An Emerging Pathogen from Rotted Chestnut in China: Gnomoniopsis daii sp. nov.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation
2.2. DNA Extraction and Phylogenetic Analysis
2.3. Morphological Identification and Characterization
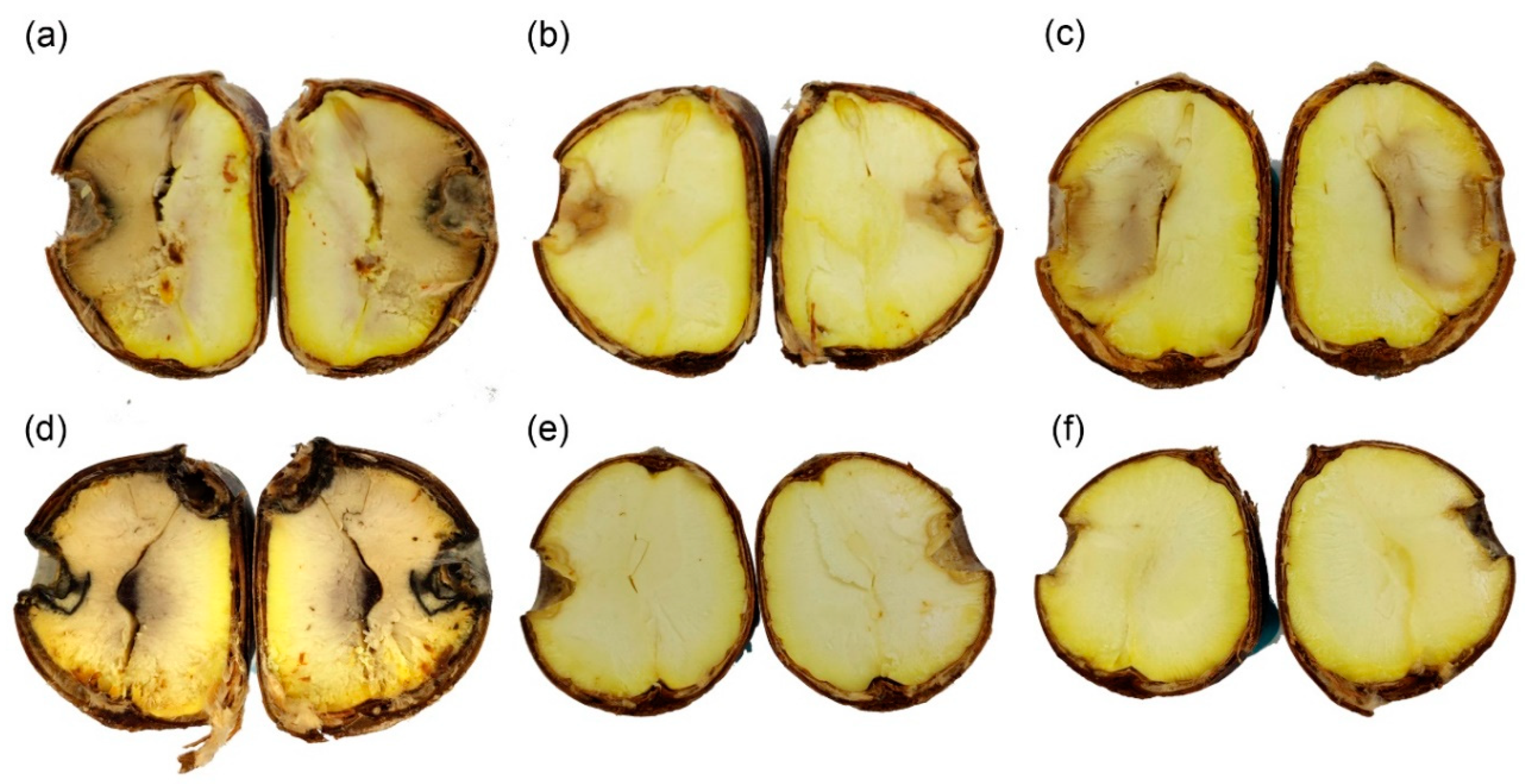
2.4. Pathogenicity Trials
3. Results
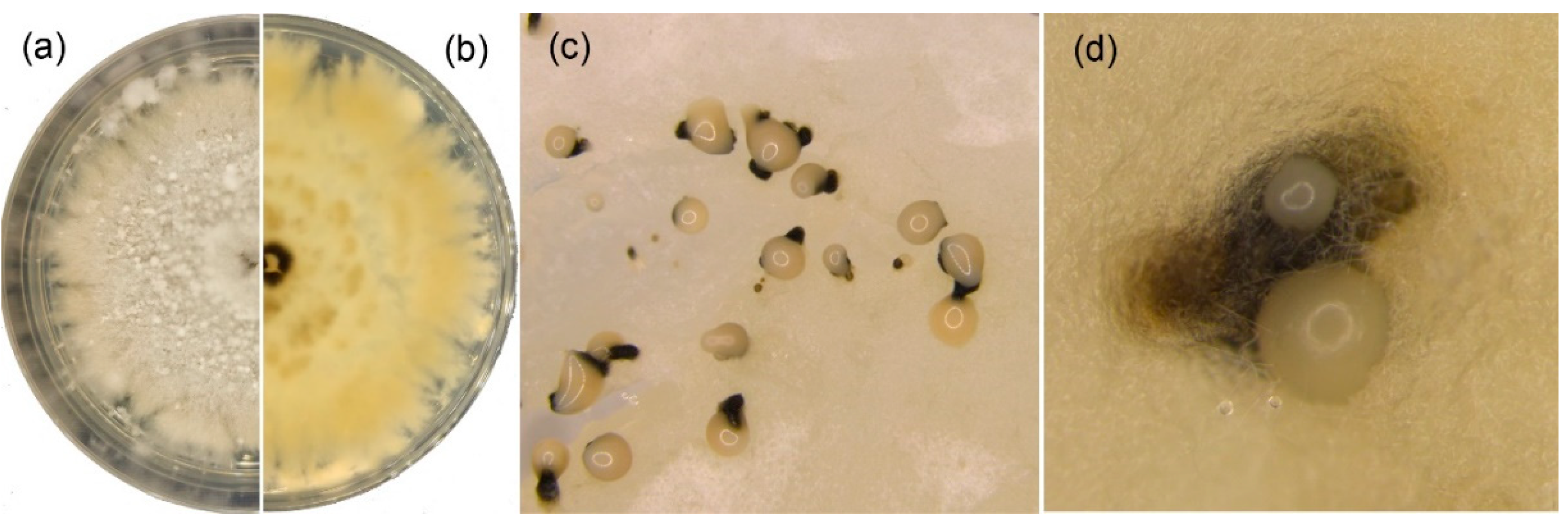
3.1. Fungal Isolation and Identification
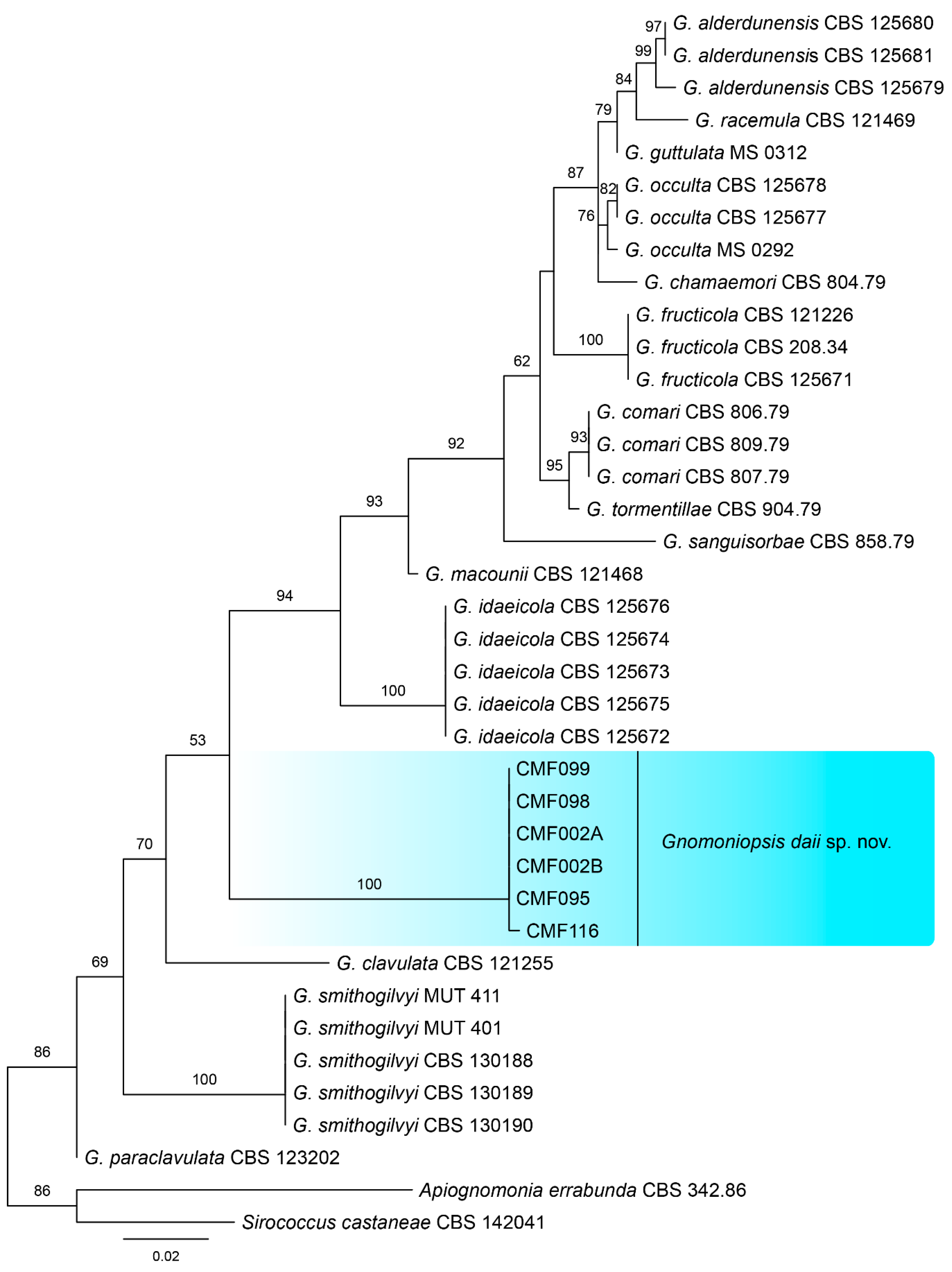
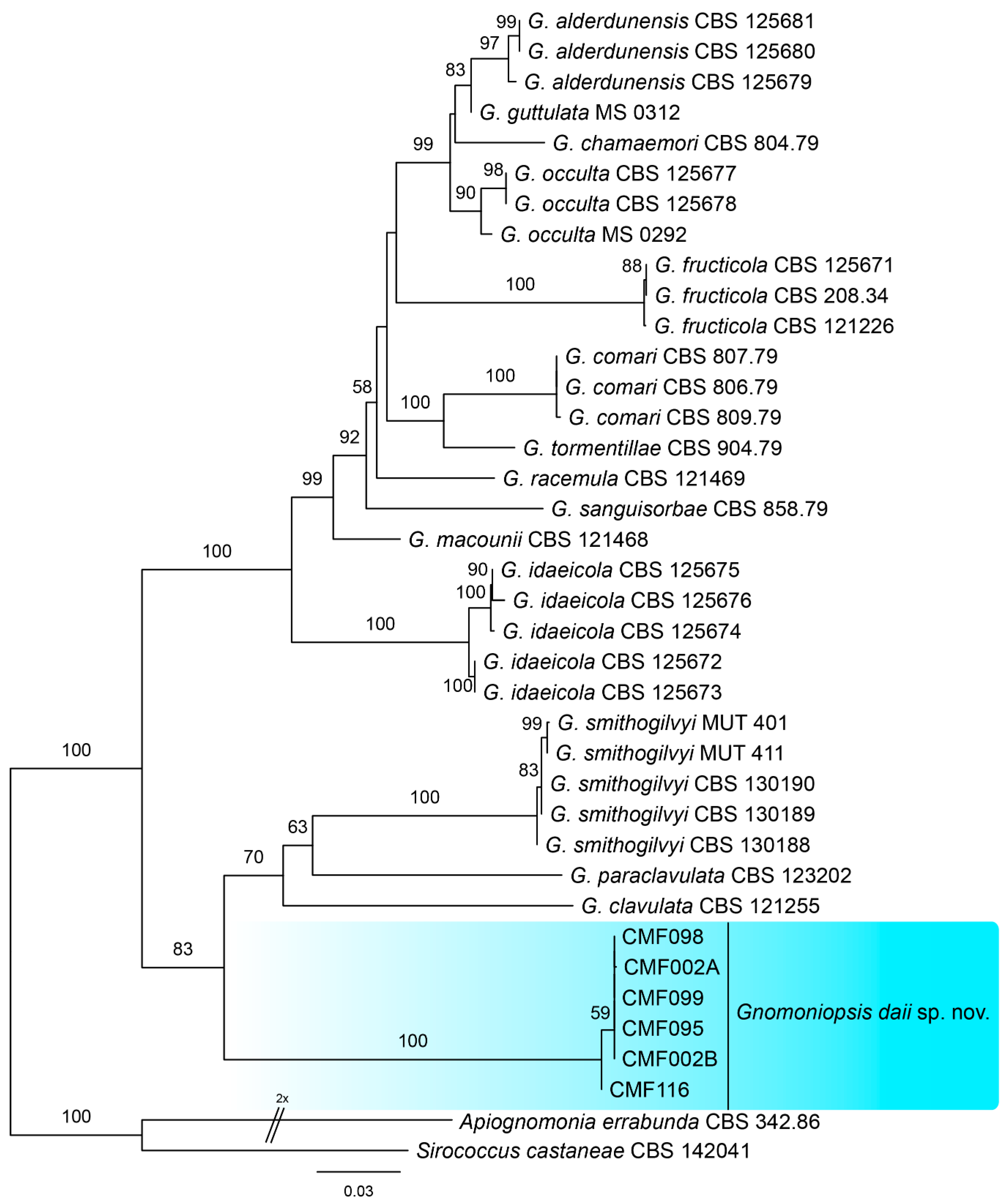
3.2. Phylogeny
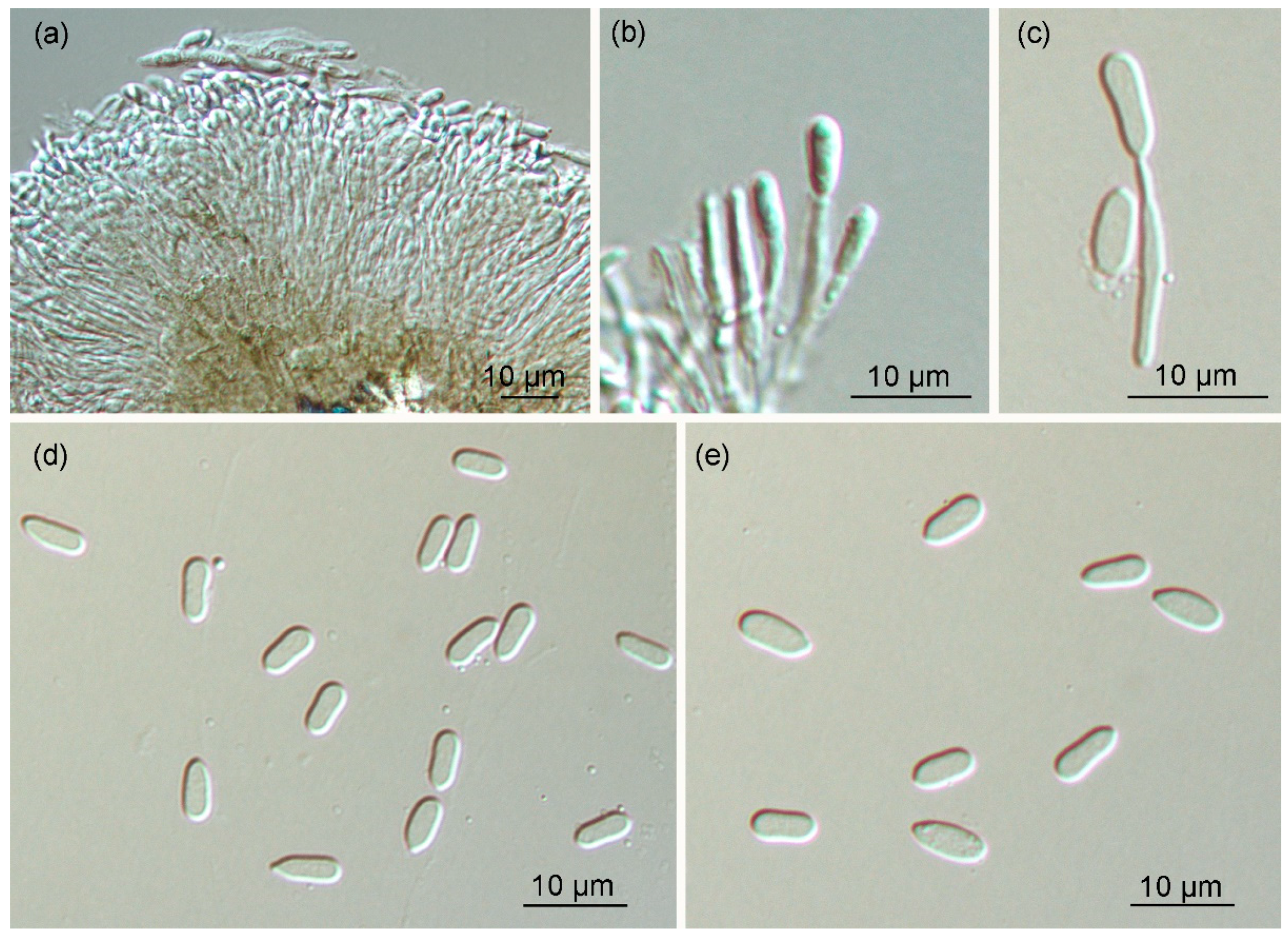
3.3. Morphology and Taxonomy
3.4. Pathogenicity Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, S.J. Situation and development strategy of chestnut industry in China. J. West China For. Sci. 2017, 46, 132–149. [Google Scholar] [CrossRef]
- Lu, C.; Guo, S.J. Analysis on the nutritional characters and comprehensive evaluation of 16 chestnut germplasm resources. Sci. Technol. Food Ind. 2017, 37, 357–376. [Google Scholar] [CrossRef]
- Wang, J.R.; Liu, M.C.; Yang, L.; Min, Q.W. Factors affecting the willingness of farmers to accept eco-compensation in the Qianxi chestnut agroforestry system, Hebei. J. Resour. Ecol. 2018, 9, 407–415. [Google Scholar] [CrossRef]
