Impact of Disturbances on the Carbon Cycle of Forest Ecosystems in Ukrainian Polissya
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Input Datasets
2.2.1. Remote Sensing Data and Ancillary Data
2.2.2. Reference Data
2.2.3. Dataset for Biomass Estimation
2.2.4. Data on Heterotrophic Soil Respiration
2.3. Analyses
2.4. Data Processing Methods
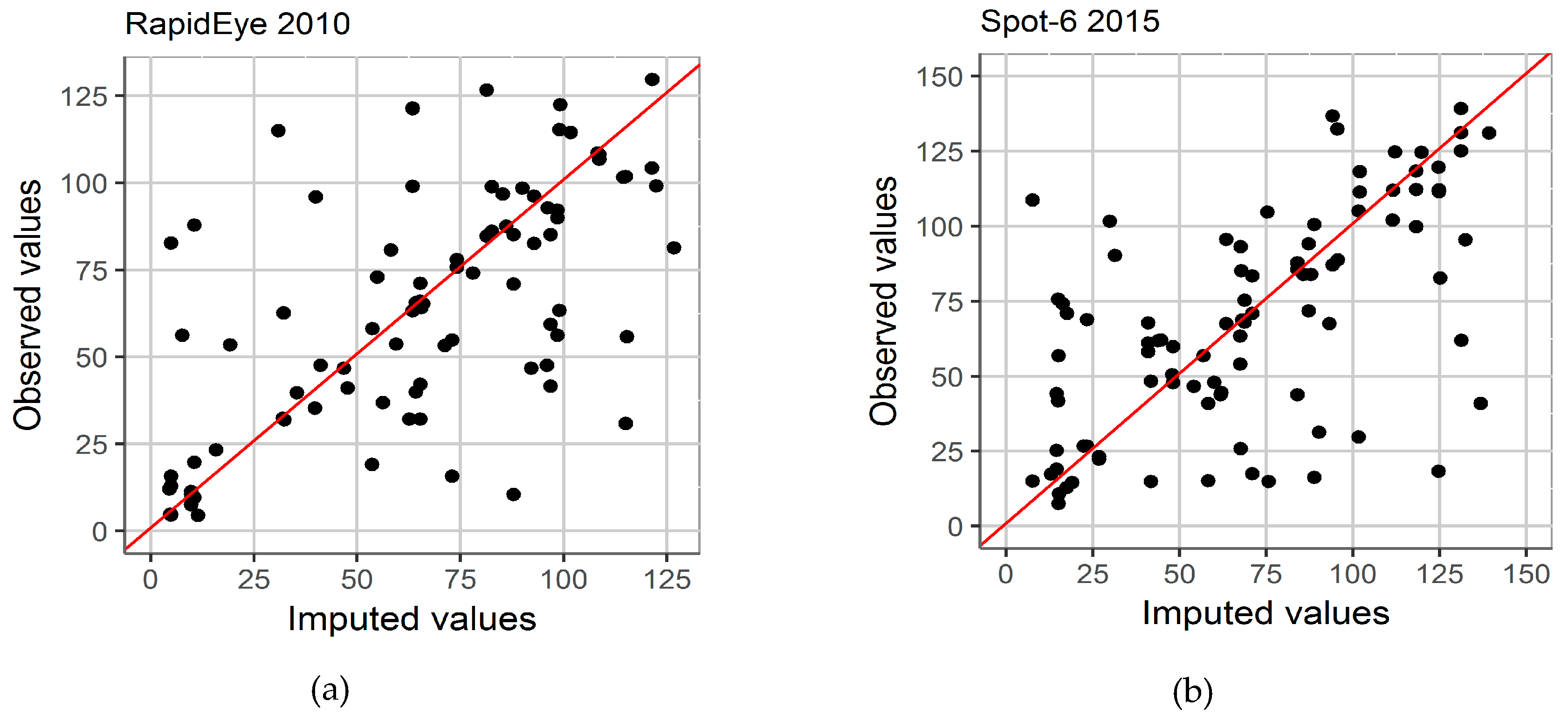
2.4.1. Image Classification Approaches
2.4.2. Carbon Budget Estimation
3. Results
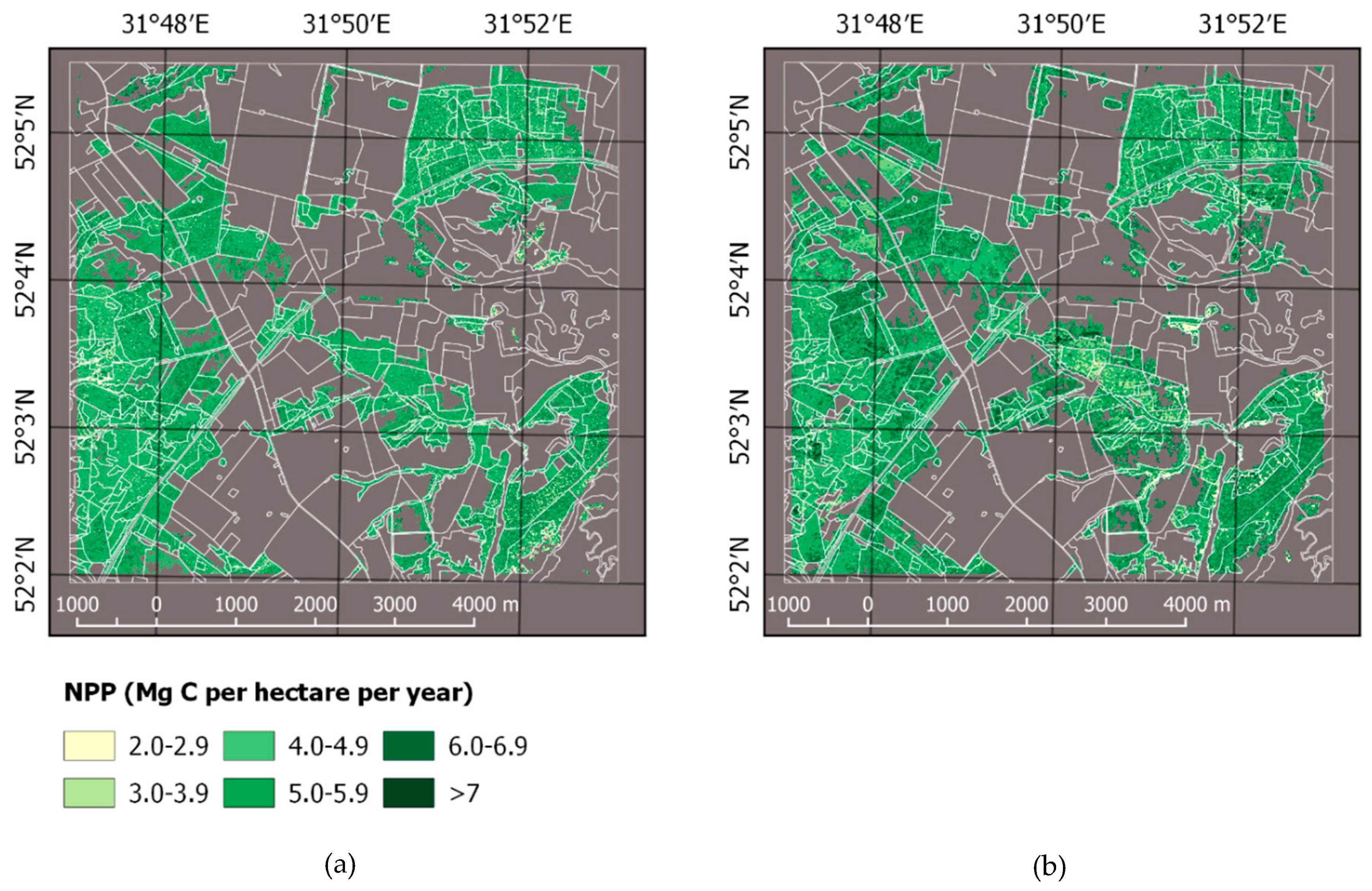
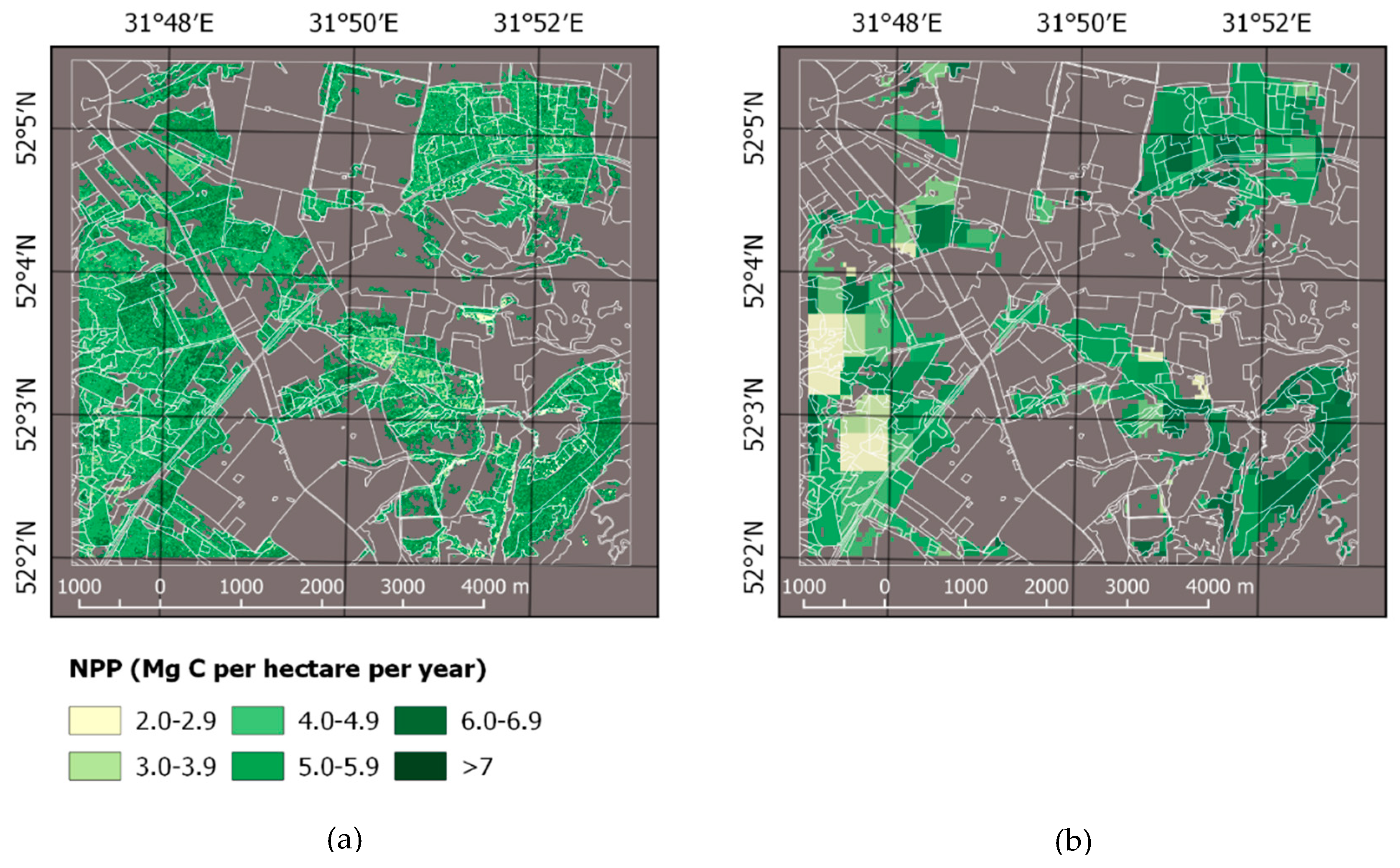
3.1. Forest Mask, Carbon Stock, and NPP
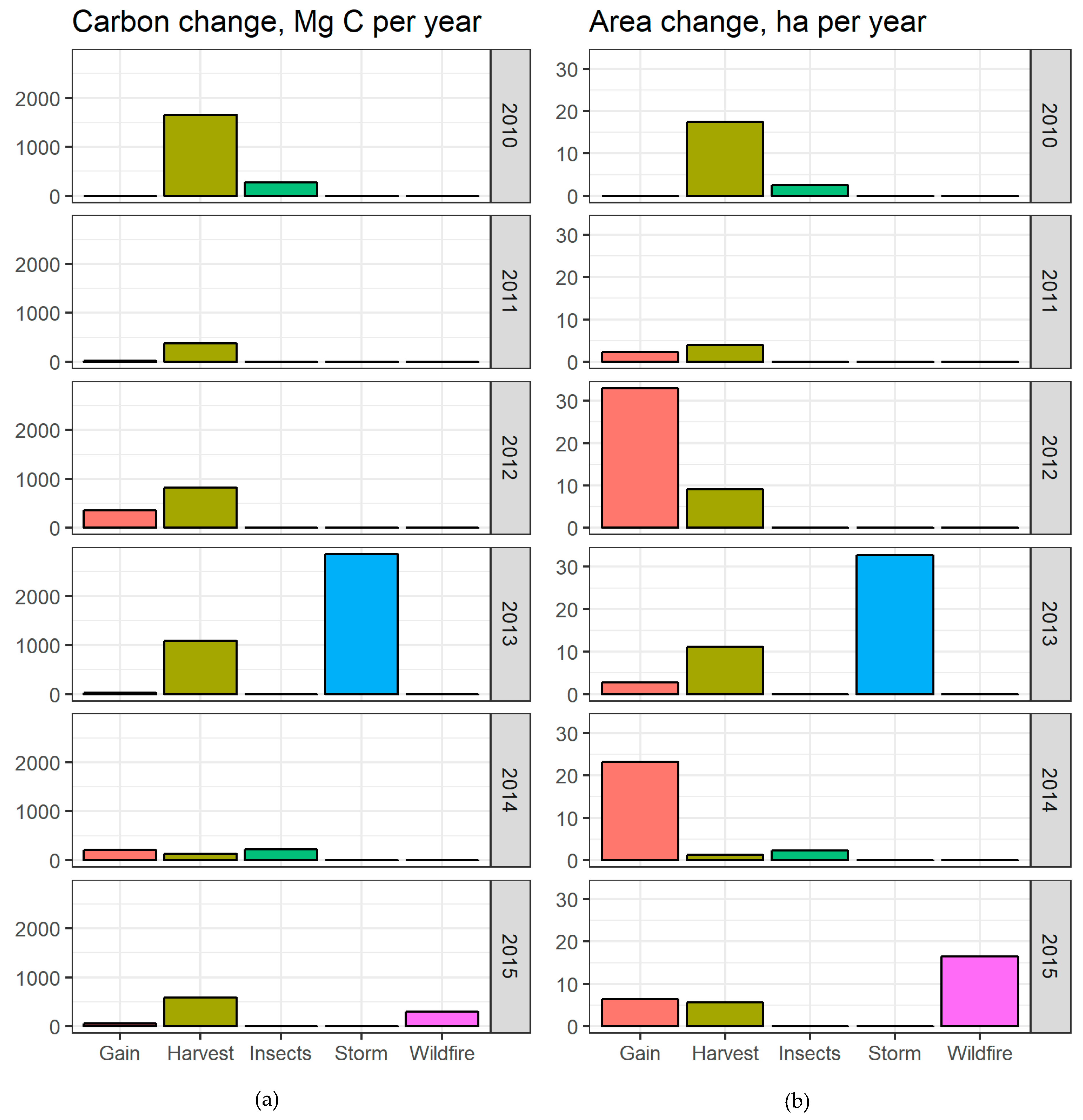
3.2. Carbon Budget Estimation and Disturbances Impact
4. Discussion
4.1. Spatial Accuracy and Reliability
4.2. Forest Land Cover and Carbon Estimation
4.3. Disturbances Impact
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Dead Biomass Fraction, Equation (No. of Used Model) | Equations Parameter Estimation | R2 | |||||
|---|---|---|---|---|---|---|---|
| a0 | a1 | a2 | a3 | a4 | a5 | ||
| Pine | |||||||
| Snags, Equation (3) | 0.497 | 1.547 | −0.679 | 0.401 | - | - | 0.82 |
| Logs, Equation (3) | 0.074 | 0.245 | 0.798 | −0.447 | - | - | 0.69 |
| Coarse branches, Equation (5) | 0.001 | 8.787 | 1.403 | 10.130 | 0.440 | 14.770 | 0.87 |
| Fine litter, Equation (3) | 0.523 | −0.574 | −0.405 | −0.815 | - | - | 0.88 |
| Birch | |||||||
| Snags, Equation (3) | 0.016 | 0.971 | 0.841 | 0.817 | - | - | 0.84 |
| Logs, Equation (3) | 0.015 | 1.387 | 0.449 | 1.151 | - | - | 0.85 |
| Coarse branches, Equation (3) | 0.002 | 1.340 | 0.903 | 1.132 | - | - | 0.87 |
| Fine litter, Equation (4) | 0.523 | −0.574 | −0.405 | −0.815 | - | - | 0.88 |
| Alder | |||||||
| Snags, Equation (3) | 0.023 | 0.587 | 1.130 | −0.290 | - | - | 0.86 |
| Logs, Equation (3) | 0.429 | 1.232 | −0.482 | 0.217 | - | - | 0.78 |
| Coarse branches, Equation (3) | 0.028 | 1.275 | 0.172 | −0.193 | - | - | 0.86 |
| Fine litter, Equation (3) | 3.521 | 0.450 | −0.214 | 0.165 | - | - | 0.74 |
| Aspen | |||||||
| Snags, Equation (3) | 0.340 | 0.241 | 0.653 | 0.177 | - | - | 0.80 |
| Logs, Equation (3) | 0.017 | −0.130 | 1.902 | 1.044 | - | - | 0.86 |
| Coarse branches, Equation (3) | 1.505 | 3.079 | −2.960 | −0.347 | - | - | 0.98 |
| Fine litter, Equation (3) | 7.197 | 0.305 | −0.279 | −0.031 | - | - | 0.72 |
| Tree Species | No. of Samplings | P0 | K ± SE | R2 |
|---|---|---|---|---|
| Birch | 41 | 512 ± 15 | 0.235 ± 0.015 | 0.72 |
| Alder | 38 | 460 ± 17 | 0.126 ± 0.017 | 0.89 |
| Aspen | 39 | 495 ± 21 | 0.259 ± 0.020 | 0.91 |
| Oak | 35 | 585 ± 19 | 0.035 ± 0.006 | 0.75 |
| Tree Species | No. of Samplings | P0 | K ± SE | R2 |
|---|---|---|---|---|
| Birch | 30 | 471 ± 15 | 0.128 ± 0.019 | 0.81 |
