypt, 2010; pp. 16–24. [Google Scholar]
- Williams, R.S. Weathering of wood. Handb. Wood Chem. Wood Compos. 2005, 7, 139–185. [Google Scholar]
- Lebow, S. Wood Preservation. In Wood Handbook- Wood as an Engineering Material; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010; p. 508. [Google Scholar]
- Lebow, S. Alternatives to Chromated Copper Arsenate for Residential Construction; Res. Pap. FPL-RP-618; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2004; p. 9.
- Hill, C.A. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: West Sussex, UK, 2006; Volume 5. [Google Scholar]
- Ringman, R.; Pilgård, A.; Brischke, C.; Richter, K. Mode of action of brown rot decay resistance in modified wood: A review. Holzforschung 2014, 68, 239–246. [Google Scholar] [CrossRef]
- Zelinka, S.L. Role of transport in wood damage mechanisms–WSE 2017 keynote lecture. Int. Wood Prod. J. 2018, 9, 50–57. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Ringman, R.; Pilgård, A.; Thybring, E.E.; Jakes, J.E.; Richter, K. The role of chemical transport in the brown-rot decay resistance of modified wood. Int. Wood Prod. J. 2016, 7, 66–70. [Google Scholar] [CrossRef]
- Jakes, J.E.; Plaza, N.; Stone, D.S.; Hunt, C.G.; Glass, S.V.; Zelinka, S.L. Mechanism of transport through wood cell wall polymers. J. For. Prod. Ind. 2013, 2, 10–13. [Google Scholar]
- Hunt, C.G.; Zelinka, S.L.; Frihart, C.R.; Lorenz, L.; Yelle, D.; Gleber, S.-C.; Vogt, S.; Jakes, J.E. Acetylation increases relative humidity threshold for ion transport in wood cell walls—A means to understanding decay resistance. Int. Biodeterior. Biodegrad. 2018, 133, 230–237. [Google Scholar] [CrossRef]
- Jakes, J.E.; Hunt, C.G.; Zelinka, S.L.; Ciesielski, P.N.; Plaza, N.Z. Effects of Moisture on Diffusion in Unmodified Wood Cell Walls: A Phenomenological Polymer Science Approach. Forests 2019, 10, 1084. [Google Scholar] [CrossRef]
- Stamm, A.J.; Hansen, L. Minimizing wood shrinkage and swelling effect of heating in various gases. Ind. Eng. Chem. 1937, 29, 831–833. [Google Scholar] [CrossRef]
- Rapp, A.O.; Brischke, C.; Welzbacher, C.R. Interrelationship between the severity of heat treatments and sieve fractions after impact ball milling: A mechanical test for quality control of thermally modified wood. Holzforschung 2006, 60, 64. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Ramsay, J.; Laine, K.; Rautkari, L.; Hughes, M. Water vapour sorption behaviour of thermally modified wood. Int. Wood Prod. J. 2013, 4, 191–196. [Google Scholar] [CrossRef]
- Jalaludin, Z.; Hill, C.A.S.; Xie, Y.; Samsi, H.W.; Husain, H.; Awang, K.; Curling, S.F. Analysis of the water vapour sorption isotherms of thermally modified acacia and sesendok. Wood Mater. Sci. Eng. 2010, 5, 194–203. [Google Scholar] [CrossRef]
- Calonego, F.W.; Severo, E.T.D.; Furtado, E.L. Decay resistance of thermally-modified Eucalyptus grandis wood at 140 C, 160 C, 180 C, 200 C and 220 C. Bioresour. Technol. 2010, 101, 9391–9394. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.L.; Kocaefe, D.; Amburgey, T.; Zhang, J. A comparative study on brown-rot fungus decay and subterranean termite resistance of thermally-modified and ACQ-C-treated wood. Holz Als Roh Und Werkst. 2007, 65, 353–358. [Google Scholar] [CrossRef]
