Thermal Insulating and Mechanical Properties of Cellulose Nanofibrils Modified Polyurethane Foam Composite as Structural Insulated Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PUF
2.3. Microstructure Characterization
2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. Mechanical Test
2.6. Thermal Conductivity Test
3. Results and Discussions
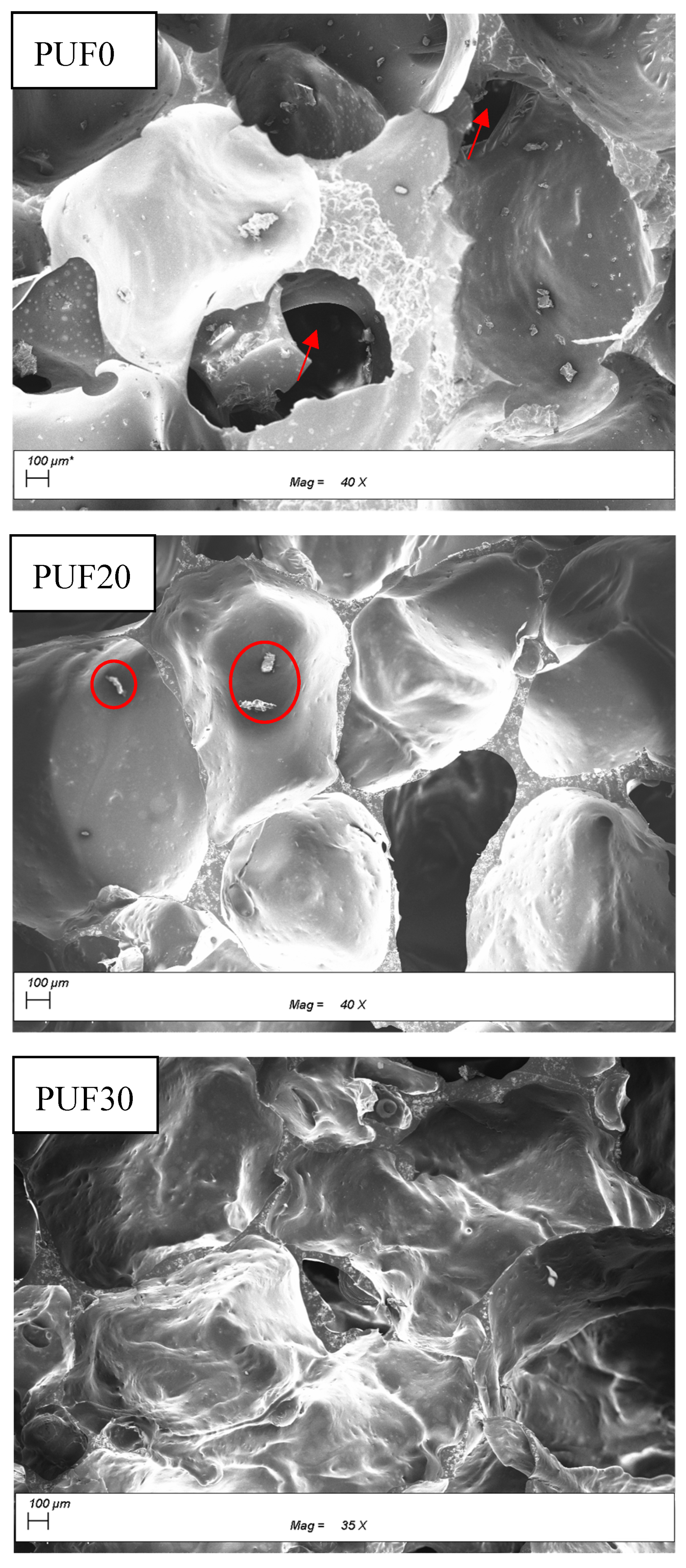
3.1. Microstructure of PUF
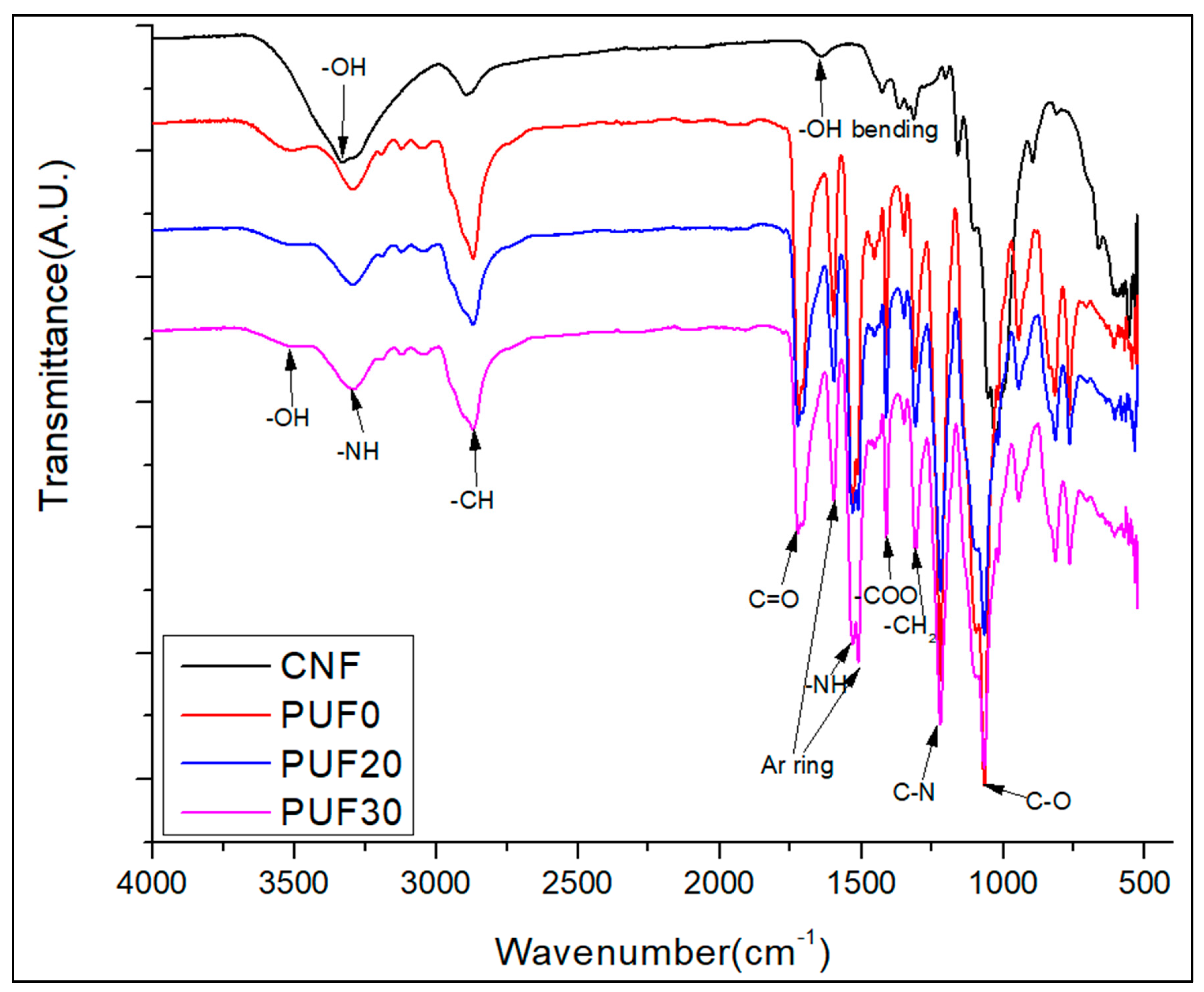
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3. Mechanical Properties of PUF
3.4. Thermal Conductivity of PUF and CNF-PUF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meng, Q.; Chen, W.; Hao, H. Numerical and experimental study of steel wire mesh and basalt fibre mesh strengthened structural insulated panel against projectile impact. Adv. Struct. Eng. 2018, 21, 1183–1196. [Google Scholar] [CrossRef]
- Chen, W.; Hao, H.; Chen, S.; Hernandez, F. Performance of composite structural insulated panel with metal skin subjected to blast loading. Mater. Des. 2015, 84, 194–203. [Google Scholar] [CrossRef]
- Kayello, A.; Ge, H.; Athienitis, A.; Rao, J. Experimental study of thermal and airtightness performance of structural insulated panel joints in cold climates. Build. Environ. 2017, 115, 345–357. [Google Scholar] [CrossRef]
- Purasinghe, R.; Dusicka, P.; Garth, J.S.; Dedek, G.; Lum, H. In-plane cyclic behavior of structural insulated panel wood walls including slit steel connectors. Eng. Struct. 2018, 174, 178–197. [Google Scholar] [CrossRef]
- Polymer Foams Market Forecast to 2019| Smithers Rapra. Available online: https://www.smithersrapra.com/news/2014/may/polymer-foam-market-to-consume-25-3-million-tonnes (accessed on 14 February 2019).
