Growth and Tree Water Deficit of Mixed Norway Spruce and European Beech at Different Heights in a Tree and under Heavy Drought
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Water Potential (Ψ Leaf)
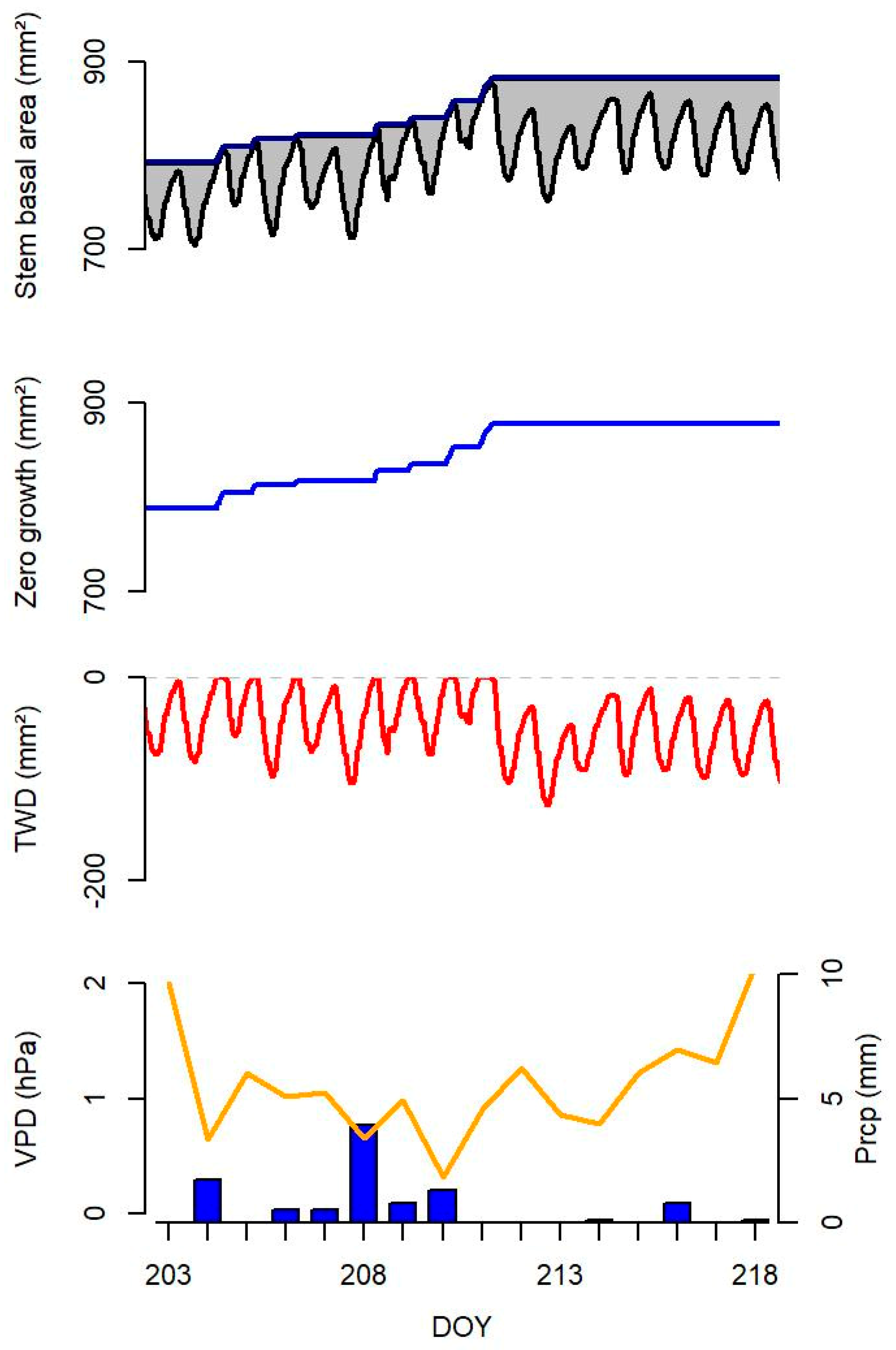
2.3. Stem Basal Area Variations (Growth and Tree Water Deficit)
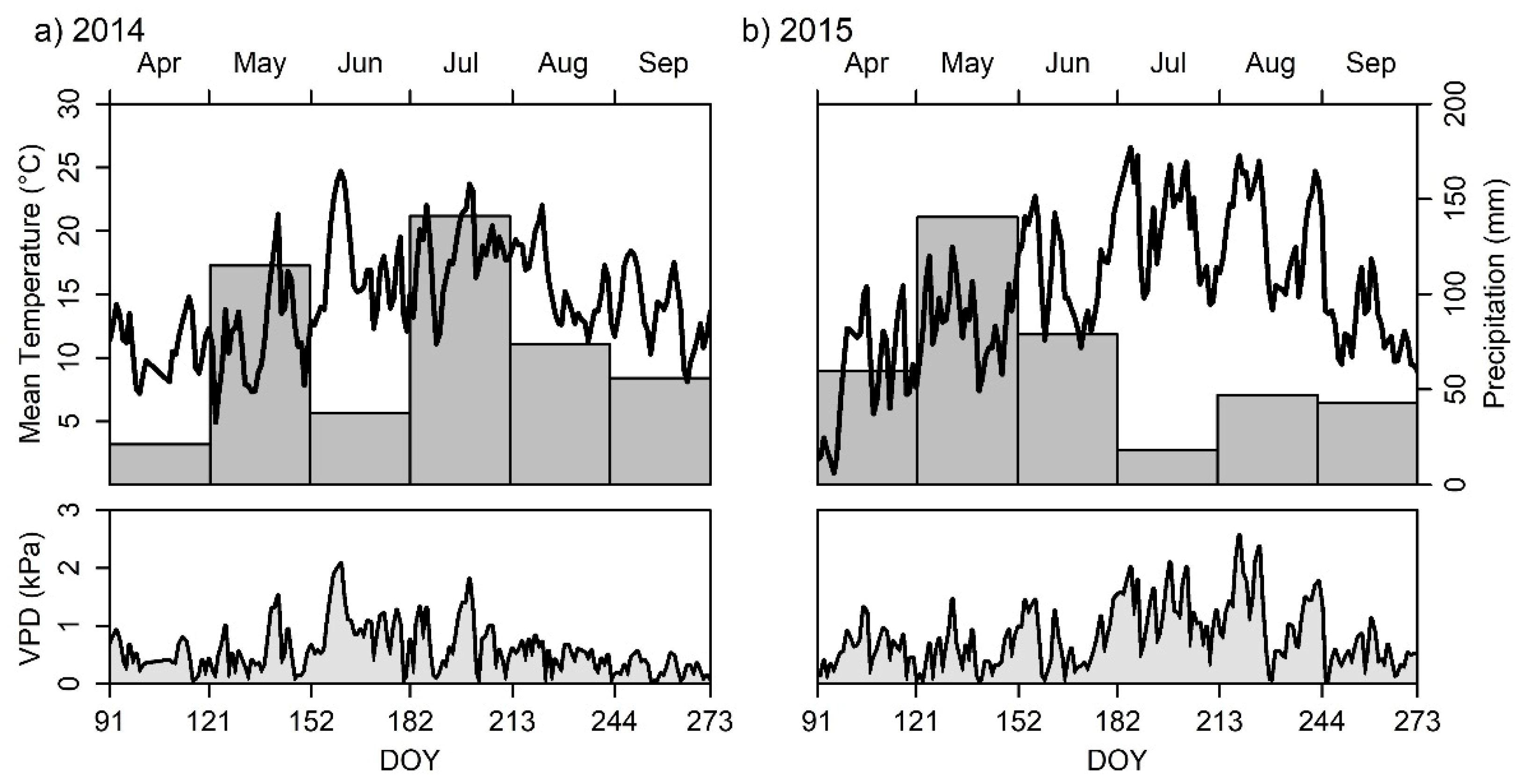
2.4. Climatic Data
2.5. Statistical Analysis
3. Results
3.1. Temperature and Precipitation in 2014 and 2015
3.2. Leaf Water Potentials
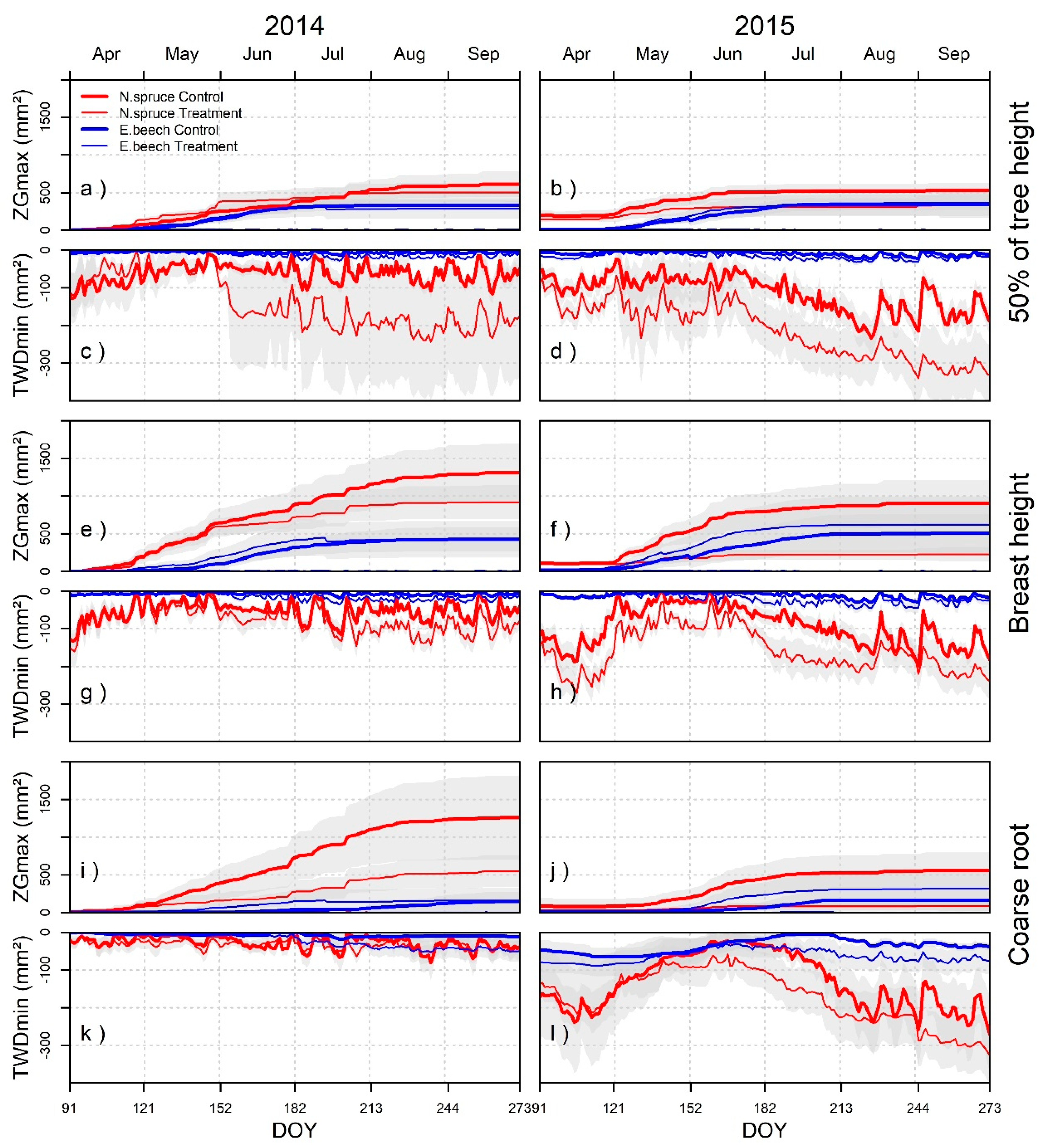
3.3. Zero Growth and Tree Water Deficit
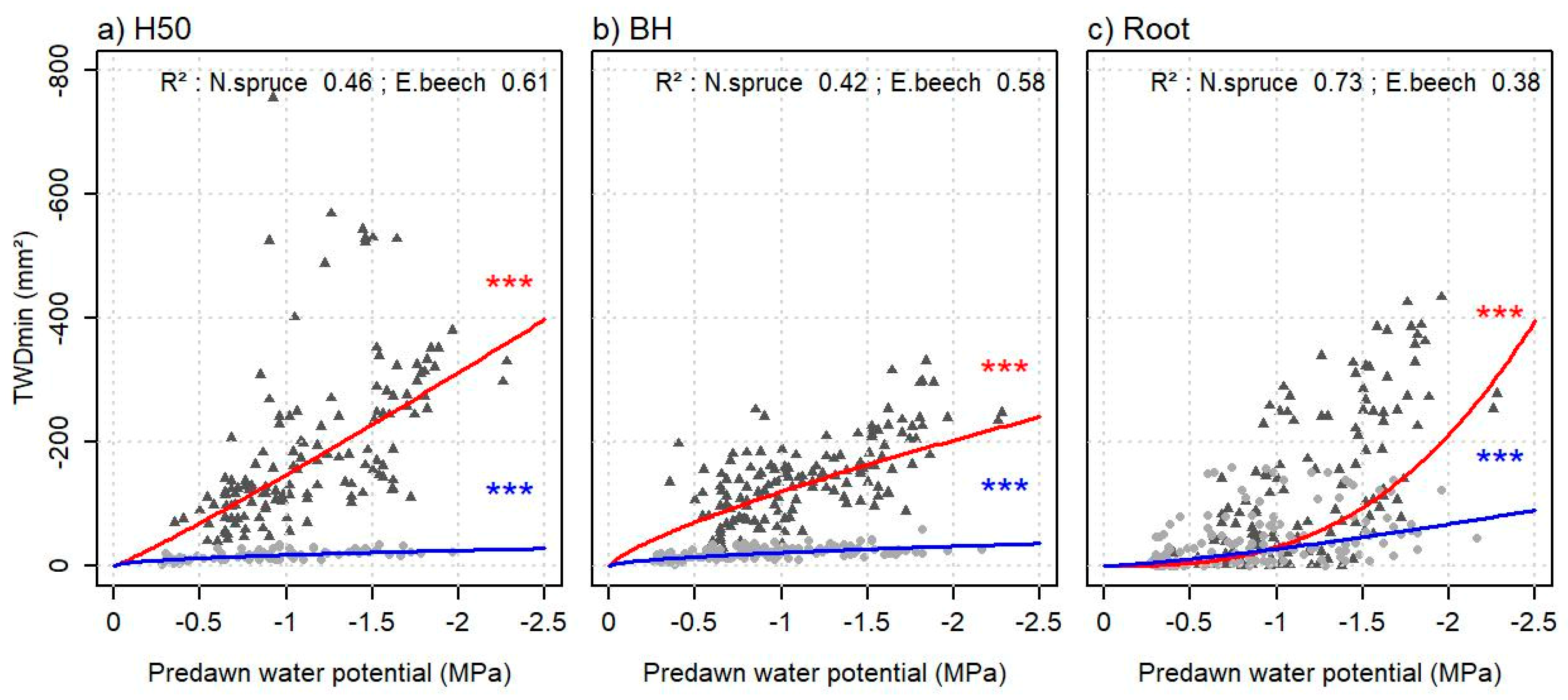
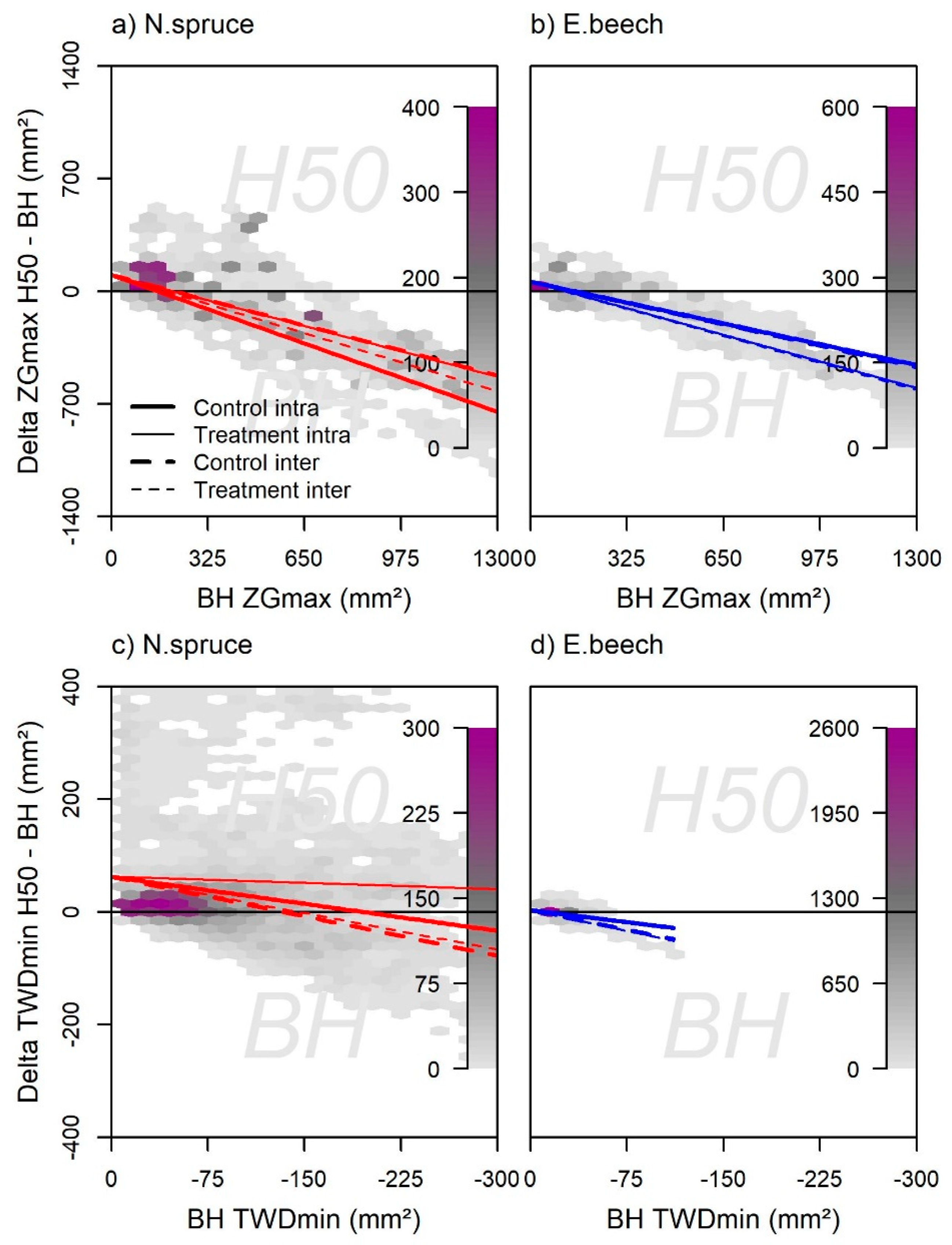
3.4. Relationship between Tree Water Deficits and Water Potentials at Different Tree Heights
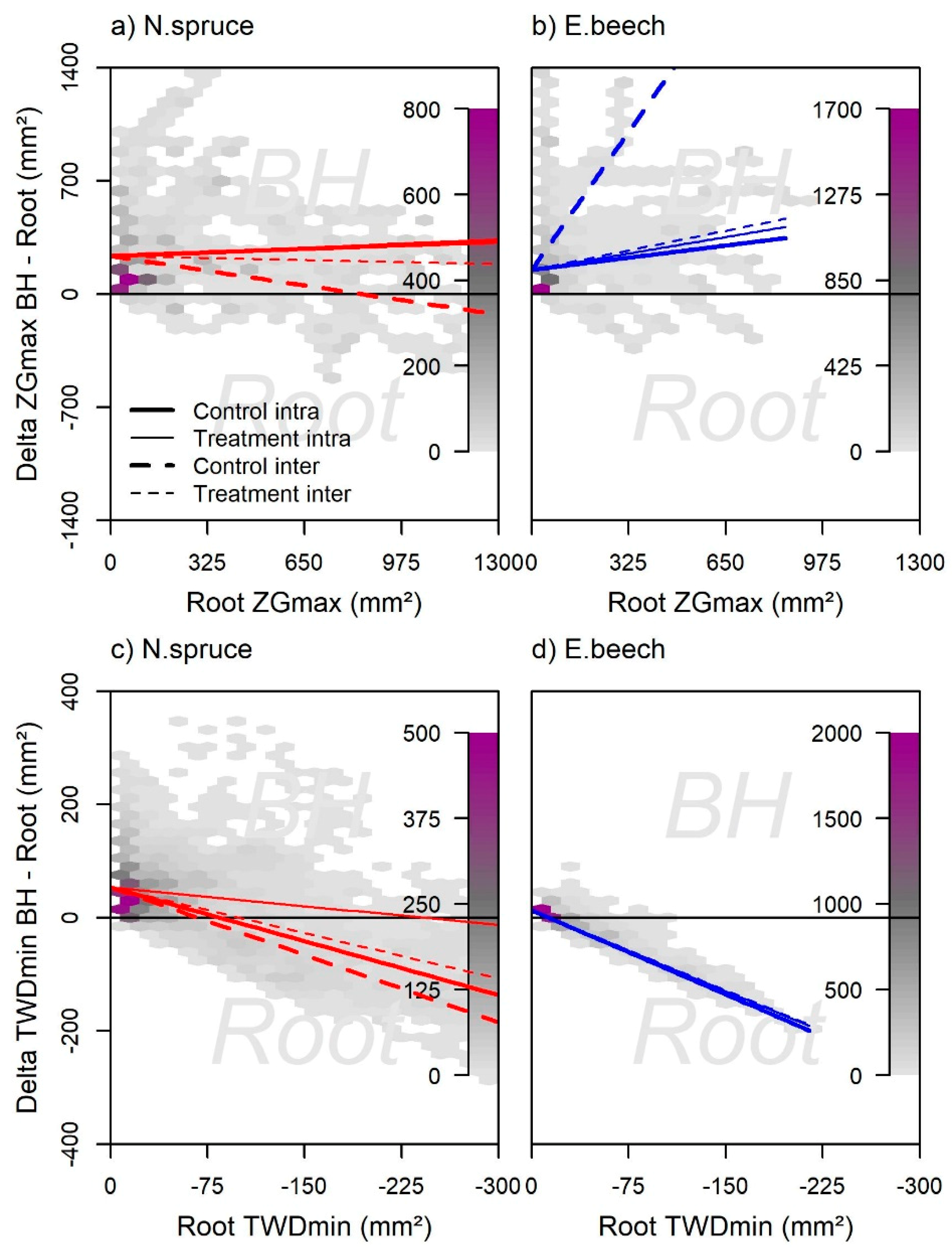
3.5. Stem and Root Growth and TWDmin in Different Tree Compartments
4. Discussion
4.1. Relationship between Tree Water Deficit and Leaf Water Potential
4.2. Root and Stem Growth and TWDmin in Different Tree Heights
4.3. Differences in Intra- and Interspecific Neighborhoods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Basis. Contribution of Working Group I to the Fifth Assessment Report of the IPCC; Cambrige University Press: Cambridge, UK, 2013; ISBN 978-1-107-66182-0. [Google Scholar]
- Meehl, G.A.; Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, J.; Beniston, M.; Fischlin, A.; Frei, C.; Goyette, S.; Jasper, K.; Pfister, C. Climate risks and their impact on agriculture and forests in Switzerland. In Climate Variability, Predictability and Climate Risks: A European Perspecive; Wanner, H., Grosjean, M., Rastlisberg, R., Xoplaki, E., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 79–102. ISBN 978-1-4020-5713-7. