Nitrogen and Phosphorus Resorption in Relation to Nutrition Limitation along the Chronosequence of Black Locust (Robinia pseudoacacia L.) Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Experimental Design and Sampling
2.3. Laboratory Analysis and Determination
2.4. Calculations and Statistical Analysis
3. Results
3.1. Leaf and Soil C, N, and P Concentrations
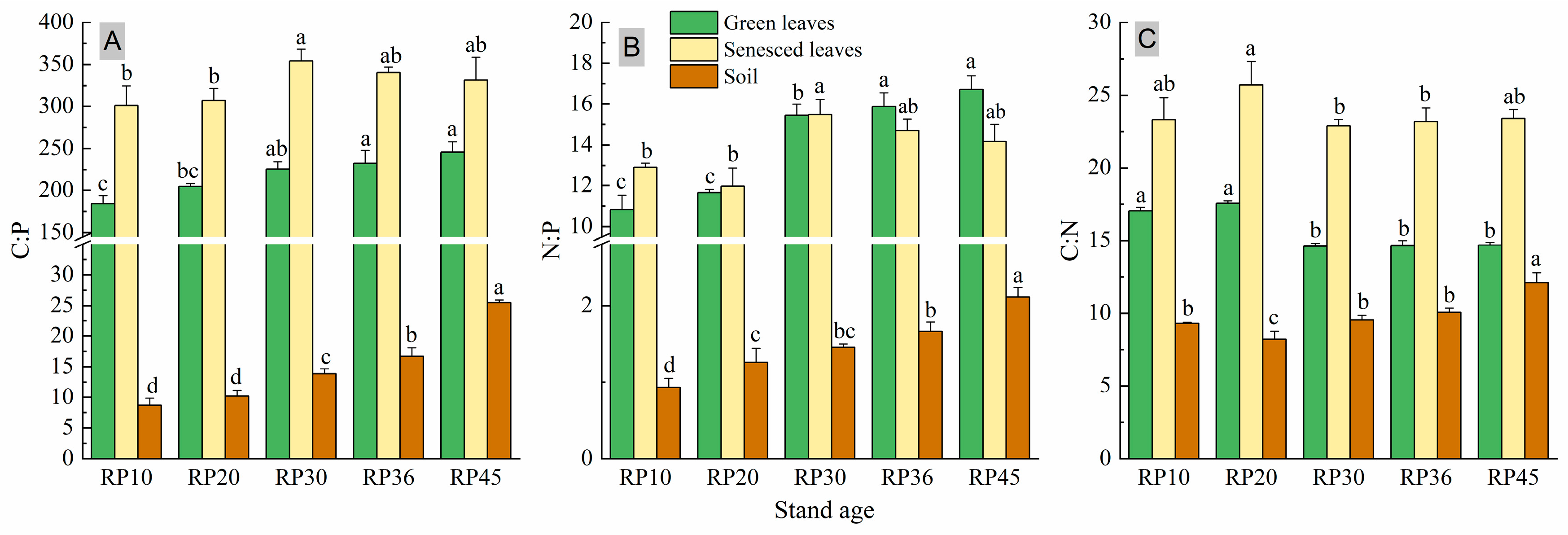
3.2. Leaf and Soil C:N:P Stoichiometry Ratios
3.3. NRE and PRE in Forests of Different Stand Ages and Their Relationship
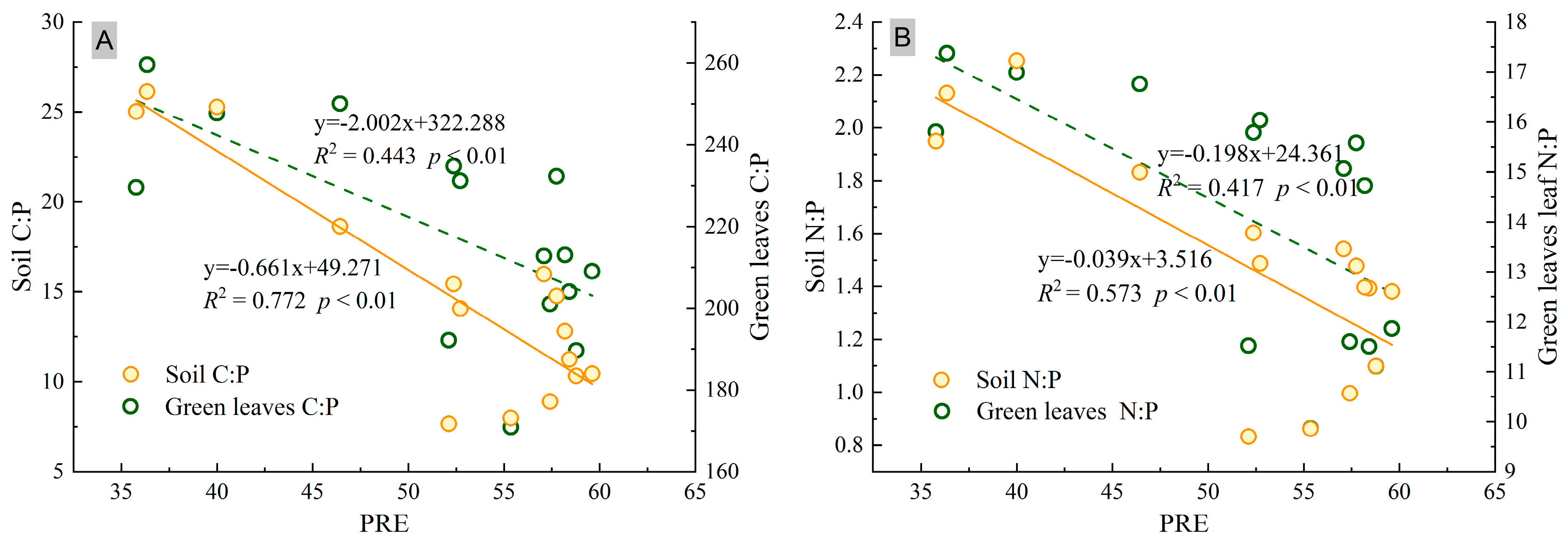
3.4. Relationships between NuRE, Nutrient Concentrations, and Stoichiometry Ratios in Leaves and Soil
4. Discussion
4.1. Leaf and Soil Nutrient Concentration and Stoichiometry of Black Locust Forests of Different Ages
4.2. N and P Resorption Correlated with Soil and Leaf Nutrients across the Plantation Chronosequence
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Jiang, L.; Liu, W.; Wang, X.; Li, S.; Yang, S.; Wang, B. Ecosystem scale trade-off in nitrogen acquisition pathways. Nat. Ecol. Evol. 2018, 2, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Reed, S.C.; Yu, Q.; Han, X. Nutrient resorption helps drive intra-specific coupling of foliar nitrogen and phosphorus under nutrient-enriched conditions. Plant Soil 2016, 398, 111–120. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; He, W.; An, L.; Xu, S. Nutrient resorption or accumulation of desert plants with contrasting sodium regulation strategies. Sci. Rep. UK 2017, 7, 17035. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen and phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Campo, J. Shift from ecosystem P to N limitation at precipitation gradient in tropical dry forests at Yucatan, Mexico. Environ. Res. Lett. 2016, 11, 95006. [Google Scholar] [CrossRef]
- Mediavilla, S.; García-Iglesias, J.; González-Zurdo, P.; Escudero, A. Nitrogen resorption efficiency in mature trees and seedlings of four tree species co-occurring in a Mediterranean environment. Plant Soil 2014, 385, 205–215. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Duan, W. Temporal variations in phosphorus fractions and phosphatase activities in rhizosphere and bulk soil during the development of Larix olgensis plantations. J. Plant Nutr. Soil Sci. 2015, 179, 67–77. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.; Zhu, J.; Yang, K.; Yu, L.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2018, 422, 385–396. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Changes in nitrogen resorption of trembling aspen (Populus tremuloides) with stand development. Plant Soil 2010, 327, 121–129. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Yang, K. Leaf nitrogen and phosphorus resorption of woody species in response to climatic conditions and soil nutrients: A meta-analysis. J. For. Res. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Lu, J.; Duan, B.; Yang, M.; Yang, H.; Yang, H. Research progress in nitrogen and phosphorus resorption from senesced leaves and the influence of ontogenetic and environmental factors. Acta Pratacult. Sin. 2018, 27, 178–188. [Google Scholar]