- Efstathiou, K. Synthesis and Characterization of a Polyurethane Prepolymer for the Development of a Novel Acrylate-Based Polymer Foam; Budapest University of Technology and Economics (BME): Budapest, Hungary, 2011. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780439839584. [Google Scholar]
- Seydibeyoglu, M.O.; Misra, M.; Mohanty, A.; Blaker, J.J.; Lee, K.-Y.; Bismarck, A.; Kazemizadeh, M. Green polyurethane nanocomposites from soy polyol and bacterial cellulose. J. Mater. Sci. 2013, 48, 2167–2175. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Du, Q. An Experimental Study on Material and Structural Properties of Structural Insulated Panels (SIPs). Appl. Mech. Mater. 2011, 147, 127–131. [Google Scholar] [CrossRef]
- Zhou, X.; Sain, M.M.; Oksman, K. Semi-rigid biopolyurethane foams based on palm-oil polyol and reinforced with cellulose nanocrystals. Compos. Part Appl. Sci. Manuf. 2016, 83, 56–62. [Google Scholar] [CrossRef]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical Properties of Polyurethanes Produced from Polyols from Seed Oils: II. Foams. J. Am. Oil Chem. Soc. 2007, 84, 65–72. [Google Scholar] [CrossRef]
- Petrović, Z.S. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Leng, W.; Li, J.; Cai, Z. Synthesis and Characterization of Cellulose Nanofibril-Reinforced Polyurethane Foam. Polymers 2017, 9, 597. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Li, Q.; Liu, Y.; Li, J. Ultralight and highly flexible aerogels with long cellulose I nanofibers. Soft Matter 2011, 7, 10360. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jing, S.; Wang, S.; Zhuo, H.; Zhong, L.; Peng, X.; Sun, R. Flexible nanocomposites with ultrahigh specific areal capacitance and tunable properties based on a cellulose derived nanofiber-carbon sheet framework coated with polyaniline. J. Mater. Chem. A 2016, 4, 13352–13362. [Google Scholar] [CrossRef]
- Silva, T.C.F.; Habibi, Y.; Colodette, J.L.; Elder, T.; Lucia, L.A. A fundamental investigation of the microarchitecture and mechanical properties of tempo-oxidized nanofibrillated cellulose (NFC)-based aerogels. Cellulose 2012, 19, 1945–1956. [Google Scholar] [CrossRef]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Pagnocelli, M.; Baldasso, C.; Zattera, A.J. Producing aerogels from silanized cellulose nanofiber suspension. Cellulose 2017, 24, 769–779. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; He, X.; Xiao, M.; Zhang, W.; Lu, C. A super biosorbent from dendrimer poly(amidoamine)-grafted cellulose nanofibril aerogels for effective removal of Cr(vi). J. Mater. Chem. A 2015, 3, 14703–14711. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Barari, B.; Ellingham, T.K.; Ghamhia, I.I.; Pillai, K.M.; El-Hajjar, R.; Turng, L.-S.; Sabo, R. Mechanical characterization of scalable cellulose nano-fiber based composites made using liquid composite molding process. Compos. Part B Eng. 2016, 84, 277–284. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Q.; Zhu, J.; Li, J.; Cai, Z.; Chen, L.; Gong, S. Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv. 2016, 6, 96518–96526. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative Study of Aerogels Obtained from Differently Prepared Nanocellulose Fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Young, T.M.; Liu, P.; Contescu, C.I.; Huang, B.; Wang, S. Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 2015, 22, 435–447. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, X.; Wu, Z.; Wang, S.; Young, T.M. Optimization of cellulose nanofibrils carbon aerogel fabrication using response surface methodology. Eur. Polym. J. 2015, 73, 137–148. [Google Scholar] [CrossRef]