- He, W.; Shen, R.X.; Wang, X.J. Pathogenicity of pathogens contributing to dry rot of Chinese chestnut and their infection process. J. Beijing For. Univ. 2001, 23, 36–39. [Google Scholar]
- He, W.; Yin, W.L.; Shen, R.X.; Wang, X.J. Latent infection of fungal pathogens and pathogenesis of Chinese chestnut seed rot. Sci. Silvae Sin. 2004, 40, 96–102. [Google Scholar]
- Hou, B.L.; Zhang, Z.M.; Yang, X.M.; Shang, J.L.; Xu, F.S.; Ma, C.F. The study on the kernel spot diseases of Chinese chestnut in Hebei. J. Hebei Agric. Univ. 1988, 11, 11–22. [Google Scholar]
- Zhu, J.R.; Lian, Y.Y.; Hua, Z.Y. Preliminary exploration into the cause of Chinese chestnut putrity in storage. J. Zhejiang For. Technol. 1992, 12, 44–46. [Google Scholar]
- Crous, P.W.; Summerell, B.A.; Shivas, R.G.; Burgess, T.I.; Decock, C.A.; Dreyer, L.L.; Granke, L.L.; Guest, D.I.; Hardy, G.E.St.J.; Hausbeck, M.K.; et al. Fungal Planet Description Sheets: 107–127. Persoonia 2012, 28, 138–182. [Google Scholar] [CrossRef]
- Dennert, F.G.; Broggini, G.A.; Gessler, C.; Storari, M. Gnomoniopsis castanea is the main agent of chestnut nut rot in Switzerland. Phytopathol. Mediterr. 2015, 54, 199–211. [Google Scholar]
- Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Franceschini, A.; Alves, A.; Abdollahzadeh, J.; Phillips, A.J.L. Phylogeny, morphology and pathogenicity of Botryosphaeriaceae, Diatrypaceae and Gnomoniaceae associated with branch diseases of hazelnut in Sardinia (Italy). Eur. J. Plant. Pathol. 2016, 146, 259–279. [Google Scholar] [CrossRef]