| Alder | 51 | 420 ± 14 | 0.072 ± 0.011 | 0.93 |
| Aspen | 33 | 450 ± 17 | 0.136 ± 0.021 | 0.96 |
| Oak | 50 | 535 ± 21 | 0.020 ± 0.004 | 0.68 |

References
- Field, C.B.; Raupach, M.R. The Global Carbon Cycle: Integrating Humans, Climate, and the Natural World; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Grassi, G.; House, J.; Dentener, F.; Federici, S.; den Elzen, M.; Penman, J. The key role of forests in meeting climate targets requires science for credible mitigation. Nat. Clim. Chang. 2017, 7, 220–226. [Google Scholar] [CrossRef]
- Schaphoff, S.; Reyer, C.P.O.; Schepaschenko, D.; Gerten, D.; Shvidenko, A. Tamm review: Observed and projected climate change impacts on Russia’s forests and its carbon balance. For. Ecol. Manag. 2016, 361, 432–444. [Google Scholar] [CrossRef]
- Shvidenko, A.; Schepaschenko, D.; Kraxner, F.; Fritz, S. Full verified carbon account of forest ecosystems as a fuzzy system: An attempt to assess uncertainty. In Proceedings of the 4th International Workshop on Uncertainty in Atmospheric Emissions, Krakow, Poland, 7–9 October 2015; Systems Research Institute, Polish Academy of Sciences: Warsaw, Poland, 2015; pp. 1–8. [Google Scholar]
- Lesiv, M.; Shvidenko, A.; Schepaschenko, D.; See, L.; Fritz, S. A spatial assessment of the forest carbon budget for Ukraine. Mitig. Adapt. Strateg. Glob. Chang. 2018. [Google Scholar] [CrossRef]
- Shvidenko, A.; Buksha, I.; Krakovska, S.; Lakyda, P. Vulnerability of ukrainian forests to climate change. Sustainability 2017, 9, 1152. [Google Scholar] [CrossRef]
- Groisman, P.; Lyalko, V. Earth Systems Change over Eastern Europe; Akademperiodyka: Kyiv, Ukraine, 2012. [Google Scholar]
- Franklin, J.F.; Spies, T.A.; Pelt, R.V.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Angelstam, P.; Kuuluvainen, T. Boreal forest disturbance regimes, successional dynamics and landscape structures: A European perspective. Ecol. Bull. 2004, 51, 117–136. [Google Scholar]
- Kuemmerle, T.; Olofsson, P.; Chaskovskyy, O.; Baumann, M.; Ostapowicz, K.; Woodcock, C.E.; Houghton, R.A.; Hostert, P.; Keeton, W.S.; Radeloff, V.C. Post-soviet farmland abandonment, forest recovery, and carbon sequestration in western Ukraine. Glob. Chang. Biol. 2011, 17, 1335–1349. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Spies, T.A. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 2014, 24, 2063–2077. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Thom, D.; Seidl, R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests: Disturbance impacts on biodiversity and services. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef] [PubMed]