- Olsson, R.T.; Azizi Samir, M.A.S.; Salazar-Alvarez, G.; Belova, L.; Ström, V.; Berglund, L.A.; Ikkala, O.; Nogués, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Anoshkin, I.V.; Nasibulin, A.G.; Ras, R.H.A.; Nonappa, N.; Laine, J.; Kauppinen, E.I.; Ikkala, O. Electrical behaviour of native cellulose nanofibril/carbon nanotube hybrid aerogels under cyclic compression. RSC Adv. 2016, 6, 89051–89056. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Zheng, Q.; Cai, Z.; Turng, L.-S.; Xia, H.; Gong, S. Poly(vinyl alcohol)/Cellulose Nanofibril Hybrid Aerogels with an Aligned Microtubular Porous Structure and Their Composites with Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2015, 7, 7436–7444. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, E.; Zafar, F. Polyurethane: An Introduction. In Polyurethane; Zafar, F., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0726-2. [Google Scholar]
- Li, Y.; Ragauskas, A.J. Cellulose nano whiskers as a reinforcing filler in polyurethanes. Algae 2011, 75, 10–15. [Google Scholar]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Srithep, Y.; Turng, L.-S.; Sabo, R.; Clemons, C. Nanofibrillated cellulose (NFC) reinforced polyvinyl alcohol (PVOH) nanocomposites: Properties, solubility of carbon dioxide, and foaming. Cellulose 2012, 19, 1209–1223. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Ragauskas, A.J. Rigid polyurethane foam reinforced with cellulose whiskers: Synthesis and characterization. Nano-Micro Lett. 2010, 2, 89–94. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Rauhe, J.C.; Jensen, L.R.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M.Á. Evaluation of the thermal conductivity and mechanical properties of water blown polyurethane rigid foams reinforced with carbon nanofibers. Eur. Polym. J. 2018, 108, 98–106. [Google Scholar] [CrossRef]
- Fu, J.; He, C.; Huang, J.; Chen, Z.; Wang, S. Cellulose nanofibril reinforced silica aerogels: Optimization of the preparation process evaluated by a response surface methodology. RSC Adv. 2016, 6, 100326–100333. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Furtwengler, P.; Matadi Boumbimba, R.; Sarbu, A.; Avérous, L. Novel Rigid Polyisocyanurate Foams from Synthesized Biobased Polyester Polyol with Enhanced Properties. ACS Sustain. Chem. Eng. 2018, 6, 6577–6589. [Google Scholar] [CrossRef]
- Mariappan, T.; Khastgir, D.; Singha, N.; Manjunath, B.S.; Naik, Y.P. Mechanical, Morphological and Thermal Properties of Rigid Polyurethane Foam: Effect of the Fillers. Cell. Polym. 2007, 26, 245–259. [Google Scholar]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Strankowski, M.; Włodarczyk, D.; Piszczyk, Ł.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. J. Spectrosc. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ren, Z.; Yin, Z.; Qian, H.; Ma, D. Amide II and Amide III Bands in Polyurethane Model Soft and Hard Segments. Polym. Bull. 2008, 60, 97–101. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High bio-content polyurethane (PU) foam made from bio-polyol and cellulose nanocrystals (CNCs) via microwave liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Yusuf, A.K.; Mamza, P.A.P.; Ahmed, A.S.; Agunwa, U. Physico-Mechanical Properties of Rigid Polyurethane Foams Synthesized From Modified Castor Oil Polyols. Int. J. Sci. Res. Publ. 2016, 6, 9. [Google Scholar]
- Ali, E.S.; Zubir, S.A. The Mechanical Properties of Medium Density Rigid Polyurethane Biofoam. MATEC Web Conf. 2016, 39, 01009. [Google Scholar] [CrossRef]
- Ugarte, L.; Gómez-Fernández, S.; Peña-Rodríuez, C.; Prociak, A.; Corcuera, M.A.; Eceiza, A. Tailoring Mechanical Properties of Rigid Polyurethane Foams by Sorbitol and Corn Derived Biopolyol Mixtures. ACS Sustain. Chem. Eng. 2015, 3, 3382–3387. [Google Scholar] [CrossRef]
- Goods, S.H.; Neuschwanger, C.L.; Whinnery, L.L. Mechanical Properties of a Structural Polyurethane Foam and the Effect of Particulate Loading. MRS Proc. 1998, 521. [Google Scholar] [CrossRef]
- Fan, H.; Tekeei, A.; Suppes, G.J.; Hsieh, F.-H. Properties of Biobased Rigid Polyurethane Foams Reinforced with Fillers: Microspheres and Nanoclay. Int. J. Polym. Sci. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Stirna, U.; Beverte, I.; Yakushin, V.; Cabulis, U. Mechanical properties of rigid polyurethane foams at room and cryogenic temperatures. J. Cell. Plast. 2011, 47, 337–355. [Google Scholar] [CrossRef]
- Technical Data for Structural Insulated Panels | Foard Panel. Available online: https://www.foardpanel.com/technical-data/ (accessed on 13 February 2019).
