Abstract
Tropical cyclones (hurricanes and typhoons) cause large-scale disturbances in forest ecosystems all over the world. In the summer of 2016, a strong tropical cyclone named Lionrock created windthrow patches in the area of more than 400 km2 on the forested eastern slopes of the Sikhote-Alin Range, in the Russian Far East. Such large-scale forest destruction by wind had never been recorded in the area prior to this event. We examined the tropical cyclone impact upon the forest composition, structure and tree mortality rates on two study sites (1 ha and 0.5 ha in size)—a contiguous windthrow patch site, and a site with partial canopy damage. Korean pine (Pinus koraiensis Siebold and Zucc.), Manchurian fir (Abies nephrolepis Trautv.) and Dahurian larch (Larix cajanderi Mayr.) were the primary tree species represented in the affected forest communities. Combined with the partial canopy damage, 7.7% of trees were blown down by the disturbance event. We determined that this one event mortality rate nearly equaled the average mortality rate for a ten year period for these forests (8.5 ± 4.0%) under normal conditions (no large-scale disturbances). Within a contiguous windthrow patch, tree mortality was determined to be 52.6%, which is significantly higher than the cumulative tree loss for the previous 50 years (42.4%). A substantial portion of thinner-stemmed trees (DBH (diameter measured at breast height) < 30 cm) were wind snapped, and those with larger diameters (DBH > 60 cm) were uprooted. Our results indicate that the probability of tree loss due to catastrophic wind loads increases as a result of the decrease in local density. We believe that tree loss estimates should include the impacts within contiguous patches of windthrows, as well as the patches with only partial tree canopy damage. Strong wind impact forecasting is possible with accounting for species composition within the stand sites and their spatial structure.
Keywords:
forest structure; windthrow; wind; disturbance; mortality; tropical cyclone; Korean pine; Lionrock 1. Introduction
Impact on the forest ecosystem caused by strong winds during typhoons (referred to as hurricanes in the Western Hemisphere) have been reported in many studies [1,2]. Wind disturbances are recognized to produce a key effect on forest dynamics and stability [3,4,5,6,7,8]. Strong winds are the cause of tree crown damages that lead to tree death in temperate and boreal forests.
As a consequence, changes in forest structure, as well as tree stand and understory species composition follow, accompanied by an increase of fire hazards and a decrease of carbon storage capacity after salvage logging [9,10,11]. In contrast, tropical forests are located in the areas where typhoons are more common, and tree mortality rates caused by strong wind are comparable to baseline mortality in temperate and boreal forests. This can be explained by the complex structure of tropical forest ecosystems adapted to regular heavy wind loads, where wind disturbances play a significant role in the formation and maintenance of the forests’ high biodiversity [12,13].
The Russian Far East is located in the area affected by cyclones originating in the tropical parts of the Pacific Ocean. Their biggest impacts fall upon the coast continental and island territories of East Asia including China, Korea and Japan [14]. Gradually losing their intensity, the most powerful tropical cyclones are capable of reaching 56–58 degrees north. In spite of the wind speed reduction at high latitudes [15], where these typhoons are termed “tropical depressions” as per World Meteorological Organization (WMO) classification, they are still able to significantly impact boreal forest ecosystems [16]. Strong winds will be able to make considerable effect on boreal forest ecosystems in the future, since frequency and poleward migration of tropical cyclones increased considerably during the last 50 years [17,18] due to global climate changes [19,20].
Massive windthrow events in the Middle Sikhote-Alin Mountain Range (Primorsky Region, Russia) were caused by tropical cyclone Lionrock that began its activity on 15 August, 2016 in the western part of the Pacific Ocean. Gradually increasing in strength, the tropical depression approached the east coast of Japan on 19 August. The typhoon changed its trajectory twice, and then crossed the territory of Japan entering from the Pacific coastal side on the 29th of August, causing much destruction and economic loss. On the night of 31 August (local time, Greenwich Mean Time + 10), tropical cyclone Lionrock reached the coastline of the continent (the eastern macroslope of the Middle Sikhote-Alin Range, Russian Far East) with wind gusts of 30 m·s−1 (108 km·h−1 or 67 mph) and subsequently moved further to Northeast China, transforming into an extratropical cyclone [21]. Cumulative precipitation for the cyclone event amounted to 70 mm [22]. According to various estimates, the overall area of windthrows on the eastern macroslope of the Sikhote-Alin Range adds up to about 400 km2. Wind disturbances of such scale had never been recorded in the Primorsky Region.
Recently, a number of authors reported on the probable shift of tropical cyclone trajectories to the North [18,23,24,25], which is attributed to global climate change. Thus, the probability of strong wind impact on temperate and boreal forest dynamics in the south of the Russian Far East, previously free of such extreme events, will possibly increase in the near future.
In this paper, we report on our study to estimate the impact of tropical cyclone Lionrock on the structure of the zonal forests of the region, based on observations within the windthrow patch. The core of these analyses was an evaluation of four hypotheses related to forest impacts as follows: (1) Tree death probability is species dependent; (2) tree death probability is size dependent; (3) hollowed and rotten trees are more likely to fall; (4) windthrow distribution is grouped into patches. To test these hypotheses, we analyzed the spatial structure of a tree stand on a one ha permanent plot which was established at a location of strong wind disturbance. We also provided a quantitative assessment of the cyclone-damaged trees.
2. Materials and Methods
2.1. Study Area
This study was conducted in the Sikhote-Alin Nature Reserve (45°20′ N, 136°09′ E) located in the central part of the Sikhote-Alin Range, Russian Far East (Figure 1 and Figure S1). The climate of this region is monsoon with a cold dry winter, rainy summer and seasonal changes in wind direction. For the period 1981 to 2019, the mean annual temperature was +3.9 °C. January was the coldest month (−12.3 °C average) and August was the warmest (+18.0 °C average). Mean annual precipitation was 828 mm, most of which fell from May to September [26].