- Pasche, S.; Calmin, G.; Auderset, G.; Crovadore, J.; Pelleteret, P.; Mauch-Mani, B.; Barja, F.; Paul, B.; Jermini, M.; Lefort, F. Gnomoniopsis smithogilvyi causes chestnut canker symptoms in Castanea sativa shoots in Switzerland. Fungal Genet. Biol. 2016, 87, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Seddaiu, S.; Cerboneschi, A.; Sechi, C.; Mello, A. Gnomoniopsis castaneae associated with Dryocosmus kuriphilus galls in chestnut stands in Sardinia (Italy). IFOREST 2017, 10, 440–445. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Guest, D.I. The infection process of chestnut rot, an important disease caused by Gnomoniopsis smithogilvyi (Gnomoniaceae, Diaporthales) in Oceania and Europe. Australas. Plant. Pathol. 2017, 46, 397–405. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Liew, E.C.Y.; Guest, D.I. Survey of the incidence of chestnut rot in south-eastern Australia. Australas. Plant. Pathol. 2013, 42, 63–72. [Google Scholar] [CrossRef]
- Şimşek, S.A.; Katircioğlu, Y.Z.; Serçe, Ç.U.; Çakar, D.; Rigling, D.; Maden, S. Phytophthora species associated with dieback of sweet chestnut in Western Turkey. For. Pathol. 2019, 49, e12533. [Google Scholar] [CrossRef]