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Rötzer, T.; Liao, Y.; Goergen, K.; Schüler, G.; Pretzsch, H. Modelling the impact of climate change on the productivity and water-use efficiency of a central European beech forest. Clim. Res. 2013, 58, 81–95. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Dieler, J.; Pretzsch, H. Morphological plasticity of European beech (Fagus sylvatica L.) in pure and mixed-species stands. For. Ecol. Manag. 2013, 295, 97–108. [Google Scholar] [CrossRef]
- Chapin, F.S. The Mineral Nutrition of Wild Plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Thornley, J.H.M. A balanced quantitative model for root: Shoot ratios in vegetative plants. Ann. Bot. 1972, 36, 431–441. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Rötzer, T.; Seifert, T.; Pretzsch, H. Modelling above and below ground carbon dynamics in a mixed beech and spruce stand influenced by climate. Eur. J. Res. 2009, 128, 171–182. [Google Scholar] [CrossRef]
- Zweifel, R.; Item, H.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 2, 869–877. [Google Scholar] [CrossRef]
- Steppe, K.; De Pauw, D.J.W.; Lemeur, R.; Vanrolleghem, P.A. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006, 26, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, N.; Eugster, W.; Zweifel, R.; Buchmann, N.; Kahmen, A. Temperate tree species show identical response in tree water deficit but different sensitivities in sap flow to summer soil drying. Tree Physiol. 2016, 36, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.; Zweifel, R.; Kahmen, A. Daily stem diameter variations can predict the canopy water status of mature temperate trees. Tree Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, M.F.; García-Orellana, Y.; Conejero, W.; Ruiz-Sánchez, M.C.; Mounzer, O.; Alarcón, J.J.; Torrecillas, A. Relationships between Climatic Variables and Sap Flow, Stem Water Potential and Maximum Daily Trunk Shrinkage in Lemon Trees. Plant. Soil 2006, 279, 229–242. [Google Scholar] [CrossRef]
