Abstract
Non-native plant species have become serious pests in Hawaii’s delicate island ecosystems. It is necessary to control invasive plants. The herbicides hexazinone and tebuthiuron were evaluated for defoliation efficacy to control several major invasive plants and for non-target effects on native plants at Site I in a rainforest at 1200 m elevation and Site II in a mesic area at 640 m elevation on the island of Kauai, Hawaii. The invasive weed species in the sites included daisy fleabane (Erigeron karvinskianus DC.), faya tree (Myrica faya Ait.), strawberry guava (Psidium cattleyanum Sabine), banana passion fruit (Passiflora mollissima Bailey), vaseygrass (Paspalum urvillei Steud.), and highbush blackberry (Rubus argutus Link. 1822). Native plants included ohia lehua (Metrosideros polymorpha Gaudich.), naupaka (Scaevola cerasifolia Labill.), pilo (Hedyotis mannii), hona (Urera glabra (Hook. & Arn.)), aalii (Dodonaea viscosa Jacq.), and amau (Sadleria sp.). The results showed that broadcast applications of hexazinone granules and tebuthiuron pellets were effective on some of those invasive species. Herbicidal tolerance varied among the native species. For example, D. viscosa showed high tolerance to hexazinone. S. cerasifolia was susceptible to hexazinone, but not to tebuthiuron. The inconsistent defoliation of Sadleria sp. occurred among different applications rates of the two herbicides. M. polymorpha, particularly when it was small, could tolerate hexazinone and tebuthiuron. U. glabra was severely injured by the two herbicides. H. mannii was moderately tolerant to hexazinone, but fairly sensitive to tebuthiuron. The invasive loblolly pine (Pinus taeda L.) was highly tolerant to hexazinone, but was very sensitive to tebuthiuron. M. faya was very sensitive to hexazinone, but very tolerant to tebuthiuron. P. cattleyanum was sensitive to both herbicides. Six and nine months after hexazinone and tebuthiuron treatment, respectively, native plants were transplanted into the Sites to observe injury from residual herbicides. Approximately less than 10% mortality was observed for the out-planted native species three months after planting (MAP), indicating that the native species showed less injury in the early period of transplant. The mortality of the three endangered species Kauai hau kuahiwi (Hibiscadelphis distans), Kauai delissea (Delissea rhytidosperma H.Mann) and kawawaenohu (Alsinidendron lynchnoides), however, increased as the MAP increased. Overall, broadcast treatments of hexazinone and tebuthiuron at rates higher than 1 kg active ingredient per hectare would be problematic. The dissipation half-life values of hexazinone and tebuthiuron in the 1–15 cm layer of soils at the two sites were approximately 7 days and greater than 180 days, respectively.
Keywords:
alien plant; efficacy; exotic plant; hexazinone; invasive plant; native plant; tebuthiuron 1. Introduction
The invasion of non-native plants is a global phenomenon, which poses a significant and widespread threat to local biodiversity and the ecological balance [1,2]. Non-native plant species have particularly become serious pests in some delicate island ecosystems. It is necessary to control invasive plants in the special island environment. The deliberate and unintentional introduction of ornamental and other species for horticulture, forestry, agriculture and other sectors aggravates the seriousness of the problem [3]. The invasive weed species continue to expand their ranges and newly introduced weeds further threaten the integrity of natural ecosystems. Those weeds out-compete desirable native plants, reduce the diversity of wildlife habitat and increase the risks of wild fires and of erosion [4,5]. It is desirable to control the invasive plant species with minimum damage to the native plants [6]. The common landscape-scale strategies to control invasive plant species, such as fire, herbicide and mowing, are not all suitable, because the native species often share similar physiology and phenology with the invaders [6,7]. The responses of native species to herbicides depend on application time and rate [8,9]. The post-emergent herbicides such as glyphosate, triclopyr, and fluazifop are widely used by land managers to control weeds in natural areas [10,11]. Though effective, these herbicides do not provide pre-emergent activity needed for long term suppression of weeds emerging from the enormous seed bank in the soil [12]. Although pre-emergent herbicides pose a threat to native species as well, they would be valuable in severely degraded forests where major renovation is needed and where natives are scarce or where natives are tolerant of the herbicides [13]. This may require waiting periods until the herbicides are detoxified, in which case knowledge of the half-lives of herbicides would be critical for planning [14,15,16]. Understanding residual efficacy of pre-emergent herbicides is, therefore, necessary to justify their use. Because of environmental concerns, the fate of herbicides in forest ecosystems also needs to be understood.
Over one hundred non-native plant species have become serious pests in native ecosystems in Hawaii. Invasive weeds threaten over 300 endangered or threatened species in Hawaii. Two herbicides with pre- and post-emergent activity and registered for forest use are hexazinone (3-cyclohexyl-6-(dimethylamino)-1,3,5-triazine-2,4(1H,3H)-dione) and tebuthiuron (N-[5-(1,1-dimethylethyl)-1,3,4-thiadiazol-2-yl]-N,N’-dimethylurea), which are both photosynthetic inhibitors and are used for weed control. Hexazinone is nonselective, active in foliar and soil applications, and is quite mobile in soils. When hexazinone was used to control smutgrass (Sporobolus indicus (L.) R.Br.) and Napiergrass (Pennisetum purpureum Schumach. 1827), it could reduce the overall yields of Bermuda grass (Cynodon dactylon (L.) Pers.), and bahiagrass (Paspalum notatum Flüggé) [17,18,19]. Tebuthiuron is selective against dicots, although grass injury can be resulted from applications at high doses. Tebuthiuron is active in soil applications against many woody species such as common guava (Psidium guajava L.), Brazilian peppertree (Schinus terebinthifolius Raddi), duck's eye (Ardisia elliptica Thunb.), and white leadtree (Leucaena leucocephala (Lam.) de Wit) [20]. Tebuthiuron is rather immobile due to its low water solubility and absorptivity to soil clays and organic matter.