- Shvidenko, A.; Lakyda, P.; Schepaschenko, D.; Vasylyshyn, R.; Marchuk, Y. Carbon, Climate and Land-Use in Ukraine: Forest Sector: A Monograph; FOP Havryshenko V. M.: Korsun-Shevchenkivskyi, Ukraine, 2016. [Google Scholar]
- Bilous, A. Methodology of the research dead biomass of forest. Biol. Resour. Nat. Manag. 2014, 6, 134–145. [Google Scholar]
- Zibtsev, S.V.; Goldammer, J.G.; Robinson, S.; Borsuk, O.A. Fires in nuclear forests: Silent threats to the environment and human security. Unasylva 2015, 66, 40–51. [Google Scholar]
- Evangeliou, N.; Balkanski, Y.; Cozic, A.; Hao, W.M.; Mouillot, F.; Thonicke, K.; Paugam, R.; Zibtsev, S.; Mousseau, T.A.; Wang, R.; et al. Fire evolution in the radioactive forests of ukraine and belarus: Future risks for the population and the environment. Ecol. Monogr. 2015, 85, 49–72. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Burton, P.J.; Franklin, J.F. Salvage Logging and Its Ecological Consequences; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.-Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Fernandes, P.M.; Fonseca, T.F.; Gillet, F.; Jönsson, A.M.; Merganičová, K.; Netherer, S.; Arpaci, A.; Bontemps, J.-D.; Bugmann, H.; et al. Modelling natural disturbances in forest ecosystems: A review. Ecol. Model. 2011, 222, 903–924. [Google Scholar] [CrossRef]
- Lakyda, P. Live Biomass of Ukraine’s Forests; Zbruch: Ternopil, Ukraine, 2002. [Google Scholar]
- Latifi, H.; Fassnacht, F.E.; Hartig, F.; Berger, C.; Hernández, J.; Corvalán, P.; Koch, B. Stratified aboveground forest biomass estimation by remote sensing data. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 229–241. [Google Scholar] [CrossRef]
- Mcroberts, R.; Tomppo, E. Remote sensing support for national forest inventories. Remote Sens. Environ. 2007, 110, 412–419. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- McRoberts, R.E. Estimating forest attribute parameters for small areas using nearest neighbors techniques. For. Ecol. Manag. 2012, 272, 3–12. [Google Scholar] [CrossRef]
- Ohmann, J.L.; Gregory, M.J. Predictive mapping of forest composition and structure with direct gradient analysis and nearest-neighbor imputation in coastal Oregon, USA. Can. J. For. Res. 2002, 32, 725–741. [Google Scholar] [CrossRef]
- Beaudoin, A.; Bernier, P.Y.; Guindon, L.; Villemaire, P.; Guo, X.J.; Stinson, G.; Bergeron, T.; Magnussen, S.; Hall, R.J. Mapping attributes of Canada’s forests at moderate resolution through kNN and MODIS imagery. Can. J. For. Res. 2014, 44, 521–532. [Google Scholar] [CrossRef]