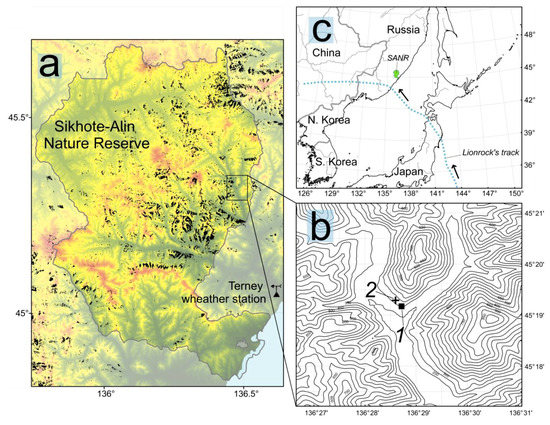
Figure 1.
Of the permanent plots PP1 (first permanent plot)and PP2 (second permanent plot) (b) in the Sikhote-Alin Nature Reserve (SANR). The dashed line (c) stands for tropical cyclone Lionrock trajectory. Forest areaswith windtrow disturbance are denoted as black (a) (based on data provided by the GlobalForestWatch: https://www.globalforestwatch.org/).
According to the Köppen–Geiger climate classification, the climate type here is humid continental, characterized by a warm summer (Dwb) [27]. The bioclimate type is temperate/boreal marine [28].
Zonal forest communities are represented by the Korean Pine-broadleaved forest (Pinus koraiensis Siebold and Zucc.). Mountain forests within the Sikhote-Alin Nature Reserve boundaries have not suffered catastrophic disturbances in recent times and therefore, the old-growth forest ecosystems are dominated by Pinus koraiensis. Other species, including Picea ajanensis Fisch. and Carr., Abies nephrolepis (Trautv.) Maxim., Larix cajanderi Mayr,Tilia amurensis Rupr., Quercus mongolica Fisch. ex Ledeb., Acer mono Maxim. ex Rupr. and Betula costata Trautv. are also present. Understory species include Corylus mandshurica Maxim., Ribes maximoviczianum Kom., Lonicera chrysantha Turcz. ex Ledeb, L. maximowiczii (Rupr.) Regel, Rosa acicularis Lindl., Spiraea betulifolia Pall., and Actinidia kolomikta (Maxim. andRupr.) Maxim.
2.2. Field Methods
The first permanent plot (PP1, 100 m × 100 m) was established in 2017 within the windthrow patch of the mountain old-growth Korean Pine-broadleaved forest at 270 m a.s.l. on a slope with southern exposure. The study was conducted in 2017–2018. After marking the permanent plot with laser range finder TruPulse 200 (USA), we determined local coordinates within 0.01 m (1 cm) of all trees having a diameter measured at breast height (DBH) greater than or equal to 6 cm. For dead trees, DBH was also measured and the type of mortality noted as uprooted or snapped. Locations of the uprooted trees were determined at the center of their root pits, while the coordinates for the snapped trees were taken at the base of the trunk. Both height and DBH were measured for each living tree with the tree health status (healthy or damaged). The trees killed prior to cyclone Lioncrock were recorded separately. For Pinus koraiensis and Larix cajanderi trees, both living and dead, core samples were collected using increment borers (Haglof, Sweden). Each sample was inspected visually for stem rot. Cores were collected only from medium to large canopy trees to be sure that these trees fell due to the direct tropical cyclone influence, and not because of the impact of other falling trees.
The baseline mortality rate of Korean Pine-broadleaved forest was estimated on the second 0.5 ha permanent plot (PP2, 50 m × 100 m) established in 1967 and located at a 100 m distance from the windthrow patch used as the described above permanent plot. There was no massive tree disturbance by tropical cyclone Lionrock in this part of the forest. We used available data on tree mortality and recorded diameters (DBH ≥ 6 cm) observed every 5–10 years for the 50 years period 1967 to 2016 inclusive, as well as most recent data collected in 2017. Then, we calculated mean baseline mortality as loss of stem density and total basal area values per each 10 year relative to total stem density and total basal area at the time of measurement in 1967 (data converted to one ha). Subsequently, the mean 10-year baseline mortality rate was calculated and accepted as a natural disturbance regime without large-scale windthrow events and later compared with tropical cyclone-induced mortality on PP2.
2.3. Statistical Analyses
We analyzed the size structure of each tree species (DBH distribution) with a sufficient number of individuals: Abies nephrolepis (n = 383), Pinus koraiensis (n = 246), Larix cajanderi (n = 35). Broadleaved species (Acer mono, Betula costata, Quercu smongolica and Tilia amurensis Mill., n = 81) were combined, and their overall size structure was also calculated. Stem density and basal area of the tree stand before and after the disturbance were also taken into account. Significance of differences between mean values was estimated using t-test. Before applying t-test, all data samples were tested against normality using the Kolmogorov-Smirnov test. Further, using t-test, we compared pre- and post-disturbance tree populations, healthy and damaged ones, and finally, uprooted and snapped tree populations.
Chi-squared test (χ2) was applied to test dependence between tree diameter and stem rot presence. Also, we used this same chi-square test to discover the relationship between the local density of spatial tree distribution (kernel density estimation was used) and the probability of a tree to be either uprooted or snapped. Trees were separated into tree size classes depending on their diameter: small (6–29 cm), medium (30–60 cm), and large (>60 cm). Based on the Bernoulli trial scheme, tree mortality probability (P ± Δ), as dependent on size class and species, was calculated with or without the type of death categorization [29].