Although hexazinone is generally nonselective in its action, there are instances in which tolerance to hexazinone allows its selective use. Broadcast applications of hexazinone were more selective to slash pine (Pinus elliottii Engelm.) and bald cypress (Taxodium distichum (L.) Rich.) and were less damaging to Sawgras (Cladium jamaicense Crantz.) than tebuthiuron in the Florida Everglades [21]. P. notatum could recover 40 days after hexazinone treatment [22], while S. indicus was effectively controlled by all applications of hexazinone one year after treatment (YAT) [22]. Likewise, tebuthiuron may be used selectively where desirable dicots are tolerant to it. In the trials in Hawaii, the non-native Formosan koa (Acacia confuse Merr.) demonstrated tolerance to tebuthiuron. The native Acacia koa A.Gray (it is endemic to the Hawaiian Islands) may also be tolerant to tebuthiuron although A. confusa is an invasive weed. Both hexazinone and tebuthiuron may be used selectively by directed applications. Hexazinone may be applied foliarly or to the soil at the base of the target weed. Tebuthiuron may be applied to the soil. Both herbicides have demonstrated efficacy in grid or “hot spot” application to the soil [23].
In the present study, hexazinone and tebuthiuron were tested for the efficacy and non-selective effects for control of alien plants in two selected sites on Kauai, Hawaii: one representative of rainforests and the other representative of mesic areas. The effect of residual herbicides on transplanted natives and efficacy of hexazinone and tebuthiuron on other major invasive weeds were investigated.
2. Materials and Methods
2.1. Study Sites
Two sites in Kauai, Hawaii, USA were selected to study the effects of hexazinone and tebuthiuron: one representative of rainforests and the other representative of mesic areas (Figure 1). Average temperature was 20–28 °C in the two study sites. The rainforest site (Site I) was at 1200 m elevation at the rim of Kalalau Valley in the Kokee State Park on the island of Kauai. An average rainfall was 1651 mm, with high rainfall during the stormy winter months. However, there was no severe dry season and there were low clouds at this high elevation that supplemented rainfall. This trial site was enclosed by a chain link fence for a planned native plant restoration study by the Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife (DFW), Kauai Branch. The enclosure protected the site from deer, goats and pigs. The soil was described by Nakamura [24]. Briefly, the soil was andisols, Kokee silty clay loam, and was classified as medial, ferrihydritic, isothermic alic hapludands. Soil pH values ranged from 4.2 to 5.2. Concentrations of N, P, K, Ca, Mg, and Al in the soil samples were from 0.26 to 0.68, 0.2 to 3.9, 210 to 296, 212 to 806, 48 to 352, and 97 to 744 µg/g. Organic carbon content ranged from 3.42% to 11.34%. The invasive weed species in the site were daisy fleabane (Erigeron karvinskianus), faya tree (Myrica faya), strawberry guava (Psidium cattleyanum), banana passion fruit (Passiflora mollissima), vaseygrass (Paspalum urvillei), and highbush blackberry (Rubus argutus). M. faya and P. cattleyanum are two of the serious forest weeds in Hawaii. Native plants included ohia lehua (Metrosideros polymorpha), and naupaka (Scaevola cerasifolia), pilo (Hedyotis mannii), hona (Urera glabra), aalii (Dodonaea viscosa), and amau (Sadleria sp.).
Figure 1.
Locations of study areas in Kauai, Hawaii, the Pacific Ocean.
The mesic area (Site II) was on Kauhao Ridge above the Napali Coast of the island of Kauai, at an elevation of 640 m and average rainfall of 536 mm. The soil at this site was also andisols. The site was severely eroded and had been replanted ca. 1972 to loblolly pine (Pinus taeda), swamp messmate (Eucalyptus robusta) and Australian pine tree (Casuarina equisetifolia L.). The rainfall here was seasonal, with an annual dry season in the summer months. Rainfall occurred when storm systems moved through the area, usually in the winter months. Unfortunately an enclosure of the area was not feasible, thus exposing the plants to grazing by wild animals such as deer and goats.
2.2. Application of Herbicides and Analysis of Defoliation
The procedures followed at both sites were identical. There were 14 10 × 10 m large plots at each site (a total of 28 10 × 10 m plots for the two sites). The 10 × 10 m plots were separated by a 5 m buffer. Each 10 × 10 large plot was equally divided to two treatments (i.e., two subplots, each 5 × 10 m). Two large 10 × 10 m plots (i.e., 4 subplots of 5 × 10 m) were used as zero (0) controls for both hexazinone and tebuthiuron. Hexazinone (Velpar®) granules and tebuthiuron (Spike® 20P) pellets were provided by the DuPont de Nemours Company (Wilmington, Delaware, USA) and Dow AgroSciences (Indianapolis, Indiana, USA), respectively. They were weighed into four equal portions for each plot following Good Laboratory Practice procedures. For the sake of uniformity of application, the herbicides were spread by a sparger. Each application covered half the plot and two applications were perpendicular to the other two. Each herbicide was applied at 0, 1, 2 and 4 kg ha−1 into their respective plots. There were 4 replicates (i.e., 4 subplots of 5 × 10 m) for each treatment.