- Zweifel, R.; Item, H.; Häsler, R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees 2000, 15, 50–57. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Newbery, D.M. Modeling tree water deficit from microclimate: An approach to quantifying drought stress. Tree Physiol. 2005, 25, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, M.F.; Conejero, W.; Moreno, F.; Moriana, A.; Intrigliolo, D.S.; Biel, C.; Mellisho, C.D.; Pérez-Pastor, A.; Domingo, R.; Ruiz-Sánchez, M.C.; et al. Could trunk diameter sensors be used in woody crops for irrigation scheduling? A review of current knowledge and future perspectives. Agric. Water Manag. 2010, 97, 1–11. [Google Scholar] [CrossRef]
- Daudet, F.-A.; Ameglio, T.; Cochard, H.; Archilla, O.; Lacointe, A. Experimental analysis of the role of water and carbon in tree stem diameter variations. J. Exp. Bot. 2005, 56, 135–144. [Google Scholar] [CrossRef]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of Pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef]
- Klepper, B.; Browning, V.D.; Taylor, H.M. Stem Diameter in Relation to Plant Water Status. Plant. Physiol. 1971, 48, 683–685. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Water Deficits and Plant Growth. In Shrinking and Swelling of Plant Tissues; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1976; pp. 1–64. [Google Scholar]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, V.; Steppe, K. Development and verification of a water and sugar transport model using measured stem diameter variations. J. Exp. Bot. 2010, 61, 2083–2099. [Google Scholar] [CrossRef] [PubMed]