Probability assessments of tree fall during the cyclone event and its dependency on local environmental conditions were based on logistic regression [30]. The following set of predictors were examined: (1) tree diameter at breast height (DBH); (2) tree height (H); (3) local density of tree stand composition (more precisely, probability density estimation of tree presence) (P); (4) presence of a hollow in wood (W). Missing height values (snapped trees) were estimated using a Curtis model which describes the relationship between tree diameter and its height [31]. The nonlinear Curtis regression model was fitted to all available data obtained by combining datasets from both PP1 and PP2. To estimate the local density of tree distribution, we applied a 2D kernel density estimator (kde2d function) from the MASS package available in R [32]. Before fitting logistic regression, we tested data against multicollinearity using the omcdiag function from the mctest R-package. To get assessments on spatial tree structure we calculated Ripley’s L(r)- and L12(r)-functions [33]. The first is Ripley’s K-function normalized to be equal to zero for the Poisson point process, that was chosen as a model for complete spatial randomness. The second, the L12(r)-function, is a generalization of the first one, allowing researchers to make decisions regarding the mutual aggregation and segregation of points belonging to different virtual types 1 and 2. If L12(r) tends to be small, points of type 1 and 2 spatially avoid each other, and otherwise they are mutually aggregated. Ripley’s L(r)- and L12(r)-functions were used to describe spatial univariate and bivariate patterns of the trees according to Equations (1) and (2):
where A is the permanent plot area, n is the number of trees, np and nq are the number of points in class 1 and class 2, δ(rij) then is the indicator function of the mean number of neighbors within a circle around each tree with the distance r being the radius, and rij is the distance from tree i to tree j.
The Monte-Carlo method with 999 generations was used for the construction of confidence limits (null hypothesis acceptation area), null hypothesis bias estimation and to a get significant value of 1%. Functions L(r) and L12(r) were calculated using Programita software (http://programita.org/), [34]. The Null hypothesis of complete spatial randomness (univariate analysis) was tested to compare tree spatial structure before and after cyclone Lionrock impact (pre-disturbance and post-disturbance tree populations). Further on, the spatial patterns of uprooted and snapped trees were analyzed. Function value L(r) within confidence limits indicates the random distribution of trees within the analyzed distances. Values above the upper confidence limit indicate tree aggregation, while those below the lower confidence limit show a segregation of trees. Distance values between trees, critical to the downfall of living trees caused by the fall of dead trees and resulting in considerable destructions, were estimated using antecedent conditions hypothesis (bivariate analysis). The function value L12(r) within confidence limits indicates the independent interaction between the spatial distribution of trees belonging to nominal categories “1” and “2”. If the L12(r) value falls above the upper confidence limit, positive correlation takes place (trees belonging to nominal types “1” and “2” tend to be distributed close to each other). If this L12(r) value falls below the lower confidence limit, trees avoid each other in their spatial distribution. In our study, we investigated spatial relationships between uprooted (category “1”) and snapped (category “2”) trees.
3. Results
We detected 364 live trees of 12 species and 404 (52.6%) trees that were fallen on the PP1 permanent plot (Table 1). Most of them were Abies nephrolepis, Pinus koraiensis (by the level of stem density); Pinus koraiensis and Larix cajanderi (by the level of basal area). Considerable damages to tree crowns and stems of 13.8% trees (6.85 m2·ha−1) were also revealed, which will contribute to the total basal area loss in the near future, bringing it up to 66.4% from the today’s 52.6% due to damaged tree mortality.

Table 1.
Overall tree density and basal area of the trees alive prior to tropical cyclone Lionrock, as well as those damaged during the tropical cyclone activity, and cyclone-killed trees in the old-growth Korean Pine-broadleaved forest, Middle Sikhote-Alin.
Of the 404 dead trees, 157 (39.2% mortality) can be attributed to having been snapped, and 247 (60.8%) to being uprooted trees (Table 2). Fall probability showed no correlation with trees’ DBH. Mean DBH with standard deviation (SD) of all living and killed trees was found to be 25.5 cm and 23.6 cm, respectively (t-test, p = 0.08). At the same time, the mean size of uprooted trees (30.2 cm) was considerably higher (t-test, p ≤ 0.01) than the mean DBH of those snapped trees (18.3 cm).

Table 2.
Number, DBH (tree diameter at breast height ) (mean ± SD (standard deviation)) and probability estimation (P ± Δ) of snapped, uprooted and total killed trees on PP1 (first permanent plot) per 1 ha of strong disturbance resulting from tropical cyclone Lionrock, Middle Sikhote-Alin.
Results of chi-square test of the tree size classes distribution showed deviation from uniform for the killed, snapped and uprooted trees (p ≤ 0.05). There was no statistically significant difference found in tree size distribution in pre-disturbance and post-disturbance tree populations. The same statement is true for healthy and damaged trees, as well as for all species considered together and each taken separately (Figure 2; Kolmogorov-Smirnov two-sample test, p < 0.05). However, the size distribution of uprooted trees differed from that of snapped trees (Kolmogorov-Smirnov two-sample test, p < 0.05). In addition, the frequency of snapped trees was higher within the small size class DBH < 30 cm, while the frequency of uprooted trees was higher within the large size class DBH > 60 cm (Table 2). Snapped trees of the understory Abies nephrolepis layer were larger in diameter (DBH = 15.2 cm) than the mean value for the species (12.5 cm, t-test, p ≤ 0.01). Diameter of the uprooted A. nephrolepis (DBH = 12.9 cm) did not show considerable difference from the overall mean for the species (t-test, p = 0.59). The DBH of snapped and uprooted trees of Pinus koraiensis and Larix cajanderi, as well as the broadleaved species, did not differ from the tree size distribution before the Lionrock cyclone’s impact (t-test, p ≥ 0.05).