At 12 months after treatment (MAT), injuries to native and non-native plants were visually evaluated and rated by a 0%–100% defoliation scale as a result of herbicide treatment. Attached leaves that were fully chlorotic or necrotic were considered “defoliated”. Both defoliation and herbicide residue data were presented as the average values and standard deviations. The data were analyzed by one-way ANOVA and Student t test. The p values less than 0.05 were considered statistically significant. Analyses were performed with Excel.
2.3. Analysis of Herbicides in Soils
There were 912 soil samples including 192 control samples collected from 28 plots at Kalalau Rim site (high rainfall site, Site I) and Kauhao site (mesic site, Site II) located about a quarter mile east of the Kalalau lookout in Kokee State Park of Kauai at days 0, 2, 7, 21, 180 and 440 (or 365) after herbicide treatment Soil samples were collected at three depth layers (0–15, 15–30 and 30–45 cm) with four replicates for each treatment using a worm screw augers. A soil auger was first tried to collect soil samples. It was, however, very difficult to pull out the auger from the deeper soil levels and digging the auger out would have disturbed too much of the plot. The worm screw auger was thus used. At each sampling date, each plot was sub-sampled 5 times, once in each of four quadrants and at a point close to mid plot. The sub-samples within the same depth levels were combined and then doubled bagged, labeled, transported with dry ice in coolers to Lihue, Kauai. The samples were then repacked with the dry ice in insulated cartons and taken immediately to the airport for shipment to the laboratory at the University of Hawaii at Manoa in Honolulu. All samples were extracted with acetone on an accelerated solvent extractor [25] and analyzed with gas chromatography-mass spectrometry (GC-MS) for the herbicides hexazinone and tebuthiuron [25,26].
2.4. Effect of Residual Herbicides on Transplanted Native Endangered Species
Six and nine months after hexazinone and tebuthiuron treatment, respectively, native plants were transplanted into each plot and observed for injury from residual herbicides. The native plants were grown at the DFW mid-elevation nursery at Kokee, Kauai. The plants were aalii (D. viscosa), kawawaenohu (Alsinidendron lynchnoides), Kauai delissea (Dellissea rhytidosperma), and Kauai hau kuahiwi (Hibiscadelphis distans). Each plot was planted to two plants of each species, due to a limited number of plants available, except for D. rhytidosperma with four plants in each plot. The current status of these four species are threatened or endangered. Mortality counts were made at 3, 7, 10 and 12 months after transplantings (MAP). The transplanted natives were placed in holes four inches (10 cm) in diameter and six inches (15 cm) deep dug with a gasoline-powered auger. Root balls were placed in the hole and covered with excavated soil.
2.5. Efficacy of Hexazinone and Tebuthiuron on the Other Major Invasive Forest Weeds
To test the efficacy of hexazinone and tebuthiuron on invasive species that were not present in the large plots or were poorly represented there on Sites I and II, a series of preliminary trials were also conducted in Kauai. In these preliminary trials, targeted weeds in 5 m diameter circular plots in two replicates were treated with 0, 1, 2 and 4 kg ha−1 of hexazinone or tebuthiruon and evaluated for susceptibility to the herbicide treatments. Injuries to the invasive plants were visually evaluated and rated by a 0%–100% defoliation scale 8 MAT. The target invasive species were bush beardgrass (Schizachyrium condensatum), kahili ginger (Hedychium gardnerianum), Psidium cattleyanum, kikuyu grass (Pennisetum clandestinum), rose myrtle (Rhodomyrtus tomentosa), wild olive tree (Olea europaeus), and karaka nut tree (Corynocarpus laevigatus).