- De Swaef, T.; De Schepper, V.; Vandegehuchte, M.W.; Steppe, K. Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiol. 2015, 35, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant. Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.; Grace, J. Continuous measurements of water tensions in the xylem of trees based on the elastic properties of wood. Planta 1997, 202, 455–461. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Rais, A.; van de Kuilen, J.-W.G.; Pretzsch, H. Growth reaction patterns of tree height, diameter, and volume of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) under acute drought stress in Southern Germany. Eur. J. Res. 2014, 133, 1043–1056. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Enquist, B.J. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]
- Pretzsch, H. Diversity and Productivity in Forests: Evidence from Long-Term Experimental Plots. In Forest Diversity and Function: Temperate and Boreal Systems; Scherer-Lorenzen, M., Körner, C., Schulze, E.-D., Eds.; Springer: Berlin, Germany; New York, NY, USA, 2005; pp. 41–64. ISBN 3-540-22191-3. [Google Scholar]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Rötzer, T. Mixing patterns of tree species and their effects on resource allocation and growth in forest stands. Nova Acta Leopold 2013, 114, 239–254. [Google Scholar]
- Mixed-Species Forests. Ecology and Management; Pretzsch, H., Forrester, D.I., Bauhus, J., Eds.; Springer: Berlin, Germany, 2017; 653p. [Google Scholar]
- Wiedemann, E. Der gleichaltrige Fichten-Buchen-Mischbestand. Mitt. Aus Forstwirtsch. Forstwiss. 1942, 1, 1–88. [Google Scholar]