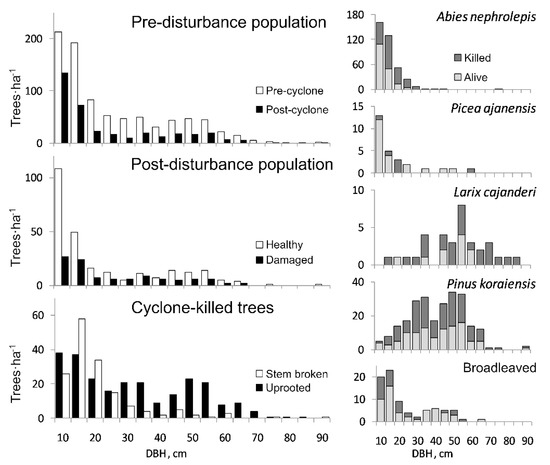
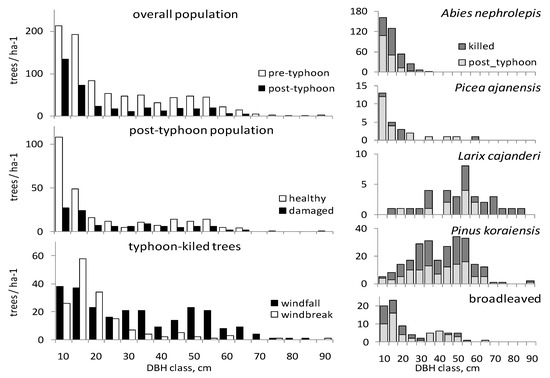
Figure 2.
Tree size distribution of pre-disturbance and post-disturbance populations on PP1 in the old-growth Korean Pine-broadleaved forest, Middle Sikhote-Alin. The distribution of trees of both disturbance types (healthy and damaged trees) is shown, as well as the ratio of killed (uprooted and snapped together) and post-disturbance living trees (healthy and damaged together) for each tree species.
The baseline mortality level during the 50-year period prior to tropical cyclone Lionrock in the Korean Pine-broadleaved forest was 42.4% of stem density or 26.5% of total basal area (Table 3). Mean baseline mortality per 10 years increased insignificantly during the study period, not exceeding the values of 13.7% of stem density and 7.2% of total basal area per 10 years. The mortality rate resulting from tropical cyclone Lionrock equals that for a 10 year period.

Table 3.
Baseline mortality rates on PP2 over 50 years before the Lionrock cyclone’s disturbance and tree loss caused by the tropical cyclone in old-growth Korean Pine-broadleaved forest, Middle Sikhote-Alin.
After the analysis of 147 trees of Pinu skoraiensis and Larix cajanderi (65% of total number of medium and large size trees for these species, before the Lionrock cyclone’s impact), we detected that dead trees were less affected by stem rot in comparison with living trees, regardless of damage type and species (Table 4).

Table 4.
The ratio of living to killed trees with and without stem rot (hollow) in the old-growth Korean Pine-broadleaved forest after the Lionrock cyclone’s impact, Middle Sikhote-Alin.
A logistic regression model was used to examine the influence of a set of parameters on tree mortality probability caused by tropical cyclone Lionrock. Using the omcdiag helper function, it was found that the covariance matrix determinant significantly (minimum significance level 0.05) differs from zero in all studied cases (Table 5).

Table 5.
Logistic regression model of cyclone-induced tree mortality. Accuracy evaluation was performed using leave-one-out cross-validation (LOOCV) scheme.
Using fewer numbers of predictors and all possible combinations did not improve the model. Use of the stem rot infection parameter allowed for a 5% change in model precision for Pinus koraiensis (accuracy changes from 15% to 20%), and did not change it for Larix cajanderi.
The spatial structure of trees present before the disturbance event is determined to be grouped at all distance levels up to 38 m (Figure 3). Uprooted trees resulting from tropical cyclone Lionrock were also grouped. The maximum distance of the uprooted tree distribution patterns was 39 m (about 4800 m2). The spatial structure of snapped trees is more regular than the distribution of uprooted trees. The results of bivariate analysis showed the impact of uprooted trees on snapped and damaged trees on small scales (up to 3 m and 8 m, respectively).
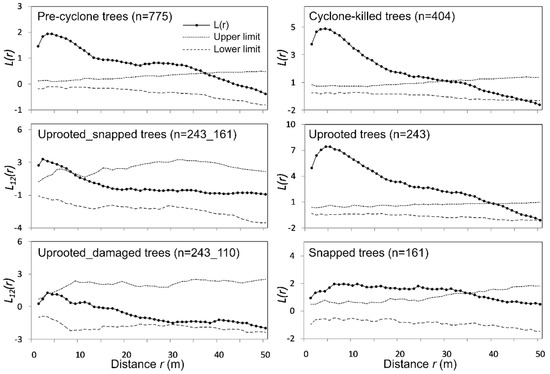
Figure 3.
Ripley’s L(r)-function patterns for pre-typhoon, typhoon-killed and typhoon-damaged tree populations in the Middle Sikhote-Alin. Dashed lines of 999 randomly generated processes indicate 99% confidence limits.
4. Discussion
On the eastern macroslope of the Sikhote-Alin Mountain Range, we estimated the total area of windthrow patches equalled 400 km2, of which, 20% occurred in the Korean Pine-broadleaved forest [35]. On the Sikhote-Alin Nature Reserve territory, tropical cyclone Lionrock formed a large-scale mosaic of complete windthrow patches (several ha in size) and forested areas with the fall of individual trees.