- Wiyono, P.; Suprobo, P.; Kristijanto, H. Characterization of physical and mechanical properties of rigid polyurethane foam. ARPN J. Eng. Appl. Sci. 2016, 11, 8. [Google Scholar]
- Kirpluks, M.; Cabulis, U.; Zeltins, V.; Stiebra, L.; Avots, A. Rigid Polyurethane Foam Thermal Insulation Protected with Mineral Intumescent Mat. Autex Res. J. 2014, 14. [Google Scholar] [CrossRef]
- Wu, J.-W.; Sung, W.-F.; Chu, H.-S. Thermal conductivity of polyurethane foams. Int. J. Heat Mass Transf. 1999, 42, 2211–2217. [Google Scholar] [CrossRef]
- Beltrán, A.A.; Boyacá, L.A. Production of rigid polyurethane foams from soy-based polyols. Lat. Am. Appl. Res. 2011, 41, 75–80. [Google Scholar]
- Jarfelt, U.; Ramnäs, O. Thermal conductivity of polyurethane foam Best performance. In Proceedings of the 10th International Symposium on District Heating and Cooling, Hannover, Germany, 3–5 September 2006; pp. 1–12. [Google Scholar]
- Traeger, R.K. Physical Properties of Rigid Polyurethane Foams. J. Cell. Plast. 1967, 3, 405–418. [Google Scholar] [CrossRef]
- Fleurent, H.; Thijs, S. The Use of Pentanes as Blowing Agent in Rigid Polyurethane Foam. J. Cell. Plast. 1995, 31, 580–599. [Google Scholar] [CrossRef]


| Chemicals | Parts by Weight (Pbw) | Equivalent Weight (Eq.wt.) | Role | ||
|---|---|---|---|---|---|
| PUF0 | PUF20 | PUF30 | |||
| PEG-400 | 100 | 80 | 70 | 198 | Polyol |
| Spray-dried CNF | 0 | 20 | 30 | 186 | Polyol, reinforcing agent |
| DABCO T12 | 3 | 3 | 3 | 0 | Catalyst |
| DABCO DC5357 | 1 | 1 | 1 | 100 | Surfactant |
| Deionized water | 0.8 | 0.8 | 0.8 | 9 | Blowing agent |
| PAPITM 27 | 88 | 89 | 90 | 133 | Reactive prepolymer |
| Sample ID | Density | Closed Cell Content | Mean Cell Size | Thermal Conductivity |
|---|---|---|---|---|
| g/cm3 | % | µm | W/mK | |
| PUF0 | 0.059 ± 0.0009 | 89.1 ± 1.01 | 741 ± 98 | 0.04390 ± 0.0015 |
| PUF20 | 0.050 ± 0.0008 | 91.2 ± 0.63 | 634 ± 79 | 0.03014 ± 0.00089 |
| PUF30 | 0.051 ± 0.0011 | 91.9 ± 0.54 | 589 ± 73 | 0.02724 ± 0.00087 |
| Sample ID | Specific Bending Modulus | Specific Bending Strength | Specific Compression Modulus | Specific Compression Strength | Specific Tensile Modulus | Specific Tensile Strength |
|---|---|---|---|---|---|---|
| Gpa*cm3/g | MPa*cm3/g | MPa*cm3/g | MPa*cm3/g | MPa*cm3/g | MPa*cm3/g | |
| PUF0 | 9.38 ± 0.95 | 103.07 ± 4.44 | 11.90 ± 0.99 | 1.52 ± 0.10 | 12.62 ± 0.57 | 1.41 ± 0.089 |
| PUF20 | 18.95 ± 0.88 | 177.38 ± 6.30 | 37.05 ± 1.35 | 3.67 ± 0.18 | 29.14 ± 1.19 | 1.62 ± 0.073 |
| PUF30 | 34.95 ± 0.93 | 319.00 ± 10.37 | 51.25 ± 0.58 | 4.55 ± 0.14 | 127.31 ± 3.81 | 3.4 ± 0.072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, W.; Pan, B. Thermal Insulating and Mechanical Properties of Cellulose Nanofibrils Modified Polyurethane Foam Composite as Structural Insulated Material. Forests 2019, 10, 200. https://doi.org/10.3390/f10020200
Leng W, Pan B. Thermal Insulating and Mechanical Properties of Cellulose Nanofibrils Modified Polyurethane Foam Composite as Structural Insulated Material. Forests. 2019; 10(2):200. https://doi.org/10.3390/f10020200
Chicago/Turabian StyleLeng, Weiqi, and Biao Pan. 2019. "Thermal Insulating and Mechanical Properties of Cellulose Nanofibrils Modified Polyurethane Foam Composite as Structural Insulated Material" Forests 10, no. 2: 200. https://doi.org/10.3390/f10020200
APA StyleLeng, W., & Pan, B. (2019). Thermal Insulating and Mechanical Properties of Cellulose Nanofibrils Modified Polyurethane Foam Composite as Structural Insulated Material. Forests, 10(2), 200. https://doi.org/10.3390/f10020200