- Du, B.; Ji, H.; Peng, C.; Liu, X.; Liu, C. Altitudinal patterns of leaf stoichiometry and nutrient resorption in Quercus variabilis in the Baotianman Mountains, China. Plant Soil 2017, 413, 193–202. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, W.; Yu, M.; Wang, G.G.; Wu, T. Latitudinal patterns of leaf N, P stoichiometry and nutrient resorption of Metasequoia glyptostroboides along the eastern coastline of China. Sci. Total Environ. 2018, 618, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, J.; Yang, H.; Zhang, X.; Luo, C.; Zhao, Y. Resorption of nitrogen, phosphorus and potassium from leaves of lucerne stands of different ages. Plant Soil 2014, 383, 301–312. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Peng, S.; Peñuelas, J.; Zeng, H.; Piao, S. Age-Related Modulation of the Nitrogen Resorption Efficiency Response to Growth Requirements and Soil Nitrogen Availability in a Temperate Pine Plantation. Ecosystems 2016, 19, 698–709. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wang, J.; Guo, Z.; Wang, G.G.; Zeng, D.; Wu, T. Tree stoichiometry and nutrient resorption along a chronosequence of Metasequoia glyptostroboides forests in coastal China. For. Ecol. Manag. 2018, 430, 445–450. [Google Scholar] [CrossRef]
- Qiu, L.; Hu, H.; Lin, Y.; Ge, L.; Wang, K.; He, Z.; Dong, Q. Nutrient resorption effciency and C:N:P stoichiometry of Eucalyptus urophylla × E. grandis of different ages in a sandy coastal plain area. Chin. J. Appl. Environ. Biol. 2017, 23, 739–744. [Google Scholar]
- Zhou, L.; Addo-Danso, S.D.; Wu, P.; Li, S.; Zou, X.; Zhang, Y.; Ma, X. Leaf resorption efficiency in relation to foliar and soil nutrient concentrations and stoichiometry of Cunninghamia lanceolata with stand development in southern China. J. Soil Sediment 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Meng, F.; Luo, Q.; Wang, Q.; Zhang, X.; Qi, Z.; Xu, F.; Lei, X.; Cao, Y.; Chow, W.S.; Sun, G. Physiological and proteomic responses to salt stress in chloroplasts of diploid and tetraploid black locust (Robinia pseudoacacia L.). Sci. Rep. UK 2016, 6, 23098. [Google Scholar] [CrossRef]
- Sitzia, T.; Campagnaro, T.; Dainese, M.; Cierjacks, A. Plant species diversity in alien black locust stands: A paired comparison with native stands across a north-Mediterranean range expansion. For. Ecol. Manag. 2012, 285, 85–91. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Cao, Y. Ecosystem carbon and nitrogen storage following farmland afforestation with black locust (Robinia pseudoacacia) on the Loess Plateau, China. J. For. Res. 2018, 29, 761–771. [Google Scholar] [CrossRef]