Tropical cyclones in the high latitudes of northeastern Asia are typically rare, because they are blocked by the cold air masses of the Sea of Okhotsk. As a result, when a rare event such as this occurs it may play a much more significant role in the forest ecosystem dynamics of the mainland coastal and insular areas of the Russian Far East. Although the results of our study represent an isolated and first example of a tropical cyclone impact (manifesting itself in strong wind gusts and heavy precipitation within a short time period) on the forest ecosystems of the Sikhote-Alin Mountain Range, it provides a valuable general understanding of the possible impact levels capable of causing disturbance events in this region.
Factors that play a key role in tropical cyclone impact on forest ecosystems differ from each other depending on the scale of the study. Wind speed and total precipitation are considered to be the main factors at the regional scale [36,37], while at the landscape scale, it is the overall topographic site-specific characteristics and plant community-related factors that play the key role [38]. Tree size, species and tree health can influence the rate and type of mortality at the community scale [39,40]. During tropical cyclone Lionrock, the maximum wind speed recorded at the nearest weather station (Ternei) was 30 m·s−1. However, our study plots were located 500 m higher in elevation and 10 km away from the station. A number of studies [41] indicate that wind strength increases with elevation, therefore making it impossible to extrapolate the wind speed registered at the weather station to the actual study areas.
The results of our study show that the impact of the tropical cyclone Lionrock led to the appearance of windthrow patches, decreasing tree stem density by 52.6% and basal area by 58.5%. Everham and Brokaw [10] reported that trees significantly damaged by strong winds die off. In this study, we found that the proportion of standing cyclone-damaged trees was 13.8%. Thus, taking into account direct and indirect “losses”, we come to the conclusion that the Lionrock cyclone’s impact on the forest ecosystem was quite large, with more than 70% loss of pre-disturbance living trees within windthrow patches.
We established that the tree mortality rate corresponds to the similar previously estimated disturbances from strong winds, storms, hurricanes and typhoons found in temperate and boreal forest ecosystems. Foster [4] provides information about the 75% mortality rate in New England forests in the northeastern United States. Sheffield and Thompson [42] recorded a 66% mortality in South Carolina (U.S.) after Hurricane Hugo. Mortality levels ranged from 23.3% to 63.4% of stem density and from 29.5% to 86.8% of basal area as a result of the 1999 catastrophic storm in boreal forests in northeastern Minnesota [40]. Detailed studies of forest ecosystems in northeastern Poland identified tree mortality of 49% of stem density and 48% of basal area caused by the 2002 hurricane [43]. Death rate of trees caused by typhoons is lower in tropical rainforests [44,45]. Thus, Bellingham [46] found 8% tree mortality in Jamaican forests; Whigham, et al. [47] registered 11.2% in Mexico forests 23 months after Hurricane Gilbert, and Walker [44] discovered 7% of tree deaths in Puerto Rico during a one year period following Hurricane Hugo.
In this study, we found that the baseline mortality of the forest under discussion was 4%–14% of stem density and 2%–7% of basal area per 10 year period over the past 50 years. Similar baseline mortality rate, determining the mode of natural disturbances, is typical of coniferous–hardwood forest ecosystems of temperate and boreal forest zones [48]. Higher tree basal mortality rates in temperate and boreal forests are explained by simpler spatial structure and homogeneous species composition. Unlike tropical forests, the above forest ecosystems are not adapted to frequent disturbance events caused by strong winds. According to recent data, mixed composition of tree stands in rainforests is the reason for high forest sustainability [49].
A number of studies have shown that the key factor influencing tree susceptibility to wind is the tree size; large trees being more prone to fall [50]. Mortality of small trees may be low due to the shielding effect of big trees [51]. On the other hand, there is evidence that strong winds cause most damage to mid-size trees, since small trees are shielded by the canopy and large trees are more resistant to gusts [9]. According to our analysis, the overall tree loss, resulting from tropical cyclone Lionrock, impacted all size classes of trees rather equally. Overall windfall frequency of large trees differed insignificantly from that of smaller size trees.
It is known that tree disturbance type (uprooted and snapped) is species and tree size dependent [52]. In this study, the ratio of uprooted trees was generally much higher than of snapped trees. Large trees (DBH > 60 cm) were recorded into the uprooted category more (P = 0.55 ± 0.15), while small trees’ damage was more frequently of the snapped category (P = 0.27 ± 0.04). Similar results were obtained when analyzing wind damage complexes in eastern North America [52], the Caribbean [53,54] and southeastern Slovenia [55]. We are inclined to assume that the uprooting effect damage caused by tropical cyclone Lionrock is the result of strong wind, heavy precipitation combined withthin the mountain soil layer, and the shallow surface root systems of the largest conifer trees Pinus koraiensis and Larix cajanderi. Furthermore, large trees have a larger crown profile. Damages resulting in tree-snapping could be considered the result of the mechanical action of larger falling trees.
Stem rot or hollow in trees is considered by a number of researchers to be a factor increasing tree death probability due to its vitality deterioration and increased susceptibility to strong wind [50,55]. Our data does not allow us to make such conclusions for the Korean Pine-broadleaved forest, Middle Sikhote-Alin. Among the surviving Pinus koraiensis and Larix cajanderi trees, the ratio of trees with rot or hollow is significantly higher than among those killed during the tropical cyclone Lionrock. We assume that this result is the consequence of smaller crowns in trees with rot, which reduces their windfall probability. Testing this hypothesis does not appear possible in our case due to the lack of ability to identify dead tree crown size and affiliation during field work.