- Pretzsch, H.; Block, J.; Dieler, J.; Dong, P.H.; Kohnle, U.; Nagel, J.; Spellmann, H.; Zingg, A. Comparison between the productivity of pure and mixed stands of Norway spruce and European beech along an ecological gradient. Ann. For. Sci. 2010, 67, 712. [Google Scholar] [CrossRef]
- Pretzsch, H.; Rötzer, T.; Matyssek, R.; Grams, T.E.E.; Häberle, K.-H.; Pritsch, K.; Kerner, R.; Munch, J.-C. Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: From reaction pattern to mechanism. Trees 2014. [Google Scholar] [CrossRef]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.-D.; Ammer, C. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 2012, 26, 557–569. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant. Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Ammer, C.; Bickel, E.; Kolling, C. Converting Norway spruce stands with beech—A review of arguments and techniques. Austrian J. For. Sci. 2008, 125, 3–26. [Google Scholar]
- Bolte, A.; Villanueva, I. Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur. J. For. Res. 2006, 125, 15–26. [Google Scholar] [CrossRef]
- Schume, H.; Jost, G.; Hager, H. Soil water depletion and recharge patterns in mixed and pure forest stands of European beech and Norway spruce. J. Hydrol. 2004, 289, 258–274. [Google Scholar] [CrossRef]
- Cremer, M.; Prietzel, J. Die Mutter des Waldes und die Fremde: Douglasien-Buchen-Mischbestände: Aus bodenkundlicher Sicht eine attraktive Mischungsoption. LWF Aktuell 2017, 2, 24–25. [Google Scholar]
- Rötzer, T.; Häberle, K.H.; Kallenbach, C.; Matyssek, R.; Schütze, G.; Pretzsch, H. Tree species and size drive water consumption of beech/spruce forests—A simulation study highlighting growth under water limitation. Plant. Soil 2017, 183, 327. [Google Scholar] [CrossRef]