- Bilous, A.; Myroniuk, V.; Holiaka, D.; Bilous, S.; See, L.; Schepaschenko, D. Mapping growing stock volume and forest live biomass: A case study of the polissya region of Ukraine. Environ. Res. Lett. 2017, 12, e105001. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Yang, Z.; Braaten, J.; Copass, C.; Antonova, N.; Jordan, C.; Nelson, P. Attribution of disturbance change agent from Landsat time-series in support of habitat monitoring in the puget sound region, USA. Remote Sens. Environ. 2015, 166, 271–285. [Google Scholar] [CrossRef]
- Schroeder, T.A.; Schleeweis, K.G.; Moisen, G.G.; Toney, C.; Cohen, W.B.; Freeman, E.A.; Yang, Z.; Huang, C. Testing a landsat-based approach for mapping disturbance causality in U.S. forests. Remote Sens. Environ. 2017, 195, 230–243. [Google Scholar] [CrossRef]
- Oeser, J.; Pflugmacher, D.; Senf, C.; Heurich, M.; Hostert, P. Using intra-annual landsat time series for attributing forest disturbance agents in Central Europe. Forests 2017, 8, 251. [Google Scholar] [CrossRef]
- Senf, C.; Pflugmacher, D.; Hostert, P.; Seidl, R. Using landsat time series for characterizing forest disturbance dynamics in the coupled human and natural systems of Central Europe. ISPRS J. Photogramm. Remote Sens. 2017, 130, 453–463. [Google Scholar] [CrossRef]
- Olofsson, P.; Foody, G.M.; Herold, M.; Stehman, S.V.; Woodcock, C.E.; Wulder, M.A. Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 2014, 148, 42–57. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Ohmann, J.L.; Gregory, M.J.; Roberts, H.M. Scale considerations for integrating forest inventory plot data and satellite image data for regional forest mapping. Remote Sens. Environ. 2014, 151, 3–15. [Google Scholar] [CrossRef]
- Lakyda, P.; Matushevych, L. Live Biomass of Silver Birch Forest Stands in Ukrainian Polissya; NNC IAE: Kyiv, Ukraine, 2006. [Google Scholar]
- Mukhortova, L.; Schepaschenko, D.; Shvidenko, A.; McCallum, I.; Kraxner, F. Soil contribution to carbon budget of Russian forests. Agric. For. Meteorol. 2015, 200, 97–108. [Google Scholar] [CrossRef]
- Crookston, N.L.; Finley, A.O. yaImpute: An R package for k-NN imputation. J. Stat. Softw. 2008, 23. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. In Advances in Ecological Research; MacFadyen, A., Ford, E.D., Eds.; Academic Press, Inc.: Orlando, FL, USA, 1986; Volume 15, pp. 133–302. [Google Scholar]
- Shvidenko, A.; Schepaschenko, D.; Nilsson, S.; Bouloui, Y. Semi-empirical models for assessing biological productivity of Northern Eurasian forests. Ecol. Model. 2007, 204, 163–179. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Bilous, A.M.; Voloshchuk, N.M.; Buzyl, M.A.; Kovbasa, I.V. Peculiarities of Mortmass Mycobiota Formation in Soft-Deciduous Young Forests on Old-Tillage Soils of the Chernihiv Polissya. Available online: https://eurekamag.com/research/054/902/054902763.php (accessed on 1 February 2019).