According to the presently available evidence, patterns of canopy formation can vary significantly in cases of partial mortality of trees (group distribution) and after catastrophic events accompanied by strong winds (random distribution) [48]. On the other hand, the spatial distribution of uprooted trees resulting from tropical cyclone impact may depend on their initial spatial patterns in the community [37]. In the analyzed forest site, we were able to establish that the large, uprooted trees were the cause of the snapped trees in the area of their fall. At the same time, a more even distribution of the snapped trees in relation to the uprooted trees at the distances of 1 cm to 50 m can be explained by the chaotic fall of the latter. Within the surveyed Korean Pine-broadleaved forest site, we found an increase of canopy gaps of up to 4800m2 (1200 m2 in most cases), which is similar to the spatial distribution patterns in this area. For comparison, Xi et al. [36] notes that the impact of Hurricane Fran in North Carolina led to the average gap sizes of 1100 m2.
Forest disturbance caused by tropical cyclone Lionrock was the strongest on record in the Sikhote-Alin region. Our detailed study of the damaged stand on one of the windthrow patches of the zonal Korean Pine-broadleaved forest enabled us to determine that the tree mortality rate we found exceeded the baseline tree mortality established over a 50-year period, which proves the strong effect of the tropical cyclone. In addition, tropical cyclone Lionrock significantly impacted forest communities, not only within the contiguous windthrow patches (PP1), but also in other parts of the forest ecosystem (PP2), where it was determined that tree death comparable to a ten-year mortality level occurred (Figure S2). We found that large trees were more susceptible to uprooting, while small trees were more susceptible to wind breaking. The largest trees in each size class were found to be more likely to die regardless of the type of death.
Based on logistic regression models defined by the coefficients presented in Table 5, we concluded that d the ecrease of tree density in the community led to an increased death probability due to the tropical cyclone. At the same time, the absence of visible stem rot, larger tree diameter and smaller height contributed to the likelihood of tree mortality in the canopy layer. We assume that these geometric parameters are typical for healthy, large trees.
However, their near-surface root systems contribute to the insufficient resistance to strong tropical cyclones accompanied by heavy precipitation and strong wind loads. High fall resistance of trees in old-growth forest ecosystems is likely related to their grouped spatial distribution. This can also indicate a decrease of stand stability in the case of repeated strong tropical cyclone action due to lower group density, including forest communities with partial canopy violation (single falls).
Trees less susceptible to wind-induced mortality were thin-stemmed and tall, with signs of damage from stem rot. The lack of fit in the logistic model for Larix cajanderi, both with the use of single predictors and their combinations, is explained by the insufficient data set, while the Abies nephrolepis mortality (the largest data set) is determined by external factors and not by the analyzed tree metric.
5. Conclusions
In our study, we firstly made an attempt to estimate the impact of strong wind due to a tropical cyclone on the natural Korean Pine-broadleavedforest community, Middle Sikhote-Alin. It is possible that at the landscape level, the degree of damage was determined by the high, short-term precipitation and maximum observed gusts of wind during the Lionrock disturbance event). At the community scale, the tree characteristics (diameter and height), as well as tree density, were most important.
The results of our study reveal a significant impact of tropical cyclone Lionrock, not only within the windthrow patches, but also for the forest communities that sustained the least amount of damage. In spite of the disturbance of this magnitude remaining a single recorded event in the region, climate change may contribute to an increased frequency of such events, or even to their regularity in the future. In future studies directed at the structure and dynamics of the catastrophic consequences of strong typhoons, we recommend that portions of the forest ecosystem that sustain partial canopy damage be included in the assessment.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/10/11/1017/s1, Figure S1: Windthrow forest in the Middle Sikhote-Alin Mountain Range, Russian Far East; Figure S2: The character of wind-caused tree damage at PP1 (a) and PP2 (b).
Author Contributions
Author Contributions: Conceptualization, A.S.V.; Data curation, A.S.V. and S.N.B.; Formal analysis, A.S.V. and D.E.K.; Funding acquisition, K.A.K.; Investigation, A.S.V., S.N.B., M.N.G., E.A.P., M.A.S. and K.A.K.; Methodology, A.S.V. and D.E.K.; Project administration, A.S.V.;Validation, A.S.V., D.E.K. and K.A.K.; Visualization, A.S.V., D.E.K. and K.A.K.; Writing—original draft, A.S.V., K.A.K. and D.E.K.; Writing—review and editing, S.N.B., M.N.G., E.A.P. and M.A.S.
Funding
The work was supported by the Russian Science Foundation (project No.18-74-00007).