- Pretzsch, H.; Dieler, J.; Seifert, T.; Rötzer, T. Climate effects on productivity and resource-use efficiency of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica [L.]) in stands with different spatial mixing patterns. Trees 2012, 26, 1343–1360. [Google Scholar] [CrossRef]
- Brodrick, P.G.; Anderegg, L.D.L.; Asner, G.P. Forest Drought Resistance at Large Geographic Scales. Geophys. Res. Lett. 2019, 46, 2752–2760. [Google Scholar] [CrossRef]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Konings, A.G.; Gentine, P. Global variations in ecosystem-scale isohydricity. Glob. Chang. Biol. 2017, 23, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Haeni, M.; Buchmann, N.; Eugster, W. Are trees able to grow in periods of stem shrinkage? New Phytol. 2016, 211, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Bauerle, T.; Häberle, K.H.; Matyssek, R.; Schütze, G.; Rötzer, T. Tree diameter growth after root trenching in a mature mixed stand of Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]). Trees 2016, 30, 1761–1773. [Google Scholar] [CrossRef]
- Hera, U.; Roetzer, T.; Zimmermann, L.; Schulz, C.; Maier, H.; Weber, H.; Kölling, C. Klima en detail—Neue hochaufgelöste Klimakarten zur klimatischen Regionalisierung Bayerns. LWF Aktuell 2011, 86, 34–37. [Google Scholar]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Bavarian State Institute of Forestry (LWF). Bavarian Forest Ecosystem Monitoring Plot Freising (WKS). Meteorological Data; Bavarian State Institute of Forestry (LWF): Freising, Germany, 2016. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests in Linear Mixed Effects Models. R Package Version 2.0-29. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Bavarian State Institute of Forestry (LWF). Umweltmonitoring. Available online: www.lwf.bayern.de/boden-klima/umweltmonitoring (accessed on 10 July 2016).