- Hui, S.; Shao, M. Soil and water loss from the Loess Plateau in China. J. Arid Environ. 2000, 45, 9–20. [Google Scholar]
- Ma, C.; Luo, Y.; Shao, M.; Li, X.; Sun, L.; Jia, X. Environmental controls on sap flow in black locust forest in Loess Plateau, China. Sci. Rep. UK 2017, 7, 13160. [Google Scholar] [CrossRef]
- Liu, G.; Shang, G.; Yao, W.; Yang, Q.; Zhao, M.; Dang, X.; Guo, M.; Wang, G.; Wang, B. Ecological Effects of Soil Conservation in Loess Plateau. Bull. Chin. Acad. Sci. 2017, 32, 11–19. [Google Scholar]
- Deng, J.; Sun, P.; Zhao, F.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil C, N, P and Its Stratification Ratio Affected by Artificial Vegetation in Subsoil, Loess Plateau China. PLoS ONE 2016, 11, e151446. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Z.; Feng, X.; Zhang, Y.; Chen, W.; Wu, X.; Jiao, L.; Wamg, X. Ecological and physiological mechanisms of growth decline of Robinia pseudoacacia plantations in the Loess Plateau of China: A review. Chin. J. Appl. Ecol. 2018, 29, 2433–2444. [Google Scholar]
- Cao, Y.; Chen, Y. Coupling of plant and soil C:N:P stoichiometry in black locust (Robinia pseudoacacia) plantations on the Loess Plateau, China. Trees 2017, 31, 1–12. [Google Scholar] [CrossRef]
- Jia, X.; Shao, M.; Zhu, Y.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Shangguan, Z.; Zheng, S. Ecological properties of soil water and effects on forest vegetation in the Loess Plateau. Int. J. Sustain. Dev. World 2006, 13, 307–314. [Google Scholar] [CrossRef]
- Yan, T.; Lü, X.; Yang, K.; Zhu, J. Leaf nutrient dynamics and nutrient resorption: A comparison between larch plantations and adjacent secondary forests in Northeast China. J. Plant Ecol. 2015, 9, 165–173. [Google Scholar] [CrossRef]
- Bao, S. Analysis of Soil Agrochemical; Chinese Agricultural Press: Beijing, China, 2000. [Google Scholar]
- He, J.; Lu, Y.; Fu, B. Frontier of Soil Biology; Science Press: Beijing, China, 2015. [Google Scholar]
- Liu, J.; Gu, Z.; Shao, H.; Zhou, F.; Peng, S. N–P stoichiometry in soil and leaves of Pinus massoniana forest at different stand ages in the subtropical soil erosion area of China. Environ. Earth Sci. 2016, 75, 1091. [Google Scholar] [CrossRef]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine-oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Ma, R.; An, S.; Huang, Y. C, N and P stoichiometry characteristics of different-aged Robinia pseudoacacia plantations on the Loess Plateau, China. Chin. J. Appl. Ecol. 2017, 28, 2787–2793. [Google Scholar]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2016, 95, 2062–2068. [Google Scholar] [CrossRef]