Acknowledgments
Authors are deeply grateful to Viktoria Chilcote and Mark Chilcote for the translation editing and valuable comments. Authors are grateful to two anonymous reviewers for working with the first version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foster, D.R.; Boose, E.R. Patterns of forest damage resulting from catastrophic wind in central New England, USA. J. Ecol. 1992, 80, 79–98. [Google Scholar] [CrossRef]
- Veblen, T.T.; Kulakowski, D.; Eisenhart, K.S.; Baker, W.L. Subalpine forest damage from a severe windstorm in northern Colorado. Can. J. For. Res. 2001, 31, 2089–2097. [Google Scholar] [CrossRef]
- Canham, C.D.; Loucks, O.L. Catastrophic windthrow in the presettlementforests of Wisconsin. J. Ecol. 1984, 65, 803–809. [Google Scholar] [CrossRef]
- Foster, D.R. Species and stand response to catastrophic wind in central New England, USA. J. Ecol. 1988, 76, 135–151. [Google Scholar] [CrossRef]
- Peterson, C.J.; Pickett, S.T.A. Treefall and resprouting following catastrophic windthrow in an old–growth hemlock–hardwoods forest. For. Ecol. Manag. 1991, 42, 205–217. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 9, 2833–2849. [Google Scholar] [CrossRef]
- Chi, C.H.; McEwan, R.W.; Chang, C.T.; Zheng, C.; Yang, Z.; Chiang, J.M.; Lin, T.C. Typhoon disturbance mediates elevational patterns of forest structure, but not species diversity, in humid monsoon Asia. Ecosystems 2015, 18, 1410–1423. [Google Scholar] [CrossRef]
- Sommerfeld, A.; Senf, C.; Buma, B.; D’Amato, A.W.; Després, T.; Díaz–Hormazábal, I.; Fraver, S.; Frelich, L.E.; Gutiérrez, Á.G.; Hart, S.J.; et al. Patterns and drivers of recent disturbances across the temperate forest biome. Nat. Commun. 2018, 9, 4355. [Google Scholar] [CrossRef]
- Everham, E.M.; Brokaw, N.V.L. Forest damage and recovery from catastrophic wind. Bot. Rev. 1996, 62, 113–185. [Google Scholar] [CrossRef]
- Meigs, G.W.; Keeton, W.S. Intermediate-severity wind disturbance in mature temperate forests: Legacy structure, carbon storage, and stand dynamics. Ecol. Appl. 2018, 28, 798–815. [Google Scholar] [CrossRef]
- Royo, A.A.; Peterson, C.J.; Stanovick, J.S.; Carson, W.P. Evaluating the ecological impacts of salvage logging: Can natural and anthropogenic disturbances promote coexistence? Ecology 2016, 97, 1566–1582. [Google Scholar] [CrossRef]
- Bellingham, P.J. Cyclone effects on Australian rain forests: An overview. Austral. Ecol. 2008, 33, 580–584. [Google Scholar] [CrossRef]
- Tanner, E.V.J.; Rodriguez–Sanchez, F.; Healey, J.R.; Holdaway, R.J.; Bellingham, P.J. Long–term hurricane damage effects on tropical forest tree growth and mortality. Ecology 2014, 95, 2974–2983. [Google Scholar] [CrossRef]
- Lin, T.C.; Hamburg, S.P.; Lin, K.C.; Wang, L.J.; Chang, C.T.; Hsia, Y.J.; Vadeboncoeur, M.A.; McMullen, C.M.M.; Liu, C.P. Typhoon disturbance and forest dynamics: Lessons from a northwest Pacific subtropical forest. Ecosystems 2011, 14, 127–143. [Google Scholar] [CrossRef]
- Ritchie, E.A.; Elsberry, R.L. Simulations of the extratropical transition of tropical cyclones: Phasing between the upper-level trough and tropical cyclones. Mon. Weather Rev. 2007, 135, 862–876. [Google Scholar] [CrossRef]
- Dvigalo, V.N.; Melekestsev, I.V. The geological and geomorphic impact of catastrophic landslides in the Geyser Valley of Kamchatka: Aerial photogrammetry. J. Volkanol. Seismol. 2009, 3, 314–325. [Google Scholar] [CrossRef]
- Knutson, T.R.; Sirutis, J.J.; Zhao, M.; Tuleya, R.E.; Bender, M.; Vecchi, G.A.; Villarini, G.; Chavas, D. Global projections of intense tropical cyclone activity for the late twenty-first century from dynamical downscaling of CMIP5/RCP4.5 scenarios. J. Clim. 2015, 28, 7203–7224. [Google Scholar] [CrossRef]
- Altman, J.; Ukhvatkina, O.N.; Omelko, A.M.; Macek, M.; Plener, T.; Pejcha, V.; Cerny, T.; Petrik, P.; Srutek, M.; Song, J.-S.; et al. Poleward migration of the destructive effects of tropical cyclones during the 20th century. Proc. Natl. Acad. Sci. USA 2018, 115, 11543–11548. [Google Scholar] [CrossRef]
- Webster, P.J.; Holland, G.J.; Curry, J.A.; Chang, H.R. Changes in tropical cyclone number, duration and intensity in a warming environment. Science 2005, 309, 1844–1846. [Google Scholar] [CrossRef]
- Walsh, K.J.E.; McBride, J.L.; Klotzbach, P.J.; Balachandran, S.; Camargo, S.J.; Holland, G.; Knutson, T.R.; Kossin, J.P.; Lee, T.C.; Sobel, A.; et al. Tropical cyclones and climate change. Nat. Geosci. 2010, 3, 157–163. [Google Scholar] [CrossRef]
- Shuo, L.; Deqin, L.; Han, S.; Li, T.; Ming, Z. The physical mechanism and strong precipitation in Northeast China analysis during Typhoon “Lionrock” merging into extratropical cyclon. Plateau Meteorol. 2019, 38, 804–816. [Google Scholar]
- Nayak, S.; Takemi, T. Dynamical downscaling of Typhoon Lionrock (2016) for assessing the resulting hazards under global warming. J. Meteorol. Soc. Jap. 2019, 97, 69–88. [Google Scholar] [CrossRef]
- Kossin, J.P.; Emanuel, K.A.; Vecchi, G.A. The poleward migration of the location of tropical cyclone maximum intensity. Nature 2014, 509, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Schurman, J.S.; Trotsiuk, V.; Bace, R.; Cada, V.; Fraver, S.; Janda, P.; Kulakowski, D.; Labusova, J.; Mikolas, M.; Nagel, T.A.; et al. Large-scale disturbance legacies and the climate sensitivity of primary Picea abies forests. Glob. Change Biol. 2018, 24, 2169–2181. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Gromyko, M.N. Climate. In Plants, Fungi and Lichens of the Sikhote-Alin Reserve; Dalnauka: Vladivostok, Russia, 2016; pp. 14–20. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Nakamura, Y.; Krestov, P.V.; Omelko, A.M. Bioclimate and zonal vegetation in Northeast Asia: First approximation to an integrated study. Phytocoenologia 2007, 37, 443–470. [Google Scholar] [CrossRef]
- Oberle, B.; Ogle, K.; Zanne, A.E.; Wooda, C.W. When a tree falls: Controls on wood decay predict standing dead tree fall and new risks in changing forests. PLoS ONE 2018, 13, e0196712. [Google Scholar] [CrossRef]
- Walker, S.H.; Duncan, D.B. Estimation of the probability of an event as a function of several independent variables. Biometrika 1967, 54, 167–179. [Google Scholar] [CrossRef]
- Mehtätalo, L.; de-Miguel, S.; Gregoire, T.G. Modeling height-diameter curves for prediction. Can. J. For. Res. 2015, 45, 826–837. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 6 October 2019).