3. Results and Discussion
3.1. Defoliation of Plant Species by Herbicides
Difference in herbicidal sensitivity among various plant species was one of the response factors considered. The response of D. viscosa (aalii) to hexazinone was erratic 12 MAT (Figure 2). The defoliation of native D. viscosa plants for H2 (hexazinone 2 kg ha−1) treatment was significantly higher than that for H4 (hexazinone 4 kg ha−1) and H1 (hexazinone 1 kg ha−1) treatments (p < 0.05) but less than the control (C in Figure 2), suggesting that some other causes were confounding the results. The insect two-spotted leafhopper (Sophonia rufofascia) was observed to injure a large number of native and alien plants with the symptoms being chlorosis and necrosis. Furthermore, the rainfall may have leached hexazinone out of root zone of the soil profile and the recovery may have begun, if erratically. D. viscosa is an indigenous shrub plant on all the main Hawaiian Islands. The defoliation of D. viscosa plants suggested more serious injure in T1 (tebuthiuron 1 kg ha−1), T2 (tebuthiuron 2 kg ha−1) and T4 (tebuthiuron 4 kg ha−1) treatments compared to hexazinone. This may be due to the less mobility of tebuthiuron. The defoliation of Sadleria sp. (amau) was uneven but it was apparent that both herbicides severely injured this species. S. cerasifolia (naupaka) was killed by H2 (hexazinone 2 kg ha−1) and H4 (hexazinone 4 kg ha−1) treatments and demonstrated a dose-dependent response to tebuthiuron. M. polymorpha (ohia lehua) is an endemic species of flowering evergreen tree to the six largest islands of Hawaii. Small M. polymorpha plants (Figure 2) demonstrated a dose-dependent response to hexazinone, except that the M. polymorpha plants in the control plots were more severely injured than those in H1 (hexazinone 1 kg ha−1) and H2 (hexazinone 2 kg ha−1) plots. Response to tebuthiuron was erratic, because M. polymorpha was one of the species sensitive to the two-spotted leafhopper (S. rufofascia). All treatments and the control of the large M. polymorpha trees showed severe injury, with small differences between treatments (Figure 2). U. glabra (hona) in the control plots, although less severely injured than in the herbicide treated plots, were moderately injured. H. mannii (pilo) is a Hawaii’s endangered plant species. In general, H. mannii was moderately tolerant to hexazinone, but fairly sensitive to tebuthiuron although the defoliation rate of the control (approximately 25%) was fairly high (Figure 2). M. faya (faya tree), a serious forest weed, was not widely distributed throughout the plots. Nevertheless, it was apparent that it was sensitive to hexazinone but not to tebuthiuron. More than 75% defoliation of M. faya was observed under H2 and H4 treatments, while only less than 20% defoliation was observed with the tebuthiuron treatments. However, M. faya was tolerant to both hexazinone and tebuthiuron in earlier preliminary trials. P. cattleyanum (strawberry guava) was another serious invasive plant in Kauai. P. cattleyanum was sensitive to the two herbicides (Figure 2).
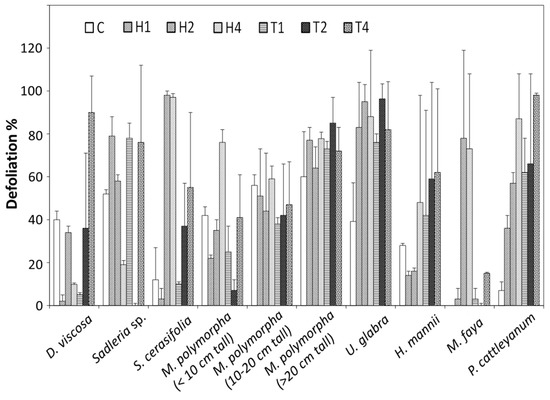
Figure 2.
Defoliation of the forest plants by hexazinone and tebuthiuron at Kalalau Rim Site, 12 MAT. The plant species were aalii (Dodonaea viscosa) (native), amau (Sadleria sp.) (native), naupaka (Scaevola cerasifolia) (native), ohia lehua (Metrosideros polymorpha) (native), hona (Urera glabra) (native), pilo (Hedyotis mannii) (native), faya tree (Myrica faya) (non-native), and strawberry guava (Psidium cattleyanum) (non-native). C (control, no hexazinone and tebuthiuron), H1 (hexazinone 1 kg ha−1), H2 (hexazinone 2 kg ha−1), H4 (hexazinone 4 kg ha−1), T1 (tebuthiuron 1 kg ha−1), T2 (tebuthiuron 2 kg ha−1) and T4 (tebuthiuron 4 kg ha−1).
Site II, on Kaohau Ridge suffered a drought and the monitored plants in the control plots suffered moderate to severe defoliation, except for P. taeda (loblolly pine) (Figure 3). Overall, injuries at 12 MAT were greater than those at 6 MAT. The small M. polymorpha (ohia lehua) plants (approximately less 10 cm tall) 12 MAT showed a response to hexazinone and tebuthiuron with greater sensitivity to the lowest rate of tebuthiuron than to the lowest rate of hexazinone (p < 0.05). The severest injury was with the highest rate of hexazinone and tebuthiuron. Besides the drought, M. polymorpha is susceptible to the two-spotted leafhopper. Casuarina equisetifolia (Australian pine tree, non-native) was moderately susceptible to hexazinone and tebuthiuron although the control was almost as severely injured. Pukeawe Styphelia tameiameiae (Cham.) F.Muell. (native), although the control plants were severely defoliated, appeared sensitive to hexazinone but not to tebuthiuron. Untreated P. taeda did not demonstrate any defoliation. It was tolerant to hexazinone, an herbicide registered for use to establish P. taeda plantings. It was very sensitive to the higher rates of tebuthiuron and nearly 100% defoliation was obtained at the highest rate of tebuthiuron.
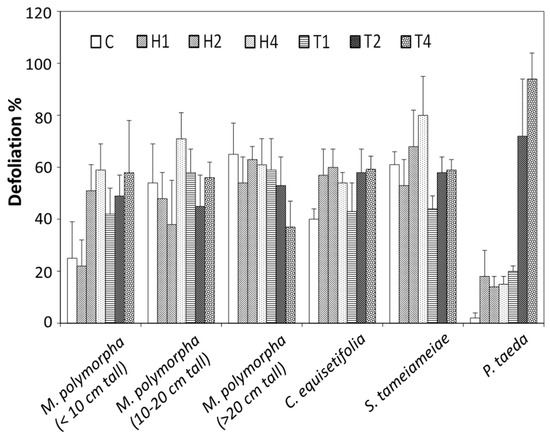
Figure 3.