- Zheng, S.; Shangguan, Z. Spatial patterns of leaf nutrient traits of the plants in the Loess Plateau of China. Trees 2007, 21, 357–370. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Zhang, X.; Yang, J.; An, S. Characteristics of Leaf Ecological Stoichiometry of Robinia pseudoacacia in Loess Plateau. Res. Soil Water Conserv. 2014, 21, 57–61. [Google Scholar]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2008, 3, 540–550. [Google Scholar] [CrossRef]
- Hayes, P.; Turner, B.L.; Lambers, H.; Laliberté, E.; Bellingham, P. Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J. Ecol. 2014, 102, 396–410. [Google Scholar] [CrossRef]
- Chapin, F.S.; Moilanen, L. Nutritional Controls Over Nitrogen and Phosphorus Resorption from Alaskan Birch Leaves. Ecology 1991, 72, 709–715. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, Y.; Xiao, H.; Zhu, R.; Luo, L.; Guo, W.; Cao, Y.; Zhang, Z.; Pan, Y.; Zheng, N.; et al. Pattern and controlling factors of terrestrial leaf litters decomposition in China. J. East China Univ. Technol. (Nat. Sci.) 2018, 41, 271–276. [Google Scholar]
- William, P.; Silver, W.L.; Burke, I.C.; Leo, G.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar]
- Gergócs, V.; Hufnagel, L. The effect of microarthropods on litter decomposition depends on litter quality. Eur. J. Soil Biol. 2016, 75, 24–30. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.L.; Wang, Q.K. Nutrient contents and resorption characteristics in needles of different age Pinus massoniana (Lamb.) before and after withering. Chin. J. Appl. Ecol. 2008, 19, 1443. [Google Scholar]
- Sardans, J.; Peñuelas, J. Trees increase their P:N ratio with size. Glob. Ecol. Biogeogr. 2015, 24, 147–156. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Glob. Ecol. Biogeogr. 2012, 22, 494–507. [Google Scholar] [CrossRef]
- Luyssaert, S.; Staelens, J.; De Schrijver, A. Does the commonly used estimator of nutrient resorption in tree foliage actually measure what it claims to? Oecologia 2005, 144, 177–186. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The Mineral Nutrition of Wild Plants Revisited: A Re-evaluation of Processes and Patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Güsewell, S. Nutrient Resorption of Wetland Graminoids Is Related to the Type of Nutrient Limitation. Funct. Ecol. 2010, 19, 344–354. [Google Scholar] [CrossRef]
- Agüero, M.L.; Puntieri, J.; Mazzarino, M.J.; Grosfeld, J.; Barroetaveña, C. Seedling response of Nothofagus species to N and P: Linking plant architecture to N/P ratio and resorption proficiency. Trees 2014, 28, 1185–1195. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in Senesced Leaves: Keys to the Search for Potential Resorption and Resorption Proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- De Marco, A.; Arena, C.; Giordano, M.; De Santo, A.V. Impact of the invasive tree black locust on soil properties of Mediterranean stone pine-holm oak forests. Plant Soil 2013, 372, 473–486. [Google Scholar] [CrossRef]