- Meyer, J.B.; Trapiello, E.; Senn-Irlet, B.; Sieber, T.N.; Cornejo, C.; Aghayeva, D.; González, A.J.; Prosperoa, S. Phylogenetic and phenotypic characterisation of Sirococcus castaneae comb. nov. (synonym Diplodina castaneae), a fungal endophyte of European chestnut. Fungal Biol. UK 2017, 121, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant. Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Walker, D.M.; Guest, D.I. The chestnut pathogen Gnomoniopsis smithogilvyi (Gnomoniaceae, Diaporthales) and its synonyms. Mycotaxon 2016, 130, 929–940. [Google Scholar] [CrossRef]
- Trapiello, E.; Feito, I.; González, A.J. First Report of Gnomoniopsis castaneae Causing Canker on Hybrid Plants of Castanea sativa× C. crenata in Spain. Plant. Dis. 2018, 102, 1040. [Google Scholar] [CrossRef]
- Visentin, I.; Gentile, S.; Valentino, D.; Gonthier, P.; Tamietti, G.; Cardinale, F. Gnomoniopsis castanea sp. nov.(Gnomoniaceae, Diaporthales) as the causal agent of nut rot in sweet chestnut. J. Plant. Pathol. 2012, 94, 411–419. [Google Scholar]
- Sogonov, M.V.; Castlebury, L.A.; Rossman, A.Y.; Mejía, L.C.; White, J.F. Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Stud. Mycol. 2008, 62, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.E. The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycol. Mem. 1978, 7, 1–232. [Google Scholar]