- Remorini, D.; Massai, R. Comparison of water status indicators for young peach trees. Irrig. Sci. 2003, 39–46. [Google Scholar] [CrossRef]
- Cohen, M.; Goldhamer, D.A.; Fereres, E.; Girona, J.; Mata, M. Assessment of peach tree responses to irrigation water ficits by continuous monitoring of trunk diameter changes. J. Hortic. Sci. Biotechnol. 2001, 76, 55–60. [Google Scholar] [CrossRef]
- Zweifel, R.; Häsler, R. Dynamics of water storage in mature subalpine Picea abies: Temporal and spatial patterns of change in stem radius. Tree Physiol. 2001, 21, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Xylem Structure and the Ascent of Sap, 2nd ed.; Tyree, M.T., Zimmermann, M.H., Eds.; Springer: Berlin, Germany; London, UK, 2011; ISBN 978-3-642-07768-5. [Google Scholar]
- Hinckley, T.M.; Lassoie, J.P.; Running, S.W. Temporal and Spatial Variations in the Water Status of Forest Trees. For. Sci Monogr. 1978, 20, 1–72. [Google Scholar]
- Kozlowski, T.T. Tree Growth; Ronald Press: New York, NY, USA, 1962. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; The University of Arizona Press: Tucson, AZ, USA, 2013; ISBN 0816526850. [Google Scholar]
- Van der Maaten-Theunissen, M.; Bouriaud, O. Climate–growth relationships at different stem heights in silver fir and Norway spruce. Can. J. Res. 2012, 42, 958–969. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Ledo, A.; Paul, K.I.; Burslem, D.F.R.P.; Ewel, J.J.; Barton, C.; Battaglia, M.; Brooksbank, K.; Carter, J.; Eid, T.H.; England, J.R.; et al. Tree size and climatic water deficit control root to shoot ratio in individual trees globally. New Phytol. 2018, 217, 8–11. [Google Scholar] [CrossRef]
- Bayer, D.; Seifert, S.; Pretzsch, H. Structural crown properties of Norway spruce (Picea abies [L.] Karst.) and European beech (Fagus sylvatica [L.]) in mixed versus pure stands revealed by terrestrial laser scanning. Trees 2013, 27, 1035–1047. [Google Scholar] [CrossRef]
- Nickel, U.T.; Weikl, F.; Kerner, R.; Schäfer, C.; Kallenbach, C.; Munch, J.C.; Pritsch, K. Quantitative losses vs. qualitative stability of ectomycorrhizal community responses to 3 years of experimental summer drought in a beech-spruce forest. Glob. Chang. Biol. 2017. [Google Scholar] [CrossRef]
- Kelty, M.J. The role of species mixtures in plantation forestry. For. Ecol. Manag. 2006, 233, 195–204. [Google Scholar] [CrossRef]
- Thurm, E.A.; Biber, P.; Pretzsch, H. Stem growth is favored at expenses of root growth in mixed stands and humid conditions for Douglas-fir (Pseudotsuga menziesii) and European beech (Fagus sylvatica). Trees 2016. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Nikolova, P.S.; Zang, C.; Pretzsch, H. Combining tree-ring analyses on stems and coarse roots to study the growth dynamics of forest trees: A case study on Norway spruce (Picea abies [L.] H. Karst). Trees 2011, 25, 859–872. [Google Scholar] [CrossRef]
- Schall, P.; Lödige, C.; Beck, M.; Ammer, C. Biomass allocation to roots and shoots is more sensitive to shade and drought in European beech than in Norway spruce seedlings. For. Ecol. Manag. 2012, 266, 246–253. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Odor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; de Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Rothe, A.; Binkley, D. Nutritional interactions in mixed species forests: A synthesis. Can. J. Res. 2001, 31, 1855–1870. [Google Scholar] [CrossRef]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Pretzsch, H.; Forrester, D.I.; Rötzer, T. Representation of species mixing in forest growth models. A review and perspective. Ecol. Model. 2015, 313, 276–292. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.; Katul, G.; Porporato, A. Onset of water stress, hysteresis in plant conductance, and hydraulic lift: Scaling soil water dynamics from millimeters to meters. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Dawson, T.E. Hydraulic lift and water use by plants: Implications for water balance, performance and plant-plant interactions. Oecologia 1993, 95, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Mölder, I.; Leuschner, C.; Leuschner, H.H. δ13C signature of tree rings and radial increment of Fagus sylvatica trees as dependent on tree neighborhood and climate. Trees 2011, 25, 215–229. [Google Scholar] [CrossRef]