- IA “Ukrderzhlisproekt”. Reference Book on Forest Fund of Ukraine According to the Data of State Forest Account in 2010; IA “Ukrderzhlisproekt”: Irpin, Ukraine, 2012. [Google Scholar]
- Meshkova, V.; Borysenko, O.; Pryhornytskyi, V. Forest growth conditions and other bark beetle-favorable characteristics of Scots pine stands. Sci. Bull. For. Acad. Sci. Ukr. 2018, 106–114. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Gazda, A.; Dobrowolska, D.; Checko, E.; Zaremba, J.; Tomski, A. Tree mortality after wind disturbance differs among tree species more than among habitat types in a lowland forest in northeastern Poland. For. Ecol. Manag. 2017, 398, 174–184. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Kozak, J.; Radeloff, V.C.; Hostert, P. Differences in forest disturbance among land ownership types in Poland during and after socialism. J. Land Use Sci. 2009, 4, 73–83. [Google Scholar] [CrossRef]
- Jonasova, M.; Prach, K. The influence of bark beetle outbreak vs. salvage logging on ground layer vegetation in Central European mountain spruce forests. Biol. Conversat. 2008, 141, 1525–1535. [Google Scholar] [CrossRef]
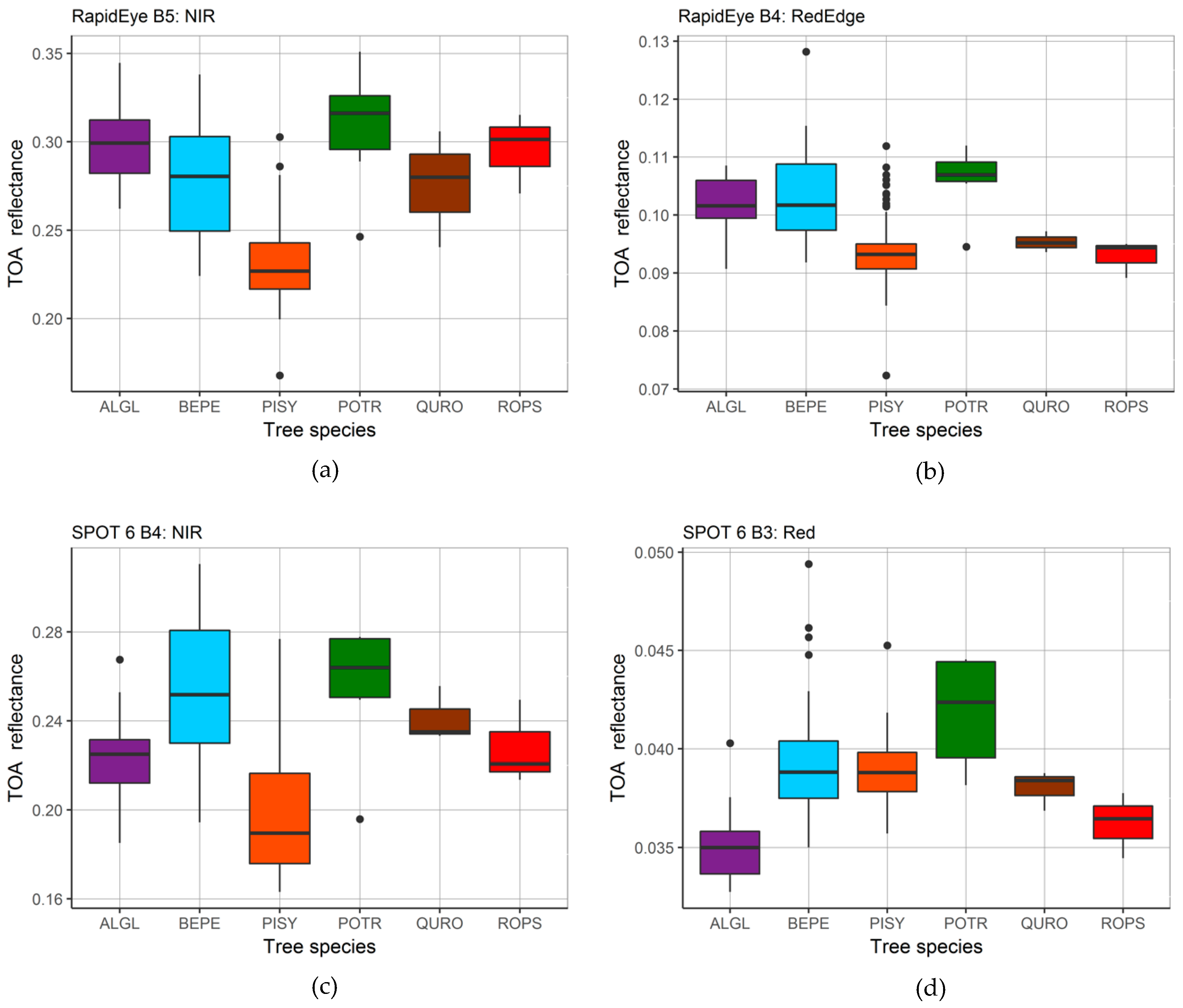
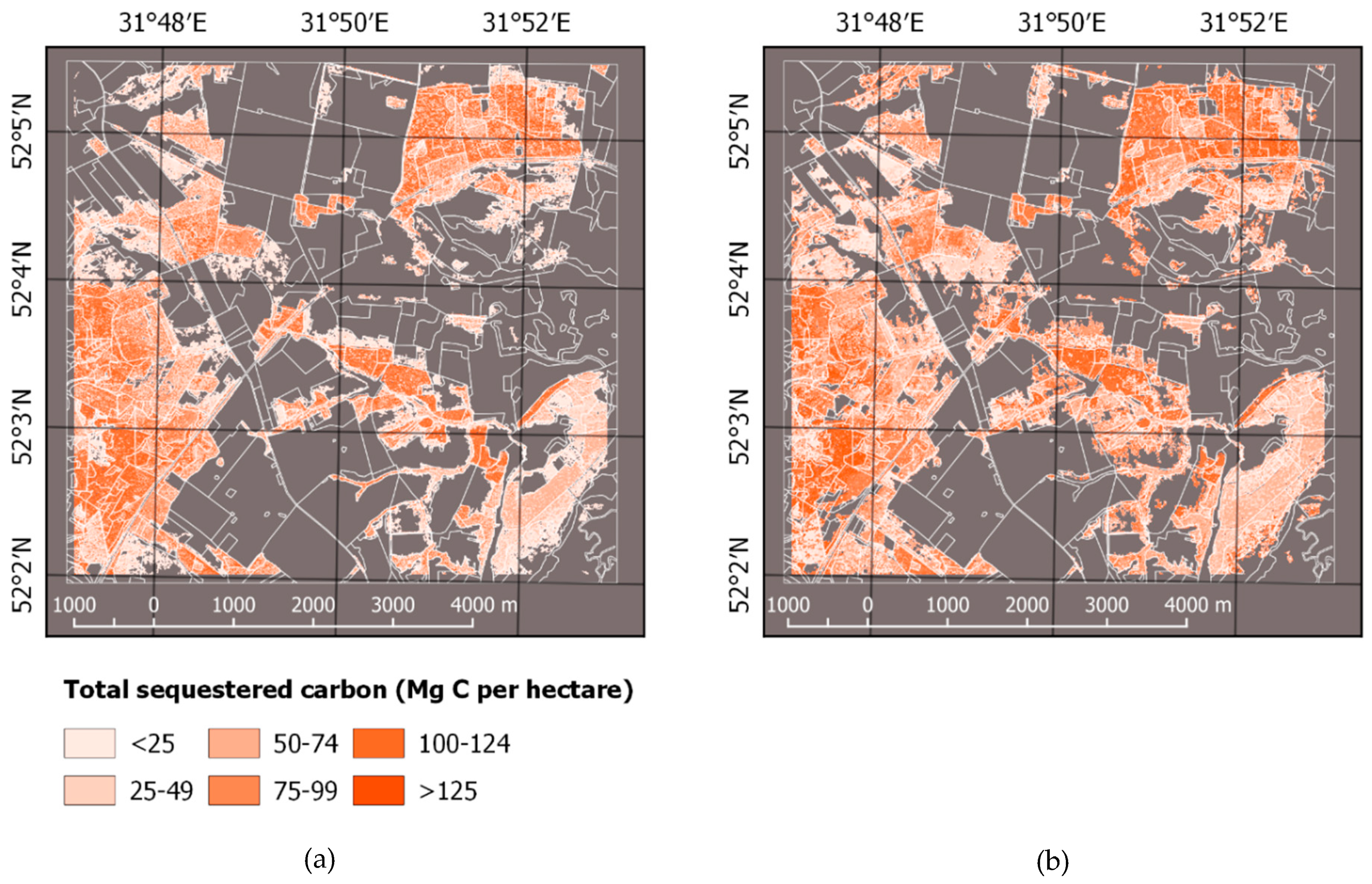
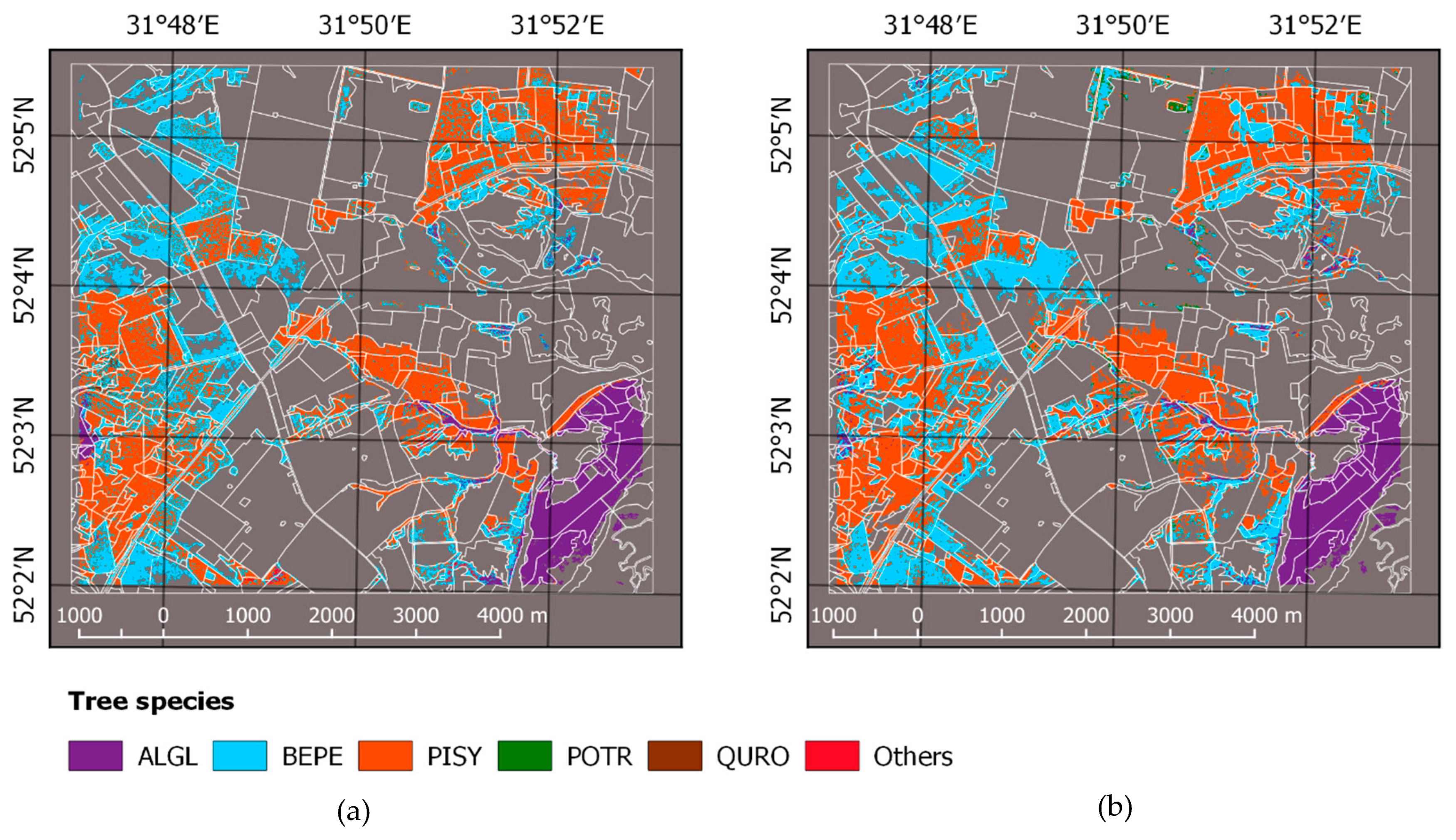
| Land Cover Class | 2010 | 2015 | ||
|---|---|---|---|---|
| area, ha | % | area, ha | % | |
| Forested area | 1660 | 38.8 | 1866 | 43.7 |
| Croplands | 830 | 19.5 | 771 | 18.1 |
| Grasslands | 1473 | 34.5 | 1077 | 25.2 |
| Shrublands | 220 | 5.2 | 460 | 10.8 |
| Wetlands | 68 | 1.6 | 59 | 1.4 |
| Water | 16 | 0.4 | 34 | 0.8 |
| Total | 4267 | 100.0 | 4267 | 100.0 |
| Tree Species Code | Latin Name | Acronym | Sample Size |
|---|---|---|---|
| 1 | Alnus glutinosa L. | ALGL | 22 |
| 2 | Betula pendula Roth | BEPE | 51 |
| 3 | Pinus sylvestris L. | PISY | 81 |
| 4 | Populus tremula L. | POTR | 6 |
| 5 | Quercus robur L. | QURO | 3 |
| 6 | Robinia pseudoacacia L. | ROPS | 3 |
| Experimental Data | Scots Pine | Silver Birch | Black Alder | Common Aspen |
|---|---|---|---|---|
| Temporary sample plots (TSPs), in total | 19 | 77 | 16 | 16 |