Defoliation of the forest plants ohia lehua (Metrosideros polymorpha) (native), Australian pine tree (Casuarina equisetifolia) (non-native), and pukeawe (Styphelia tameiameiae) (native), and loblolly pine (Pinus taeda) (non-native) by hexazinone and tebuthiuron at Kaohau site, 12 MAT. C (control, no hexazinone and tebuthiuron), H1 (hexazinone 1 kg ha−1), H2 (hexazinone 2 kg ha−1), H4 (hexazinone 4 kg ha−1), T1 (tebuthiuron 1 kg ha−1), T2 (tebuthiuron 2 kg ha−1) and T4 (tebuthiuron 4 kg ha−1).
3.2. Environmental Fate of Tebuthiuron and Hexazinone
The residual levels of tebuthiuron in the 0–15 cm layer soil at Kalalau Rim site (Site I) collected immediately after spraying at application rates of 1, 2 and 4 kg ha−1 were 896 ± 89, 1623 ± 62, and 3214 ± 97 µg g−1 dry soil weight (dw), respectively. The corresponding hexazinone residue levels in the 0–15 cm layer soil at Kalalau Rim site were 239 ± 28, 284 ± 127, and 837 ± 126 µg g−1 dw, respectively, at application rates of 1, 2 and 4 kg ha−1. It is noteworthy that there was no documented record that any herbicides were previously used in the two study sites, meaning the background concentration of both hexazinone and tebuthiuron would be undetectable. The concentrations of hexazinone and tebuthiuron were undetectable in the soil samples collected from the control plots. The concentrations of the two herbicides decreased as a function of time, but the concentrations in the deeper layer soil increased as a function of time. For example, the concentrations of tebuthiuron in 0–15 cm, 15–30 cm, and 30–45 cm layers at Kalalau Rim site were 1899 ± 119, 199 ± 42 and 135 ± 39 µg g−1 dw, respectively, 7 DAT for the application rate of 4 kg ha−1 as compared with 733 ± 219, 184 ± 47 and 257 ± 69 µg g−1, respectively, 180 DAT at the same site. The concentrations of hexazinone in 0–15 cm, 15–30 cm, and 30–45 cm layers at Kalalau Rime site were 1365 ± 749, 614 ± 363 and 205 ± 119 µg g−1 dw, respectively, 7 DAT for the application rate of 4 kg ha−1 as compared with 348 ± 174, 236 ± 54 and 229 ± 86 µg g−1 dw, respectively, 180 DAT. The dissipation half-life values (t1/5) of hexazinone and tebuthiuron in the 1–15 cm layer of soils at the two sites were approximately 7 days and greater than 180 days, respectively.
The concentrations of hexazinone in the 30–45 cm layer of soils at Kalalau Rim site (Site I) at foliar application rates of 1, 2 and 4 kg ha−1 were 109 ± 114, 259 ± 73 and 229 ± 86 µg g−1 dw, respectively, 180 DAT. The concentrations of tebuthiuron in the 30-45 cm layer of soils at Kalalau Rim site at foliar application rates of 1, 2 and 4 kg ha−1 were 43 ± 38, 137 ± 73, 257 ± 69 µg g−1 dw, respectively, 180 DAT.
The concentrations of hexazinone in the 30–45 cm layer of soils at Kauhao site (mesic site, Site II) at foliar application rates of 1, 2 and 4 kg ha−1 were 80 ± 61, 62 ± 47 and 69 ± 71 µg g−1 dw, respectively, 180 DAT. The concentrations of tebuthiuron in the 30-45 cm layer of soils at Kauhao site at foliar application rates of 1, 2 and 4 kg ha−1 were 13 ± 23, 65 ± 92 and 27 ± 27 µg g−1 dw, respectively, 180 DAT. The results showed that hexazinone was more mobile than tebuthiuron, which is agreed with their chemical and physical properties. The results also indicated that hexazinone and tebuthiuron appeared to be less mobile at Kauhao site (less rain) than at Kalalau Rim site (more rain), which is likely related to rainfall. The dissipation profiles of the two herbicides at Kauhao site were very similar to those at the Kalalau Rim site.
3.3. Effect of Residual Herbicides on Transplanted Natives
Regenerating and regaining the natural structure of native plants is important for ecological restoration [27]. The results of sensitivity of replanted natives in Kauhao site (Site II) were somewhat affected by feral deer and goats because an enclosure of the site was not feasible. At Kalalau Rim site (Site 1), approximately less than 10% mortality was observed for out-planted native species three months after planting (3 MAP), indicating the native species showed less injury in the early period of transplant (Figure 4). The mortality of H. distans, (Kauai hau kuahiwi) D. rhytidosperma (Kauai delissea) and A. lynchnoides (kawawaenohu), however, increased as the MAP increased (Figure 4). The mortality of A. lynchnoides could even reach 80% 15 MAP. The three plant species are all endemic to the island of Kauai and are assessed as endangered or critically endangered. Only D. viscosa (aalii) that is also endangered exhibited less than 20% mortality 15 MAP, indicating its high tolerance to the herbicide residues in the soil. The response of out-planted native species suggested that fertilizer application, pest control (e.g., fungi and insects) and remediation of herbicide residues may be required to regrow native plants at the sites [28,29].