- Han, W.; Tang, L.; Chen, Y.; Fang, J. Relationship between the Relative Limitation and Resorption Efficiency of Nitrogen vs. Phosphorus in Woody Plants. PLoS ONE 2013, 8, e83366. [Google Scholar] [CrossRef] [PubMed]
- Lado-Monserrat, L.; Lidón, A.; Bautista, I. Litterfall, litter decomposition and associated nutrient fluxes in Pinus halepensis: Influence of tree removal intensity in a Mediterranean forest. Eur. J. For. Res. 2015, 134, 833–844. [Google Scholar] [CrossRef]
- Spohn, M.; Zavišić, A.; Nassal, P.; Bergkemper, F.; Schulz, S.; Marhan, S.; Schloter, M.; Kandeler, E.; Polle, A. Temporal variations of phosphorus uptake by soil microbial biomass and young beech trees in two forest soils with contrasting phosphorus stocks. Soil Biol. Biochem. 2018, 117, 191–202. [Google Scholar] [CrossRef]
- Zavišić, A.; Nassal, P.; Yang, N.; Heuck, C.; Spohn, M.; Marhan, S.; Pena, R.; Kandeler, E.; Polle, A. Phosphorus availabilities in beech (Fagus sylvatica L.) forests impose habitat filtering on ectomycorrhizal communities and impact tree nutrition. Soil Biol. Biochem. 2016, 98, 127–137. [Google Scholar]
- Netzer, F.; Schmid, C.; Herschbach, C.; Rennenberg, H. Phosphorus-nutrition of European beech (Fagus sylvatica L.) during annual growth depends on tree age and P-availability in the soil. Environ. Exp. Bot. 2017, 137, 194–207. [Google Scholar] [CrossRef]
- Pistocchi, C.; Mészáros, É.; Tamburini, F.; Frossard, E.; Bünemann, E.K. Biological processes dominate phosphorus dynamics under low phosphorus availability in organic horizons of temperate forest soils. Soil Biol. Biochem. 2018, 126, 64–75. [Google Scholar] [CrossRef]

| Stand Ages | 10 | 20 | 30 | 36 | 45 |
|---|---|---|---|---|---|
| Location | 36°52′00″ N 109°21′38″ E | 36°52′08″ N 109°21′06″ E | 36°51′36″ N 109°21′06″ E | 36°51′37″ N 109°21′05″ E | 36°52′16″ N 109°20′54″ E |
| Elevation (m) | 1332.31 | 1245.2 | 1283.6 | 1255 | 1121.34 |
| Slope (°) | 37 | 30 | 35 | 30 | 28 |
| Aspect (°) | ES38 | ES20 | EN16 | EN15 | ES10 |
| Bulk density (g cm−3) | 1.09 | 1.29 | 1.05 | 1.01 | 0.98 |
| pH | 8.56 | 8.62 | 8.58 | 8.51 | 8.60 |
| Canopy density (%) | 48 | 55 | 64 | 63 | 60 |
| Stand density (trees hm−2) | 1540 | 1535 | 1350 | 1305 | 1240 |
| DBH (cm) | 6.33 | 15.32 | 17.25 | 18.27 | 19.10 |
| Degree of herb cover (%) | 46.83 | 59.13 | 83.87 | 76.85 | 73.40 |
| Number of herb species | 13 | 24 | 21 | 22 | 23 |
| Field Site | Soil (g kg−1) | Green Leaves (g kg−1) | Senesced Leaves (g kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | N | P | C | N | P | C | N | P | |