| Including estimation: snags | 19 | 77 | 16 | 16 |
| Logs | 19 | 32 | 13 | 14 |
| Coarse branches | 19 | 33 | 13 | 14 |
| Fine litter | 19 | 33 | 13 | 14 |
| Snag sample trees | 72 | 84 | 48 | 48 |
| Samplings, in total | 792 | 840 | 525 | 704 |
| Including: stems of snags | 90 | 168 | 96 | 144 |
| Logs | 270 | 420 | 195 | 210 |
| Coarse branches | 270 | 84 | 195 | 210 |
| Fine litter | 162 | 84 | 39 | 140 |
| No. | Dead Biomass Component | Description | Size |
|---|---|---|---|
| 1 | Snags | Standing stems over bark and branches of dead and live trees, if their height is >1.3 m | No limitations |
| 2 | Logs | Downed stems over bark or their parts with branches, including stumps with a height <1.3 m | No limitations |
| 3 | Litter of coarse branches | Downed branches over bark, broken from stems of live or dead trees | Diameter >1 cm |
| 4 | Fine litter | Foliage (leaves and needles), fine branches, bark, fruits and seeds on the ground | Diameter of branches ≤1 cm |
| Group of Soils | HSR, g C·m−2·year−1 |
|---|---|
| Luvisols and Greyzems—texture-differentiated soils | 290 ± 160 |
| Gleysols—over wetted mineral soils with thick (10–30 cm) organic horizon | 314 ± 214 |
| Histosols—over wetted organic soils | 268 ± 201 |
| Reference Data | Classified Data | Class Error 1 | |||||
|---|---|---|---|---|---|---|---|
| Croplands | Forests | Grasslands | Shrublands | Water Bodies | Wetlands | ||
| Croplands | 113 | 1 | 16 | 1 | 0 | 0 | 0.137 |
| Forests | 0 | 242 | 2 | 0 | 0 | 0 | 0.016 |
| Grasslands | 28 | 3 | 96 | 4 | 0 | 0 | 0.267 |
| Shrublands | 4 | 0 | 26 | 2 | 0 | 0 | 0.937 |
| Water bodies | 0 | 1 | 0 | 0 | 18 | 0 | 0.053 |
| Wetlands | 0 | 2 | 1 | 0 | 0 | 6 | 0.333 |
| Reference Data | Classified Data | Class Error 2 | |||||
|---|---|---|---|---|---|---|---|
| Croplands | Forests | Grasslands | Shrublands | Water Bodies | Wetlands | ||
| Croplands | 114 | 1 | 15 | 2 | 0 | 0 | 0.130 |
| Forests | 1 | 240 | 1 | 4 | 0 | 0 | 0.024 |
| Grasslands | 21 | 0 | 101 | 8 | 0 | 1 | 0.230 |
| Shrublands | 0 | 4 | 14 | 14 | 0 | 0 | 0.562 |
| Water boodies | 0 | 0 | 0 | 0 | 19 | 0 | 0.000 |