- Walker, D.M.; Castlebury, L.A.; Rossman, A.Y.; Sogonov, M.V.; White, J.F. Systematics of genus Gnomoniopsis (Gnomoniaceae, Diaporthales) based on a three gene phylogeny, host associations and morphology. Mycologia 2010, 102, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]

| Species | Country | Host | Strain | GenBank Accession Number | ||
|---|---|---|---|---|---|---|
| Internal Transcribed Spacer (ITS) | tub2 | tef1 | ||||
| Apiognomonia veneta | France | Platanus occidentalis | CBS 342.86 | DQ313531 | EU219235 | DQ318036 |
| Gnomoniopsis alderdunensis | USA | Rubus pedatus | CBS 125679 | GU320826 | GU320788 | GU320813 |
| Gnomoniopsis alderdunensis | USA | Rubus parviflorus | CBS 125680 | GU320825 | GU320787 | GU320801 |
| Gnomoniopsis alderdunensis | USA | Rubus parviflorus | CBS 125681 | GU320827 | GU320789 | GU320802 |
| Gnomoniopsis chamaemori | Finland | Rubus chamaemorus | CBS 804.79 | GU320817 | GU320777 | GU320809 |
| Gnomoniopsis clavulata | USA | Quercus falcata | CBS 121255 | EU254818 | EU219211 | GU320807 |
| Gnomoniopsis comari | Finland | Comarum palustre | CBS 806.79 | EU254821 | EU219156 | GU320810 |