- Candelier, K.; Thevenon, M.-F.; Petrissans, A.; Dumarcay, S.; Gerardin, P.; Petrissans, M. Control of wood thermal treatment and its effects on decay resistance: A review. Ann. For. Sci. 2016, 73, 571–583. [Google Scholar] [CrossRef]
- Rapp, A.O.; Brischke, C.; Welzbacher, C.R.; Jazayeri, L. Increased resistance of thermally modified Norway spruce timber (TMT) against brown rot decay by Oligoporus placenta–a study on the mode of protective action. Wood Res. 2008, 53, 13–25. [Google Scholar]
- Chaouch, M.; Pétrissans, M.; Pétrissans, A.; Gérardin, P. Use of wood elemental composition to predict heat treatment intensity and decay resistance of different softwood and hardwood species. Polym. Degrad. Stab. 2010, 95, 2255–2259. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Passarini, L. Corrosion of metal fasteners embedded in acetylated and untreated wood at different moisture contents. Wood Mater. Sci. Eng. 2018, 1–18. [Google Scholar] [CrossRef]
- Humar, M.; Brischke, C.; Meyer, L.; Lesar, S.; Thaler, N.; Jones, D.; Bardage, S.; Belloncle, C.; Bulcke, J.; Abascal, M. Introduction of the COST FP 1303 cooperative performance test. IRG/WP 15-20567. In Proceedings of the 46th Annual Meeting of the International Research Group on Wood Protection, Vina del Mar, Chile, 10–14 May 2015. [Google Scholar]
- Jermer, J.; Andersson, B.L.; Schalnat, J. Corrosion of fasteners in furfurylated wood- final report after 9 years exposure outdoors. IRG/WP 17-40810. In Proceedings of the 48th Annual Meeting of the International Research Group on Wood Protection, Ghent, Belgium, 4–8 June 2017. [Google Scholar]
- Jermer, J.; Andersson, B.L. Corrosion of Fasteners in Heat-Treated Wood—Progress Report after Two Years’ Exposure Outdoors; IRG/WP 05-40296; IRG Secretariat: Bangalore, India, 2005. [Google Scholar]
- Dennis, J.K.; Zou, C.; Short, N.R. Corrosion behaviour of zinc and zinc alloy coated steel in preservative treated timber. Trans. Inst. Met. Finish. 1995, 75, 96–101. [Google Scholar] [CrossRef]
- Short, N.R.; Dennis, J.K. Corrosion resistance of zinc-alloy coated steel in construction industry environments. Trans. Inst. Met. Finish. 1997, 75, 47–52. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Derome, D.; Glass, S.V. The effect of moisture content on the corrosion of fasteners embedded in wood subjected to alkaline copper quaternary treatment. Corros. Sci. 2014, 83, 67–74. [Google Scholar] [CrossRef]
- Burkholder, M. CCA, NFBA, and the post-frame building industry. Fram. Build. News 2004, 16, 6–12. [Google Scholar]
- Rush, F.A. Deck Collapse- 4403 Ocean. Drive; Town of Emerald Isle: Emerald Isle, NC, USA, 2015; p. 19. [Google Scholar]
- Zelinka, S.L.; Derome, D.; Glass, S.V. Combining hygrothermal and corrosion models to predict corrosion of metal fasteners embedded in wood. Build. Environ. 2011, 46, 2060–2068. [Google Scholar] [CrossRef]
- Baker, A.J. Corrosion of nails in CCA- and ACA-treated wood in two environments. For. Prod. J. 1992, 42, 39–41. [Google Scholar]
- Zelinka, S.L. Uncertainties in corrosion rate measurements of fasteners exposed to treated wood at 100% relative humidity. ASTM J. Test. Eval. 2007, 35, 106–109. [Google Scholar]
- Zelinka, S.L.; Rammer, D.R. Corrosion rates of fasteners in treated wood exposed to 100% relative humidity. ASCE J. Mater. Civ. Eng. 2009, 21, 758–763. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Sichel, R.J.; Stone, D.S. Exposure testing of fasteners in preservative treated wood: Gravimetric corrosion rates and corrosion product analyses. Corros. Sci. 2010, 52, 3943–3948. [Google Scholar] [CrossRef]