| RP10 | 4.65 ± 0.27 | 0.50 ± 0.03 | 0.55 ± 0.07 | 380.81 ± 12.89 | 22.38 ± 0.94 | 2.07 ± 0.06 | 352.61 ± 16.54 | 15.16 ± 0.46 | 1.18 ± 0.05 |
| RP20 | 4.97 ± 0.09 | 0.61 ± 0.05 | 0.49 ± 0.04 | 439.85 ± 13.32 | 25.05 ± 0.54 | 2.15 ± 0.04 | 348.77 ± 12.22 | 13.62 ± 1.09 | 1.14 ± 0.02 |
| RP30 | 8.26 ±0.71 | 0.87 ± 0.07 | 0.60 ± 0.04 | 467.31 ± 8.51 | 32.02 ± 0.75 | 2.07 ± 0.05 | 409.41 ± 12.37 | 17.90 ± 0.82 | 1.16 ± 0.06 |
| RP36 | 8.79 ± 0.35 | 0.87 ± 0.04 | 0.53 ± 0.03 | 475.38 ± 6.11 | 32.49 ± 0.43 | 2.17 ± 0.11 | 425.59 ± 11.48 | 18.38 ± 0.51 | 1.25 ± 0.05 |
| RP45 | 14.41 ± 0.91 | 1.20 ± 0.13 | 0.57 ± 0.03 | 421.53 ± 12.06 | 28.71 ± 0.80 | 1.72 ± 0.06 | 453.97 ± 24.16 | 19.4 ± 0.52 | 1.37 ± 0.05 |
| NRE | PRE | ||||
|---|---|---|---|---|---|
| R | p | R | p | ||
| Soil | C | −0.303 | 0.273 | −0.879 | <0.001 |
| N | −0.151 | 0.592 | −0.773 | <0.001 | |
| P | −0.116 | 0.681 | -0.258 | 0.353 | |
| C:N | −0.489 | 0.064 | −0.881 | <0.001 | |
| C:P | −0.289 | 0.296 | −0.878 | <0.001 | |
| N:P | −0.109 | 0.700 | −0.756 | <0.001 | |
| Green leaves | C | 0.711 | <0.001 | 0.127 | 0.651 |
| N | 0.432 | 0.108 | −0.188 | 0.503 | |
| P | 0.525 | 0.044 | 0.947 | <0.001 | |
| C:N | −0.041 | 0.884 | 0.489 | 0.064 | |
| C:P | 0.085 | 0.763 | −0.666 | <0.001 | |
| N:P | 0.058 | 0.836 | −0.645 | <0.001 | |
| Senesced leaves | C | −0.126 | 0.656 | −0.716 | <0.001 |
| N | −0.292 | 0.292 | −0.675 | <0.001 | |
| P | −0.529 | 0.043 | −0.930 | <0.001 | |
| C:N | 0.504 | 0.048 | 0.229 | 0.413 | |
| C:P | 0.345 | 0.209 | −0.045 | 0.873 | |
| N:P | 0.029 | 0.919 | −0.146 | 0.603 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Wang, S.; Ren, C.; Zhang, W.; Zhao, F.; Li, X.; Zhang, D.; Han, X.; Yang, G. Nitrogen and Phosphorus Resorption in Relation to Nutrition Limitation along the Chronosequence of Black Locust (Robinia pseudoacacia L.) Plantation. Forests 2019, 10, 261. https://doi.org/10.3390/f10030261
Deng J, Wang S, Ren C, Zhang W, Zhao F, Li X, Zhang D, Han X, Yang G. Nitrogen and Phosphorus Resorption in Relation to Nutrition Limitation along the Chronosequence of Black Locust (Robinia pseudoacacia L.) Plantation. Forests. 2019; 10(3):261. https://doi.org/10.3390/f10030261
Chicago/Turabian StyleDeng, Jian, Sha Wang, Chengjie Ren, Wei Zhang, Fazhu Zhao, Xianfang Li, Dan Zhang, Xinhui Han, and Gaihe Yang. 2019. "Nitrogen and Phosphorus Resorption in Relation to Nutrition Limitation along the Chronosequence of Black Locust (Robinia pseudoacacia L.) Plantation" Forests 10, no. 3: 261. https://doi.org/10.3390/f10030261
APA StyleDeng, J., Wang, S., Ren, C., Zhang, W., Zhao, F., Li, X., Zhang, D., Han, X., & Yang, G. (2019). Nitrogen and Phosphorus Resorption in Relation to Nutrition Limitation along the Chronosequence of Black Locust (Robinia pseudoacacia L.) Plantation. Forests, 10(3), 261. https://doi.org/10.3390/f10030261