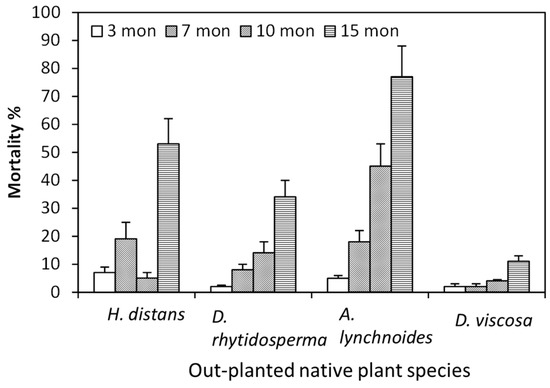
Figure 4.
Mortality of out-planted native species Kauai hau kuahiwi (Hibiscadelphis distans), Kauai delissea (Dellissea rhytidosperma), kawawaenohu (Alsinidendron lynchnoides) and aalii (Dodonaea viscosa) at Kalalau Rim site (Site I) 3, 7, 10 and 15 MAP.
3.4. Efficacy Trials of Hexazinone and Tebuthiuron on the Other Major Invasive Forest Weeds
Drought compromised the Schizachyrium trials (S. condensatum), causing 48% defoliation in the control 9 MAT (Table 1), which made the interpretation of the defoliation data difficult. Because of termination of the field trials, further observations were not possible. Trials on S. condensatum in east Kauai, where rainfall was high, indicated that it was indeed sensitive to hexazinone. Hexazinone was also found to reduce bermudagrass yield at 4 weak after treatment (WAT) in southeast of USA [18]. Hedychium gardnerianum, as commonly called kahili ginger, a rain forest pest, showed initial injury to hexazinone and tebuthiuron, but it was recovering by 9 MAT (Table 1). H. gardnerianum has thick and fleshy rhizomes that probably account for the rapid recovery. Furthermore, rainforest soils have high organic matter contents that attenuate the impact of soil-applied herbicides. The closely related Hedychium flavescens (yellow ginger) was sensitive to picloram granules, of which the registration for use in Hawaii has since been cancelled. The application of herbicide Escort® (the active ingredient metsulfuron methyl) was observed to have killed many of the smaller saplings of H. gardnerianum in cleared plots of the Hawaiian rainforest [30]. Only 10–22% defoliation was observed for A. confusa (formosan koa) under different tebuthiuron treatments, indicating A. confusa was tolerant of tebuthiuron (Table 1). Removing the invader is essential for the native Hawaiian forest to regenerate and regain its natural structure [30]. As such, further trials with saplings are required to determine the feasibility of using tebuthiuron in A. confusa establishment.

Table 1.
Response of the major invasive weeds bush beardgrass (Schizachyrium condensatum), kahili ginger (Hedychium gardnerianum) and Formosan koa (Acacia confusa)) to soil-applied with hexazinone and tebuthiuron, 9 MAT.
In general, the woody dicots (C. laevigatus, O. europaeus, R. tomentosa, and P. cattleyanum, Table 2) were very tolerant to soil-applied hexazinone and tebuthiuron. The highest defoliation 8 MAT was 60% for R. tomentosa (rose myrtle) treated by hexazinone at 4 kg ha−1. It is noteworthy that the soil-applied hexazinone and tebuthiuron were not as effective as foliar applications to control P. cattleyanum (strawberry guava) (Table 2 versus Figure 2). The 5 m diameter plots may have been too small to encompass a critical volume of the roots. Better results might have been obtained with the grid application method [31]. Good preliminary results were obtained with large pellets of hexazinone on S. terebinthifolius (Brazilian peppertree) applied in grid applications. Likewise, grid and spot gun applications of hexazinone were effective for tree-of-heaven [32] and sweet briar [33], respectively. Furthermore, grid applications would probably be necessary to avoid damage to non-target plants.

Table 2.
Response of karaka nut tree (Corynocarpus laevigatus), wild olive tree (Olea europaeus), rose myrtle (Rhodomyrtus tomentosa), strawberry guava (Psidium cattleyanum) and kikuyu grass (Pennisetum clandestinum) (a monocot) to soil-applied hexazinone and tebuthiuron, 8 MAT.
P. clandestinum (kikuyu grass), a South African native, is planted in many countries as a pasture grass. Once established, it can spread vigorously and can tolerate a wide range of climatic zones from temperate to subtropical [34]. Only 40% defoliation of P. clandestinum reached at the highest hexazinone treatment rate and while less than 20% defoliation was observed for all the tebuthiuron treatments, indicating P. clandestinum was poorly controlled by soil application of hexazinone and tebuthiuron (Table 2). The recovery was probably facilitated by the stoloniferous nature of its growth. The thick rhizomes allowed the grass to survive the treatment. Hexazinone would probably be more effective on bunch grasses such as S. indicus [22]. Tebuthiuron as expected was not effective on P. clandestinum, since it is registered for broadcast applications in pastures for control of dicot weeds. A low dosage glyphosate spray was effective to control P. clandestinum, but it should be careful to minimize any herbicide overspray reaching native plants [35]. We would propose that efficacy may be improved by grid application. Grid applications may offer more protection to non-target species and therefore, may be more practical in forest management, which warrants further investigations.