- Kear, G.; Wu, H.-Z.; Jones, M.S. Weight loss studies of fastener materials corrosion in contact with timbers treated with copper azole and alkaline copper quaternary compounds. Corros. Sci. 2009, 51, 252–262. [Google Scholar] [CrossRef]
- Kear, G.; Wú, H.Z.; Jones, M.S. The Corrosion of Metallic Fastener Materials in Untreated; CCA-, CuAz0 and ACQ-Based Timbers; BRANZ: Porirua, New Zealand, 2006; p. 281. [Google Scholar]
- Kear, G.; Wú, H.Z.; Jones, M.S. Non-accelerated weight loss studies of fastener material corrosion in contact with CCA, CuAz, and ACQ-treated timbers. In Proceedings of the Corrosion and Protection 06 Conference, Hobart, Australia, 19–22 November 2006. [Google Scholar]
- Anon. ThermoWood handbook; International ThermoWood Association: Helsinki, Finland, 2003; p. 66. [Google Scholar]
- Siteworks, T. Boulevard™ Decking Technical Data Sheet; Tournesol Siteworks: Union City, CA, USA, 2019; Available online: https://cdn.tournesol.com/media/Documents/BL119.pdf (accessed on 23 December 2019).
- Rammer, D.R.; Zelinka, S.L. Analytical determination of the surface area of a threaded fastener. ASTM J. Test. Eval. 2008, 36, 80–88. [Google Scholar]
- Rammer, D.R.; Zelinka, S.L. Optical method for measuring the surface area of a threaded fastener. Exp. Tech. 2010, 34, 36–39. [Google Scholar] [CrossRef]
- Anon. ASTM G198-17: Standard test. In Method for Determining the Relative Corrosion Performance of Driven Fasteners in Contact with Treated Wood; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Zelinka, S.L. Comparing the methodologies in ASTM G198 using combined hygrothermal-corrosion modeling. Corrosion 2013, 70, 206–213. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Jakes, J.E.; Kirker, G.T.; Passarini, L.; Hunt, C.G.; Lai, B.; Antipova, O.; Li, L.; Vogt, S. Copper distribution and oxidation states near corroded fasteners in treated wood. SN Appl. Sci. 2019, 1, 240. [Google Scholar] [CrossRef]
- Zelinka, S.L. Corrosion of metals in wood products. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: Rijeka, Croatia, 2014; Chapter 23; pp. 568–592. [Google Scholar]
- Zelinka, S.L.; Stone, D.S. Corrosion of metals in wood: Comparing the results of a rapid test method with long-term exposure tests across six wood treatments. Corros. Sci. 2011, 53, 1708–1714. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Stone, D.S. The effect of tannins and pH on the corrosion of steel in wood extracts. Mater. Corros. 2011, 62, 739–744. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Rammer, D.R.; Stone, D.S. Corrosion of metals in contact with treated wood: Developing test methods. In Proceedings of the Corrosion Conference 2008, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Zelinka, S.L.; Rammer, D.R.; Stone, D.S. Electrochemical corrosion testing of fasteners in wood extract. Corros. Sci. 2008, 50, 1251–1257. [Google Scholar] [CrossRef]
- Makkar, H. A laboratory manual for the FAO/IAEA co-ordinated research project on use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on tanniniferous tree foliage. In Animal Production and Health Sub-Progrmme, FAO/IAEA Working Document; IAEA: Vienna, Austria, 2000. [Google Scholar]