| Gnomoniopsis comari | Finland | Comarum palustre | CBS 807.79 | EU254822 | GU320779 | GU320814 |
| Gnomoniopsis comari | Switzerland | Comarum palustre | CBS 809.79 | EU254823 | GU320778 | GU320794 |
| Gnomoniopsis daii | China | Castanea mollissima | CMF002A | MN598671 | MN605519 | MN605517 |
| Gnomoniopsis daii | China | Castanea mollissima | CMF002B | MN598672 | MN605520 | MN605518 |
| Gnomoniopsis daii | China | Castanea mollissima | CMF095 | MN598673 | NA | NA |
| Gnomoniopsis daii | China | Castanea mollissima | CMF098 | MN598674 | NA | NA |
| Gnomoniopsis daii | China | Castanea mollissima | CMF099 | MN598675 | NA | NA |
| Gnomoniopsis daii | China | Castanea mollissima | CMF116 | MN598676 | NA | NA |
| Gnomoniopsis fructicola | USA | Fragaria vesca | CBS 121226 | EU254824 | EU219144 | GU320792 |
| Gnomoniopsis fructicola | France | Fragaria sp. | CBS 208.34 | EU254826 | EU219149 | GU320808 |
| Gnomoniopsis fructicola | USA | Fragaria sp. | CBS 125671 | GU320816 | GU320776 | GU320793 |
| Gnomoniopsis guttulata | Bulgaria | Agrimonia eupatoria | NA | EU254812 | NA | NA |
| Gnomoniopsis idaeicola | USA | Rubus sp. | CBS 125672 | GU320823 | GU320781 | GU320797 |
| Gnomoniopsis idaeicola | USA | Rubus pedatus | CBS 125673 | GU320824 | GU320782 | GU320798 |