- Besag, J. Contribution to the discussion of Dr. Ripley’s paper. J. R. Stat. Soc. 1977, B39, 193–195. [Google Scholar]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point Pattern Analysis in Ecology; Chapman and Hall/CRC: Boca Raton, FL, USA, 2014; p. 538. [Google Scholar]
- Gromyko, M.N. The first results of studying the catastrophic effect of typhoon Lyonrok on forest ecosystems of the Sikhote-Alin Nature Reserve. In Proceedings of the XII Far Eastern Conference of Nature Conservation Problems, Birobidzhan, Russian, 10–13 October 2017; pp. 35–37. [Google Scholar]
- Xi, W.; Peet, R.K.; Urban, D.L. Changes in forest structure, species diversity and spatial pattern following hurricane disturbance in a piedmont North Carolina forest, USA. J. Plant Ecol. 2008, 1, 43–57. [Google Scholar] [CrossRef]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef]
- Boose, E.R.; Serrano, M.I.; Foster, D.R. Landscape and regional impacts of hurricanes in Puerto Rico. Ecol. Monogr. 2004, 74, 335–352. [Google Scholar] [CrossRef]
- Lin, S.Y.; Shaner, P.J.L.; Lin, T.C. Characteristics of old–growth and secondary forests in relation to age and typhoon disturbance. Ecosystems 2018, 21, 1521–1532. [Google Scholar] [CrossRef]
- Peterson, C.J. Within-stand variation in windthrow in southern boreal forests of Minnesota: Is it predictable? Can. J. For. Res. 2004, 34, 365–375. [Google Scholar] [CrossRef]
- Tan, F.; Lim, H.S.; Abdullah, K. The effects of orography in Indochina on wind, cloud, and rainfall patterns during Typhoon Ketsana (2009). Asia Pac. J. Atmos. Sci. 2012, 48, 295–314. [Google Scholar] [CrossRef]
- Sheffield, R.M.; Thompson, M.T. Hurricane Hugo: Effects on South Carolina’s Forest Resource; USDA Forest Service: Asheville, NC, USA, 1992; p. 51.
- Szwagrzyk, J.; Gazda, A.; Dobrowolska, D.; Chećko, E.; Zaremba, J.; Tomski, A. Tree mortality after wind disturbance differs among tree species more than among habitat types in a lowland forest in northeastern Poland. For. Ecol. Manag. 2017, 398, 174–184. [Google Scholar] [CrossRef]
- Walker, L.R. Tree damage and recovery from Hurricane Hugo in Luquillo Experimental Forest, Puerto Rico. Biotropica 1991, 23, 379–385. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Kapos, V.; Varty, N.; Healey, J.R.; Tanner, E.V.J.; Kelly, D.L.; Dalling, J.W.; Burns, L.S.; Lee, D.; Sidrak, G. Hurricanes need not cause high mortality: The effects of Hurricane Gilbert on forests in Jamaica. J. Trop. Ecol. 1992, 8, 217–223. [Google Scholar] [CrossRef]
- Bellingham, P.J. Landforms influence patterns of hurricane damage: Evidence from Jamaican montane forests. Biotropica 1991, 23, 427–433. [Google Scholar] [CrossRef]
- Whigham, D.F.; Dickinson, M.B.; Brokaw, N.V. Background canopy gap and catastrophic wind disturbances in tropical forests. In Ecosystems of Disturbed Ground; Elsevier Science: Amsterdam, The Netherlands, 1999; pp. 223–252. [Google Scholar]
- Woods, K.D. Intermediate disturbance in a late-successional hemlock northern hardwood forest. J. Ecol. 2004, 92, 464–476. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez-Olabarria, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Canham, C.D.; Papaik, M.J.; Latty, E.F. Interspecific variation in susceptibility to windthrow as a function of tree size and storm severity for northern temperate tree species. Can. J. For. Res. 2001, 31, 1–10. [Google Scholar] [CrossRef]
- Imbert, D.; Labbe, P.; Rousteau, A. Hurricane damage and forest structure in Guadeloupe, French West Indies. J. Trop. Ecol. 1996, 12, 663–680. [Google Scholar] [CrossRef]
- Greenberg, C.H.; McNab, W.H. Forest disturbance in hurricane-related downbursts in the Appalachian Mountains of North Carolina. For. Ecol. Manag. 1998, 104, 179–191. [Google Scholar] [CrossRef]
- Basnet, K.; Likens, G.E.; Scatera, F.N.; Lugo, A.E. Hurricane Hugo: Damage to a tropical rain forest in Puerto Rico. J. Trop. Ecol. 1992, 8, 47–55. [Google Scholar] [CrossRef]
- Tanner, E.V.J.; Kapos, V.; Healey, J.R. Hurricane effects on forest ecosystems in the Caribbean. Biotropica 1991, 23, 513–521. [Google Scholar] [CrossRef]
- Nagel, T.A.; Diaci, J. Intermediate wind disturbance in an old-growth beech–fir forest in southeastern Slovenia. Can. J. For. Res. 2006, 36, 629–638. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).