4. Conclusions
Native and non-native plants on the two experiment sites on Kauai, Hawaii were tested for their tolerance and sensitivity, as measured by defoliation, to hexazinone and tebuthiuron at foliar application rates of 1, 2 and 4 kg ha−1. The herbicidal tolerance and sensitivity varied among the plant species and between the two herbicides. A defoliation of 40% is arbitrarily considered here as high tolerance (or low sensitivity) for qualitative description of the herbicidal effects on plants. D. viscosa (aalii, native) showed high tolerance to hexazinone. S. cerasifolia (naupaka, native) was susceptible to hexazinone, but not to tebuthiuron. The defoliation of Sadleria sp. (amau, native) by the two herbicides was inconsistent at different foliar application rates. M. polymorpha (ohia lehua, native) at a height less than 10 cm was fairly tolerant to the two herbicides. U. glabra (hona, native) was severely injured by the two herbicides. The native H. mannii (pilo) was moderately tolerant to hexazinone, but was quite sensitive to tebuthiuron.
The non-native species M. faya (faya tree) was very susceptible to hexazinone, but very tolerant to tebuthiuron. The non-native P. cattleyanum (strawberry guava) was sensitive to both herbicides. P. taeda (loblolly pine, non-native) was highly tolerant to hexazinone, but was very sensitive to tebuthiuron.
Six and nine months after hexazinone and tebuthiuron treatment, respectively, native plants were transplanted into the Sites to observe injury from residual herbicides. Approximately less than 10% mortality was observed for the out-planted native species 3 MAP, indicating that the native species showed less injury in the early period of transplant. The mortality of the native plants H. distans (Kauai hau kuahiwi), D. rhytidosperma (Kauai delissea) and A. lynchnoides (kawawaenohu), however, increased as the MAP increased, indicating phytotoxicity of the residual herbicides and a need of fertilization and pest control. D. viscosa (aalii) exhibited less than 20% mortality 15 MAP, indicating its high tolerance to the herbicide residues in the soil. The herbicidal toxicity to the out-planted plants was supported by the high concentrations of the herbicide residues detected in the soil. The dissipation half-life values (t1/5) of tebuthiuron and hexazinone in the 1–15 cm layer of soils at the two sites were approximately 7 days and greater than 180 days, respectively.
Author Contributions
P.S.M., D.A.N. and Q.X.L. designed the experiment; J.W. and Q.X.L. wrote the manuscript; D.A.N., J.A., Y.Z. and P.S.M. performed the experiments and analyzed data.
Funding
This research was supported in part by [USDA National Agricultural Pesticide Impact Assessment Program (QXL)], National Key Research and Development Program of China, grant number [2018YFD0900604], and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanley, M.E. Seedling defoliation, plant growth and flowering potential in native- and invasive-range Plantago lanceolata populations. Weed Res. 2012, 52, 252–259. [Google Scholar] [CrossRef]
- Vitousek, P.M.; DAntonio, C.M.; Loope, L.L.; Rejmanek, M.; Westbrooks, R. Introduced species: A significant component of human-caused global change. New Zeal. J. Ecol. 1997, 21, 1–16. [Google Scholar]
- Protopopova, V.; Shevera, M.; Mosyakin, S. Deliberate and unintentional introduction of invasive weeds: A case study of the alien flora of Ukraine. Euphytica 2006, 148, 17–33. [Google Scholar] [CrossRef]
- Nasim, G.; Shabbir, A. Invasive Weed Species—A Threat to Sustainable Agriculture. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 523–556. [Google Scholar]
- DiTomaso, J.M. Invasive weeds in rangelands: Species, impacts, and management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef]
- Simmons, M.T.; Windhager, S.; Power, P.; Lott, J.; Lyons, R.K.; Schwope, C. Selective and non-selective control of invasive plants: The short-term effects of growing-season prescribed fire, herbicide, and mowing in two Texas prairies. Restor. Ecol. 2007, 15, 662–669. [Google Scholar] [CrossRef]
- Lesica, P.; Martin, B. Effects of prescribed fire and season of burn on recruitment of the invasive exotic plant, Potentilla recta, in a semiarid grassland. Restor. Ecol. 2003, 11, 516–523. [Google Scholar] [CrossRef]
- Wilson, S.D.; Partel, M. Extirpation or coexistence? Management of a persistent introduced grass in a prairie restoration. Restor. Ecol. 2003, 11, 410–416. [Google Scholar] [CrossRef]
- Grilz, P.L.; Romo, J.T. Management considerations for controlling smooth brome on fescue prairie. Nat. Areas J. 1995, 15, 148–156. [Google Scholar]
- Sanderson, M.A.; Schnabel, R.R.; Curran, W.S.; Stout, W.L.; Genito, D.; Tracy, B.F. Switchgrass and big bluestem hay, biomass, and seed yield response to fire and glyphosate treatment. Agron. J. 2004, 96, 1688–1692. [Google Scholar] [CrossRef]