| Gnomoniopsis idaeicola | France | Rubus sp. | CBS 125674 | GU320820 | GU320780 | GU320796 |
| Gnomoniopsis idaeicola | USA | Rubus procerus | CBS 125675 | GU320822 | GU320783 | GU320799 |
| Gnomoniopsis idaeicola | USA | Rubus procerus | CBS 125676 | GU320821 | GU320784 | GU320811 |
| Gnomoniopsis macounii | USA | Spiraea sp. | CBS 121468 | EU254762 | EU219126 | GU320804 |
| Gnomoniopsis occulta | USA | Potentilla sp. | CBS 125677 | GU320828 | GU320785 | GU320812 |
| Gnomoniopsis occulta | USA | Potentilla sp. | CBS 125678 | GU320829 | GU320786 | GU320800 |
| Gnomoniopsis occulta | Russia | Potentilla anserina | NA | EU254811 | NA | NA |
| Gnomoniopsis paraclavulata | USA | Quercus alba | CBS 123202 | GU320830 | GU320775 | GU320815 |
| Gnomoniopsis racemula | USA | Chamerion angustifolium | CBS 121469 | EU254841 | EU219125 | GU320803 |
| Gnomoniopsis sanguisorbae | Switzerland | Sanguisorba minor | CBS 858.79 | GU320818 | GU320790 | GU320805 |
| Gnomoniopsis smithogilvyi | Australia | Castanea sp. | CBS 130190 | JQ910642 | JQ910639 | KR072534 |
| Gnomoniopsis smithogilvyi | Australia | Castanea sp. | CBS 130189 | JQ910644 | JQ910641 | KR072535 |
| Gnomoniopsis smithogilvyi | Australia | Castanea sp. | CBS 130188 | JQ910643 | JQ910640 | KR072536 |