| Wetlands | 0 | 0 | 0 | 0 | 0 | 9 | 0.000 |
| Species | Area, ha | Carbon, Gg C | ||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2015 | 2010 | 2015 | |||||
| FID | RS | FID | RS | FID | RS | FID | RS | |
| Black alder | 240 ± 5 | 202 ± 43 | 245 ± 5 | 231 ± 49 | 11.1 ± 0.7 | 7.9 ± 2.6 | 12.5 ± 0.8 | 13.4 ± 4.6 |
| Silver birch | 711 ± 14 | 614 ± 86 | 725 ± 14 | 681 ± 95 | 29.4 ± 1.6 | 25.7 ± 5.5 | 31.2 ± 1.7 | 36.4 ± 7.9 |
| Scots pine | 866 ± 17 | 829 ± 92 | 831 ± 17 | 928 ± 103 | 66.2 ± 3.3 | 66.0 ± 11.0 | 69.7 ± 3.4 | 82.2 ± 14.0 |
| Common oak | 13 ± 1 | 5 ± 3 | 13 ± 1 | 2 ± 2 | 1.2 ± 0.11 | 0.3 ± 0.2 | 1.3 ± 0.12 | 0.1 ± 0.1 |
| Common aspen | 13 ± 1 | 1 ± 1 | 14 ± 1 | 23 ± 9 | 1.0 ± 0.12 | >0.1 | 1.1 ± 0.13 | 1.3 ± 1.3 |
| Black locust | 6 ± 1 | 9 ± 6 | 6 ± 1 | 1 ± 1 | 0.4 ± 0.05 | 0.7 ± 0.7 | 0.4 ± 0.05 | >0.1 |
| Total | 1849 ± 23 | 1660 ± 129 | 1834 ± 23 | 1866 ± 146 | 109.3 ± 5.9 | 100.7 ± 11.6 | 116.2 ± 6.3 | 133.5 ± 15.7 |
| Direction of Change | Area, ha | Carbon, Gg C | ||
|---|---|---|---|---|
| FID | RS | FID | RS | |
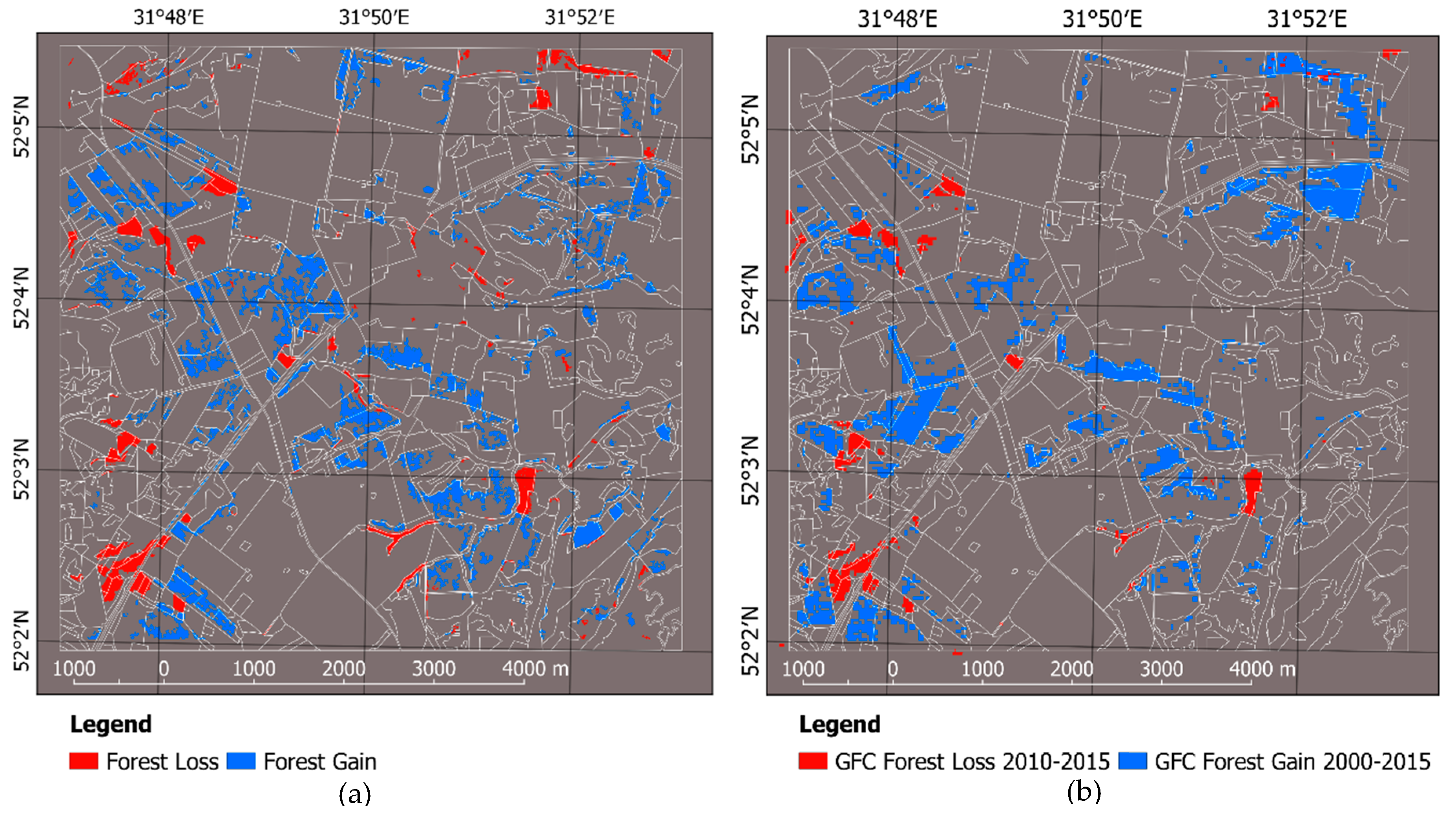
| Gain | 68 | 304 | 0.7 | 17.0 |
| Loss | 103 | 108 | 8.2 | 7.1 |
| Net change | −35 | +196 | −7.5 | +9.9 |
| Species | NPP, Gg C·year−1 | Average NPP, Mg C·ha−1·year−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2015 | 2010 | 2015 | |||||
| FID | RS | FID | RS | FID | RS | FID | RS | |
| ALGL | 1.2 | 0.8 | 1.2 | 1.0 | 4.9 | 4.6 | 4.8 | 5.0 |
| BEPE | 3.5 | 3.0 | 3.5 | 3.2 | 5.0 | 4.9 | 4.7 | 4.8 |
| PISY | 4 | 3.7 | 3.9 | 4.4 | 4.6 | 4.5 | 4.8 | 4.8 |
| QURO | >0.1 | >0.1 | 0.1 | 0.1 | 4.9 | 5.0 | 5.5 | 5.4 |
| POTR | 0.1 | >0.1 | 0.1 | >0.1 | 4.1 | 3.9 | 4.7 | 4.7 |
| ROPS | >0.1 | >0.1 | >0.1 | >0.1 | 3.9 | 4.2 | 4.3 | 4.8 |
| Total | 8.8 | 7.6 | 8.8 | 8.7 | – | |||
| Ecosystem Components | Carbon stock, Gg C | |||||
|---|---|---|---|---|---|---|
| FID | RS | |||||
| 2010 | 2015 | Changes, % | 2010 | 2015 | Changes, % | |
| Live biomass | 96.3 ± 3.5 | 103.0 ± 3.8 | +7.0 | 87.6 ± 15.1 | 116.2 ± 20.2 | +32.6 |
| Woody detritus | 6.0 ± 1.0 | 6.2 ± 1.0 | +3.3 | 5.4 ± 1.6 | 7.2 ± 2.2 | +33.3 |