- Hoss, N.E.; Al-Khatib, K.; Peterson, D.E.; Loughin, T.M. Efficacy of glyphosate, glufosinate, and imazethapyr on selected weed species. Weed Sci. 2003, 51, 110–117. [Google Scholar] [CrossRef]
- Sileshi, G.; Tessema, T. Weed Suppression in Legume Crops for Stress Management. In Climate Change and Management of Cool Season Grain Legume Crops; Yadav, S.S., Redden, R., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 243–281. [Google Scholar]
- Dixon, F.L.; Clay, D.V.; Willoughby, I. The efficacy of pre-emergence herbicides on problem weeds in woodland regeneration. Crop Protection 2006, 25, 259–268. [Google Scholar] [CrossRef]
- Cai, X.; Sheng, G.; Liu, W. Degradation and detoxification of acetochlor in soils treated by organic and thiosulfate amendments. Chemosphere 2007, 66, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Mamy, L.; Vrignaud, P.; Cheviron, N.; Perreau, F.; Belkacem, M.; Brault, A.; Breuil, S.; Delarue, G.; Pétraud, J.-P.; Touton, I.; et al. No evidence for effect of soil compaction on the degradation and impact of isoproturon. Environ. Chem. Lett. 2011, 9, 145–150. [Google Scholar] [CrossRef]
- Lin, C.H.; Lerch, R.N.; Garrett, H.E.; George, M.F. Bioremediation of atrazine-contaminated soil by forage grasses: Transformation, uptake, and detoxification. J. Environ. Qual. 2008, 37, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Sellers, B.A.; Ferrell, J.A.; MacDonald, G.E. Influence of hexazinone on Pensacola bahiagrass growth and crude protein content. Agron. J. 2008, 100, 808–812. [Google Scholar] [CrossRef]
- Wilder, B.; Ferrell, J.A.; Sellers, B.A.; MacDonald, G.E. Influence of hexazinone on 'Tifton 85' bermudagrass growth and forage quality. Weed Technol. 2008, 22, 499–501. [Google Scholar] [CrossRef]
- Cutts, G.S.; Webster, T.M.; Grey, T.L.; Vencill, W.K.; Lee, R.D.; Tubbs, R.S.; Anderson, W.F. Herbicide Effect on Napiergrass (Pennisetum purpureum) Control. Weed Sci. 2011, 59, 255–262. [Google Scholar] [CrossRef]
- Borrel, J.A.; Brown, R.L.; Slater, K. Chemical control of invasive Psidium guajava in Swaziland: A preliminary assessment of costs and efficacy. Afr. J. Agr. Res. 2011, 6, 3291–3297. [Google Scholar]
- Laroche, F.B. Managing melaleuca (Melaleuca quinquenervia) in the Everglades. Weed Technol. 1998, 12, 726–732. [Google Scholar] [CrossRef]
- Mislevy, P.; Shilling, D.G.; Martin, F.G.; Hatch, S.L. Smutgrass (Sporobolus indicus) control in bahiagrass (Paspalum notatum) pastures. Weed Technol. 1999, 13, 571–575. [Google Scholar] [CrossRef]
- Motooka, P.; Ching, L.; Nagai, G. Herbicidal Weed Control Methods for Pastures and Natural Areas of Hawaii; University of Hawaii: Honolulu, HI, USA, 2002; p. 36. [Google Scholar]
- Nakamura, S. Soil Survey of Kalalau Rim Endangered Plant Exclosure and Outplanting Site; USDA Natural Resources Conservation Service: Honolulu, HI, USA, 1998. [Google Scholar]
- Zhu, Y.; Yanagihara, K.; Guo, F.; Li, Q.X. Pressurized fluid extraction for quantitative recovery of chloroacetanilide and nitrogen heterocyclic herbicides in soil. J. Agric. Food Chem. 2000, 48, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.X. Movement of bromacil and hexazinone in soils of Hawaiian pineapple fields. Chemosphere 2002, 49, 669–674. [Google Scholar] [CrossRef]
- Uprety, Y.; Asselin, H.; Bergeron, Y.; Doyon, F.; Boucher, J.-F. Contribution of traditional knowledge to ecological restoration: Practices and applications. Ecoscience 2012, 19, 225–237. [Google Scholar] [CrossRef]
- Choi, Y.D.; Temperton, V.M.; Allen, E.B.; Grootjans, A.P.; Halassy, M.; Hobbs, R.J.; Naeth, M.A.; Torok, K. Ecological restoration for future sustainability in a changing environment. Ecoscience 2008, 15, 53–64. [Google Scholar] [CrossRef]
- Paquin, D.; Campbell, S.; Li, Q.X. Phytoremediation in sub-tropical Hawaii—A review of over 100 plant species. Remediation 2004, 14, 127–139. [Google Scholar] [CrossRef]
- Minden, V.; Jacobi, J.D.; Porembski, S.; Boehmer, H.J. Effects of invasive alien kahili ginger (Hedychium gardnerianum) on native plant species regeneration in a Hawaiian rainforest. Appl. Vegetat. Sci. 2010, 13, 5–14. [Google Scholar] [CrossRef]
- Scifres, C.J. Woody plant control in the Post Oak Savannah of Texas with hexazinone. J. Range Manag. 1982, 35, 401–404. [Google Scholar] [CrossRef]
- Pritchard, G.H. Spot-gun application of hexazinone for the control of tree of heaven. Proc. 6th Australian Weeds Conf. 1981, 1, 113–114. [Google Scholar]
- Meeklah, F.A.; Mitchell, R.B. Evaluation of the spot gun technique for control of sweet briar. Proc. 6th Australian Weeds Conf. 1981, 1, 99–103. [Google Scholar]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; East-West Center/University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Hutton, I.; Coenraads, R.; Auld, T.D.; Denham, A.J.; Ooi, M.K.; Brown, D. Herbicide impacts on exotic grasses and a population of the critically endangered herb Calystegia affinis (Convolvulaceae) on Lord Howe Island. Cunninghamia 2008, 10, 539–545. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).