| Gnomoniopsis smithogilvyi | Italy | Castanea sativa | MUT 401 | HM142946 | KR072532 | KR072537 |
| Gnomoniopsis smithogilvyi | New Zealand | Castanea sativa | MUT 411 | HM142948 | KR072533 | KR072538 |
| Gnomoniopsis tormentillae | Switzerland | Potentilla sp. | CBS 904.79 | EU254856 | EU219165 | GU320795 |
| Sirococcus castaneae | Switzerland | Castanea sativa | CBS 142041 | KX929744 | KX958443 | KX929710 |
| Strain | No. of Affected Nuts | No. of Asymptomatic Nuts |
|---|---|---|
| CMF002A | 26 | 4 |
| CMF002B | 24 | 6 |
| CMF095 | 22 | 8 |
| CMF098 | 23 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Tian, C. An Emerging Pathogen from Rotted Chestnut in China: Gnomoniopsis daii sp. nov. Forests 2019, 10, 1016. https://doi.org/10.3390/f10111016
Jiang N, Tian C. An Emerging Pathogen from Rotted Chestnut in China: Gnomoniopsis daii sp. nov. Forests. 2019; 10(11):1016. https://doi.org/10.3390/f10111016
Chicago/Turabian StyleJiang, Ning, and Chengming Tian. 2019. "An Emerging Pathogen from Rotted Chestnut in China: Gnomoniopsis daii sp. nov." Forests 10, no. 11: 1016. https://doi.org/10.3390/f10111016
APA StyleJiang, N., & Tian, C. (2019). An Emerging Pathogen from Rotted Chestnut in China: Gnomoniopsis daii sp. nov. Forests, 10(11), 1016. https://doi.org/10.3390/f10111016