- Makkar, H.P. Use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on tanniniferous tree foliage: Achievements, result implications, and future research. Anim. Feed Sci. Technol. 2005, 122, 3–12. [Google Scholar] [CrossRef]
- Makkar, H.P.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Kodama, T. Corrosion of wrought carbon steels. In ASM Metals Handbook, Volume 13B Corrosion: Matierals; Cramer, S.D., Covino, B.S., Eds.; ASM International: Materials Park, OH, USA, 2003; pp. 5–10. [Google Scholar]
- Zelinka, S.L.; Glass, S.V.; Boardman, C.R.; Derome, D. Comparison of the corrosion of fasteners embedded in wood measured in outdoor exposure with the predictions from a combined hygrothermal-corrosion model. Corros. Sci. 2016, 102, 178–185. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion of Zinc and Zinc Alloys. In ASM Handbok Volume 13B, Corrosion: Materials; Cramer, S.D., Covino, B.S., Eds.; ASM International: Materials Park, OH, USA, 2003; pp. 402–417. [Google Scholar]
- Zhang, X.G.; Hwang, J.; Wu, W.K. Corrosion testing of steel and zinc. In Proceedings of the 4th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (GALVATECH’98), Makuhari, Japan, 20–23 September 1998. [Google Scholar]
- Smith, C.A. Corrosion of metals by wood. Anti Corros. Methods Mater. 1982, 29, 16–17. [Google Scholar]
- Farmer, R.H. Corrosion of metals in association with wood. Part 2. Corrosion of metals in contact with wood. Wood 1962, 27, 443–446. [Google Scholar]
- Bartel-Kornacka, E.T. Corrosion of iron by Ghana timbers. Wood 1967, 32, 39–42. [Google Scholar]
- Knotkova-Cermakova, D.; Vlckova, J. Corrosive effect of plastics, rubber and wood on metals in confined spaces. Br. Corros. J. 1971, 6, 17–22. [Google Scholar] [CrossRef]
| Carbon Steel | Stainless Steel | Galvanized Coating | |
|---|---|---|---|
| Carbon | 0.191 | 0.040 | − |
| Silicon | 0.130 | 0.419 | 0.415 |
| Manganese | 0.750 | 1.680 | − |
| Phosphorus | 0.007 | 0.025 | − |
| Sulfur | 0.007 | 0.020 | − |
| Chromium | 0.022 | 18.110 | − |
| Nickel | − | 8.830 | − |
| Molybdenum | − | 0.253 | − |
| Copper | 0.040 | 0.181 | − |
| Cobalt | 0.004 | 0.106 | 0.037 |
| Tin | 0.001 | 0.013 | 0.032 |
| Bismuth | 0.006 | − | 0.186 |
| Zinc | − | 0.018 | balance |
| Iron | balance | balance | 2.815 |
| Species | Treatment | Final Moisture Content (Standard Deviation) |
|---|---|---|
| Ash | Untreated | 36% (8%) |
| Thermally Modified | 25% (8%) | |
| Thermally Modified w/Oil Treatment | 32% (4%) | |
| Oak | Untreated | 18% (3%) |
| Thermally Modified | 16% (1%) | |
| Thermally Modified w/Oil Treatment | 16% (1%) | |
| Pine | Untreated | 27% (1%) |
| Preservative-Treated | 49% (6%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelinka, S.L.; Passarini, L.; Matt, F.J.; Kirker, G.T. Corrosiveness of Thermally Modified Wood. Forests 2020, 11, 50. https://doi.org/10.3390/f11010050
Zelinka SL, Passarini L, Matt FJ, Kirker GT. Corrosiveness of Thermally Modified Wood. Forests. 2020; 11(1):50. https://doi.org/10.3390/f11010050
Chicago/Turabian StyleZelinka, Samuel L., Leandro Passarini, Frederick J. Matt, and Grant T. Kirker. 2020. "Corrosiveness of Thermally Modified Wood" Forests 11, no. 1: 50. https://doi.org/10.3390/f11010050
APA StyleZelinka, S. L., Passarini, L., Matt, F. J., & Kirker, G. T. (2020). Corrosiveness of Thermally Modified Wood. Forests, 11(1), 50. https://doi.org/10.3390/f11010050