| Area | N | n | BA | V | hq | dq | |
|---|---|---|---|---|---|---|---|
| (m²) | (m²) | (m³) | (m) | (cm) | |||
| Drought Treatment | |||||||
| Spruce | 301 | 12 | 29.7 | 422 | 29.3 | 34.8 | |
| Beech | 352 | 12 | 22.9 | 309 | 26.1 | 29.1 | |
| Total | 145 | 653 | 24 | 52.6 | 730 | ||
| Control | |||||||
| Spruce | 310 | 12 | 28.8 | 400 | 28.7 | 33.8 | |
| Beech | 356 | 12 | 22.6 | 305 | 26 | 28.7 | |
| Total | 144 | 666 | 24 | 51.4 | 705 |
| log(TWDmin) | ||||||
|---|---|---|---|---|---|---|
| H50 | BH | Root | ||||
| Species | N. Spruce | E. Beech | N. Spruce | E. Beech | N. Spruce | E. Beech |
| Intercept | 4.995 *** | 2.876 *** | 4.786 *** | 3.071 *** | 3.412 *** | 3.317 *** |
| log(LWPpre) | 1.081 *** | 0.501 *** | 0.760 *** | 0.563 *** | 2.804 *** | 1.292 *** |
| R2 | 0.46 | 0.61 | 0.42 | 0.58 | 0.73 | 0.38 |
| RMSE | 126.93 | 6.17 | 50.40 | 7.93 | 112.27 | 44.47 |
| N | 138 | 79 | 144 | 134 | 138 | 140 |
| Position | H50–BH | BH–Root | ||||||
|---|---|---|---|---|---|---|---|---|
| ZGmax/TWDmin | ZGmax | TWDmin | ZGmax | TWDmin | ||||
| Art | (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) |
| N. Spruce | E. Beech | N. Spruce | E. Beech | N. Spruce | E. Beech | N. Spruce | E. Beech | |
| Intercept | 100.961 *** | 59.753 * | 62.534 ** | 2.908 (*) | 235.5 ** | 149.09 ** | 53.718 *** | 13.17 *** |
| BH | −0.481 *** | −0.408 *** | −0.468 *** | −0.463 *** | ||||
| Treat × BH | −0.075 *** | −0.109 *** | 0.037 | −0.028 (*) | ||||
| Mixture × BH | −0.173 *** | 0.01 | 0.148 ** | 0.18 *** | ||||
| Mixture × Treat × BH | 0.243 *** | −0.001 | 0.209 ** | 0.026 | ||||
| Root | −0.279 *** | 2.63 *** | −0.796 *** | −0.992 *** | ||||
| Treat × Root | 0.241 *** | −2.257 *** | 0.258 *** | 0.048 *** | ||||
| Mixture × Root | 0.347 *** | −2.398 *** | 0.161 *** | 0.001 | ||||
| Mixture × Treat × Root | −0.23 *** | 2.338 *** | 0.155 *** | −0.011 | ||||
| R2 | 0.95 | 0.94 | 0.56 | 0.77 | 0.73 | 0.67 | 0.81 | 0.94 |
| RMSE | 69.6 | 49.9 | 59.9 | 4.2 | 182.1 | 194.8 | 42.9 | 9.6 |
| N | 7924 | 4866 | 7924 | 4866 | 8290 | 7940 | 8290 | 7940 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, C.; Rötzer, T.; Thurm, E.A.; Biber, P.; Kallenbach, C.; Pretzsch, H. Growth and Tree Water Deficit of Mixed Norway Spruce and European Beech at Different Heights in a Tree and under Heavy Drought. Forests 2019, 10, 577. https://doi.org/10.3390/f10070577
Schäfer C, Rötzer T, Thurm EA, Biber P, Kallenbach C, Pretzsch H. Growth and Tree Water Deficit of Mixed Norway Spruce and European Beech at Different Heights in a Tree and under Heavy Drought. Forests. 2019; 10(7):577. https://doi.org/10.3390/f10070577
Chicago/Turabian StyleSchäfer, Cynthia, Thomas Rötzer, Eric Andreas Thurm, Peter Biber, Christian Kallenbach, and Hans Pretzsch. 2019. "Growth and Tree Water Deficit of Mixed Norway Spruce and European Beech at Different Heights in a Tree and under Heavy Drought" Forests 10, no. 7: 577. https://doi.org/10.3390/f10070577
APA StyleSchäfer, C., Rötzer, T., Thurm, E. A., Biber, P., Kallenbach, C., & Pretzsch, H. (2019). Growth and Tree Water Deficit of Mixed Norway Spruce and European Beech at Different Heights in a Tree and under Heavy Drought. Forests, 10(7), 577. https://doi.org/10.3390/f10070577