| Soil | 461.0 ± 253.6 | 458.2 ± 252.0 | −0.6 | 416.2 ± 228.9 | 468.4 ± 257.6 | +12.5 |
| Total | 563.3 ± 253.6 | 567.4 ± 252.0 | +0.7 | 509.2 ± 229.4 | 591.8 ± 258.4 | +16.2 |
| Carbon Fluxes of Forest Ecosystems | Annual Value of Flux (Except DIST), Mg C year−1 | |||
|---|---|---|---|---|
| FID | RS | |||
| 2010 | 2015 | 2010 | 2015 | |
| Net primary production (NPP) | 8886 ± 678 | 8750 ± 671 | 7748 ± 1262 | 9008 ± 1498 |
| Heterotrophic soil respiration (HSR) | 5340 ± 3364 | 5320 ± 3352 | 4778 ± 3345 | 5371 ± 3760 |
| Decomposition of CWD (DEC) | 297 ± 91 | 303 ± 91 | 336 ± 147 | 360 ± 160 |
| Lateral fluxes into lithosphere and hydrosphere (LAT) | 444 ± 333 | 438 ± 329 | 387 ± 290 | 450 ± 338 |
| Loss caused by natural disturbances and harvest (DIST) | 8311 ± 1247 | 7073 ± 2334 | ||
| Net | +5426 | +5610 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakyda, P.; Shvidenko, A.; Bilous, A.; Myroniuk, V.; Matsala, M.; Zibtsev, S.; Schepaschenko, D.; Holiaka, D.; Vasylyshyn, R.; Lakyda, I.; et al. Impact of Disturbances on the Carbon Cycle of Forest Ecosystems in Ukrainian Polissya. Forests 2019, 10, 337. https://doi.org/10.3390/f10040337
Lakyda P, Shvidenko A, Bilous A, Myroniuk V, Matsala M, Zibtsev S, Schepaschenko D, Holiaka D, Vasylyshyn R, Lakyda I, et al. Impact of Disturbances on the Carbon Cycle of Forest Ecosystems in Ukrainian Polissya. Forests. 2019; 10(4):337. https://doi.org/10.3390/f10040337
Chicago/Turabian StyleLakyda, Petro, Anatoly Shvidenko, Andrii Bilous, Viktor Myroniuk, Maksym Matsala, Sergiy Zibtsev, Dmitry Schepaschenko, Dmytrii Holiaka, Roman Vasylyshyn, Ivan Lakyda, and et al. 2019. "Impact of Disturbances on the Carbon Cycle of Forest Ecosystems in Ukrainian Polissya" Forests 10, no. 4: 337. https://doi.org/10.3390/f10040337
APA StyleLakyda, P., Shvidenko, A., Bilous, A., Myroniuk, V., Matsala, M., Zibtsev, S., Schepaschenko, D., Holiaka, D., Vasylyshyn, R., Lakyda, I., Diachuk, P., & Kraxner, F. (2019). Impact of Disturbances on the Carbon Cycle of Forest Ecosystems in Ukrainian Polissya. Forests, 10(4), 337. https://doi.org/10.3390/f10040337









