Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids
Abstract
:1. Introduction
2. Nanofluid Characterization
2.1. Material and Sample Preparation
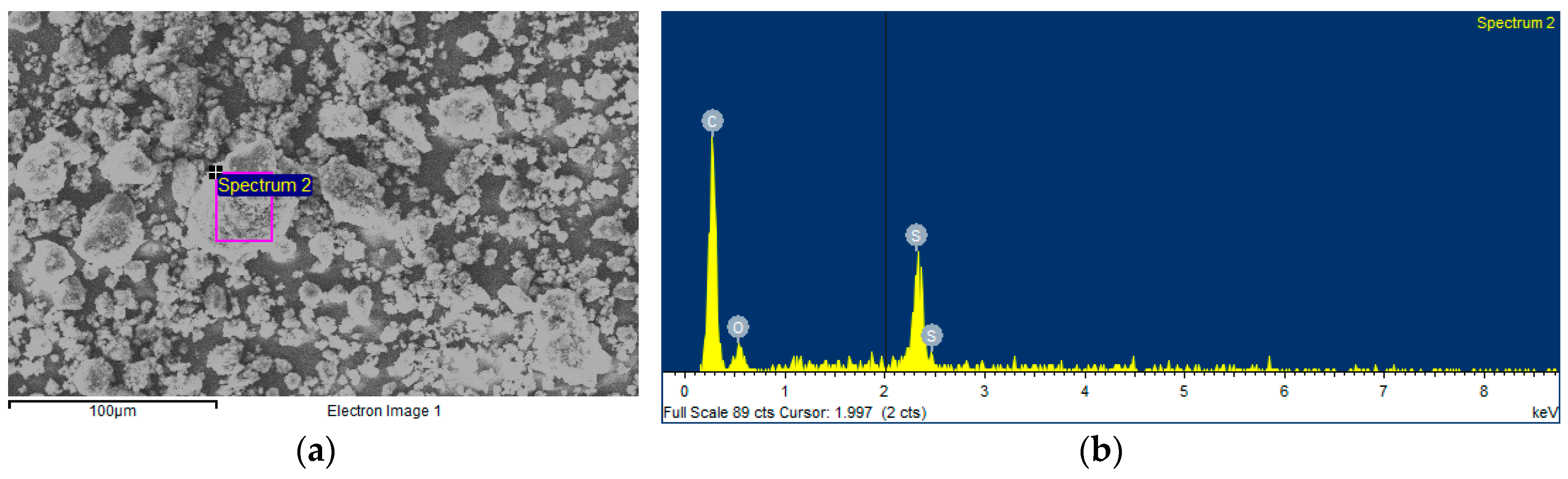
2.2. Nanopowder Characterization
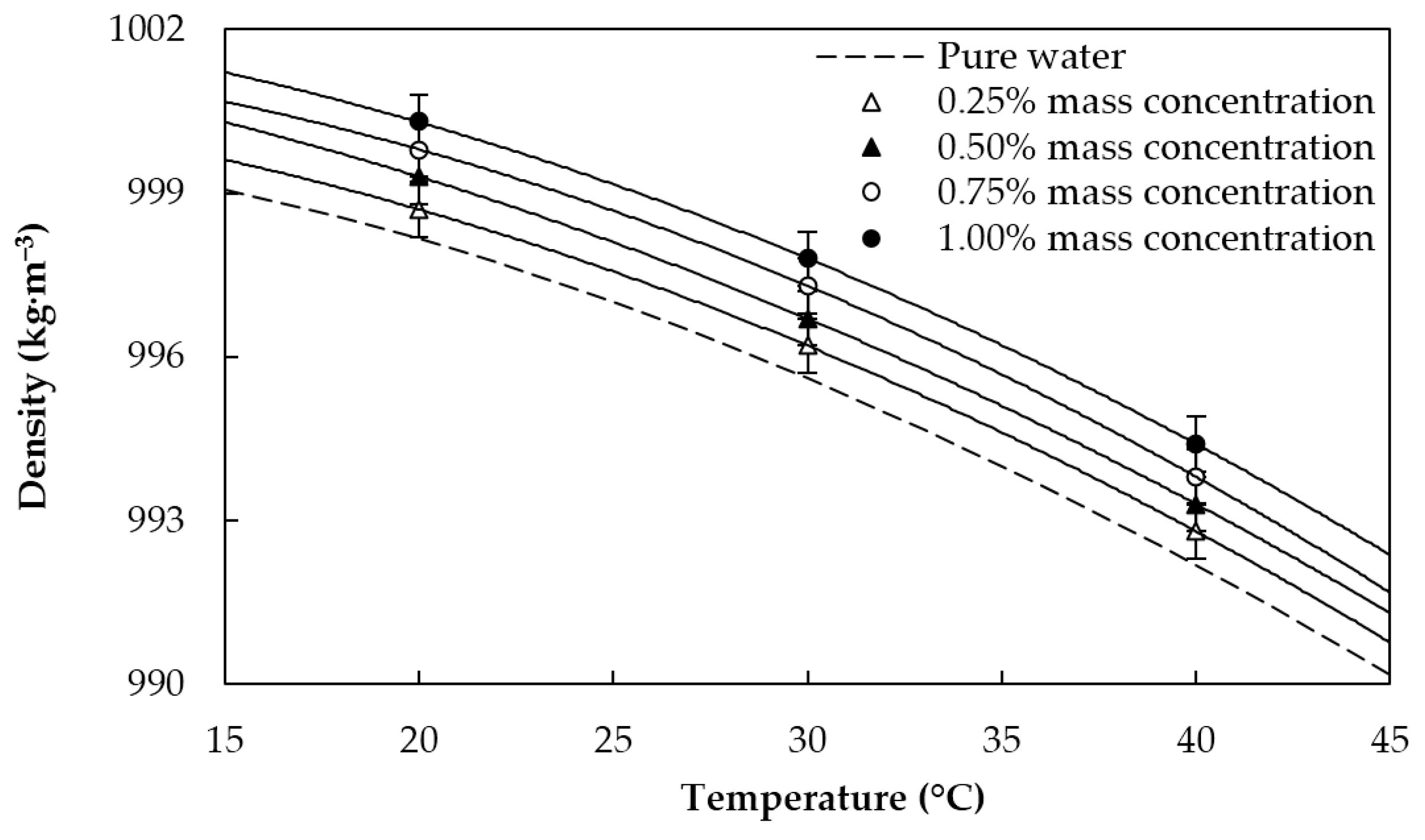
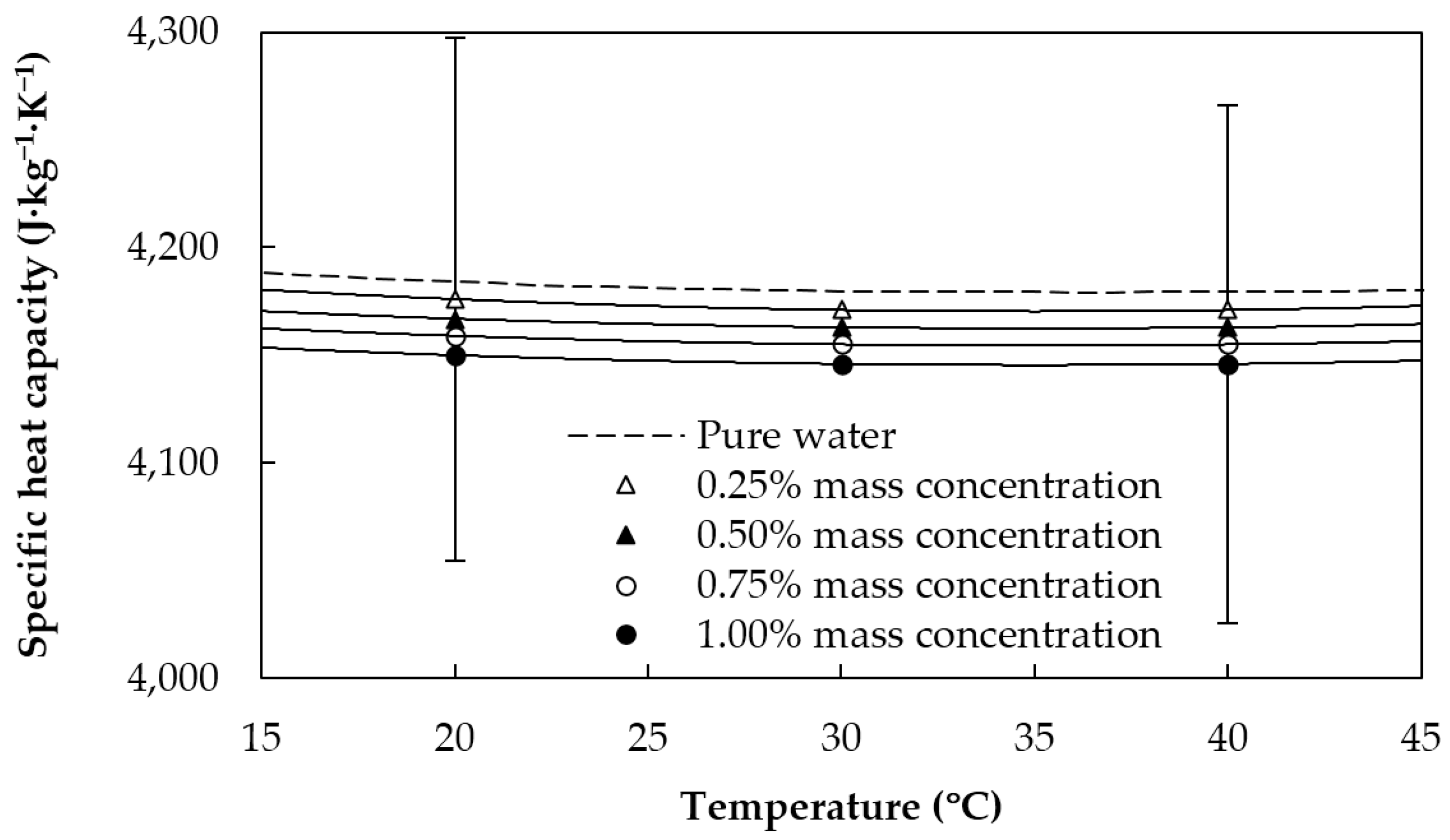
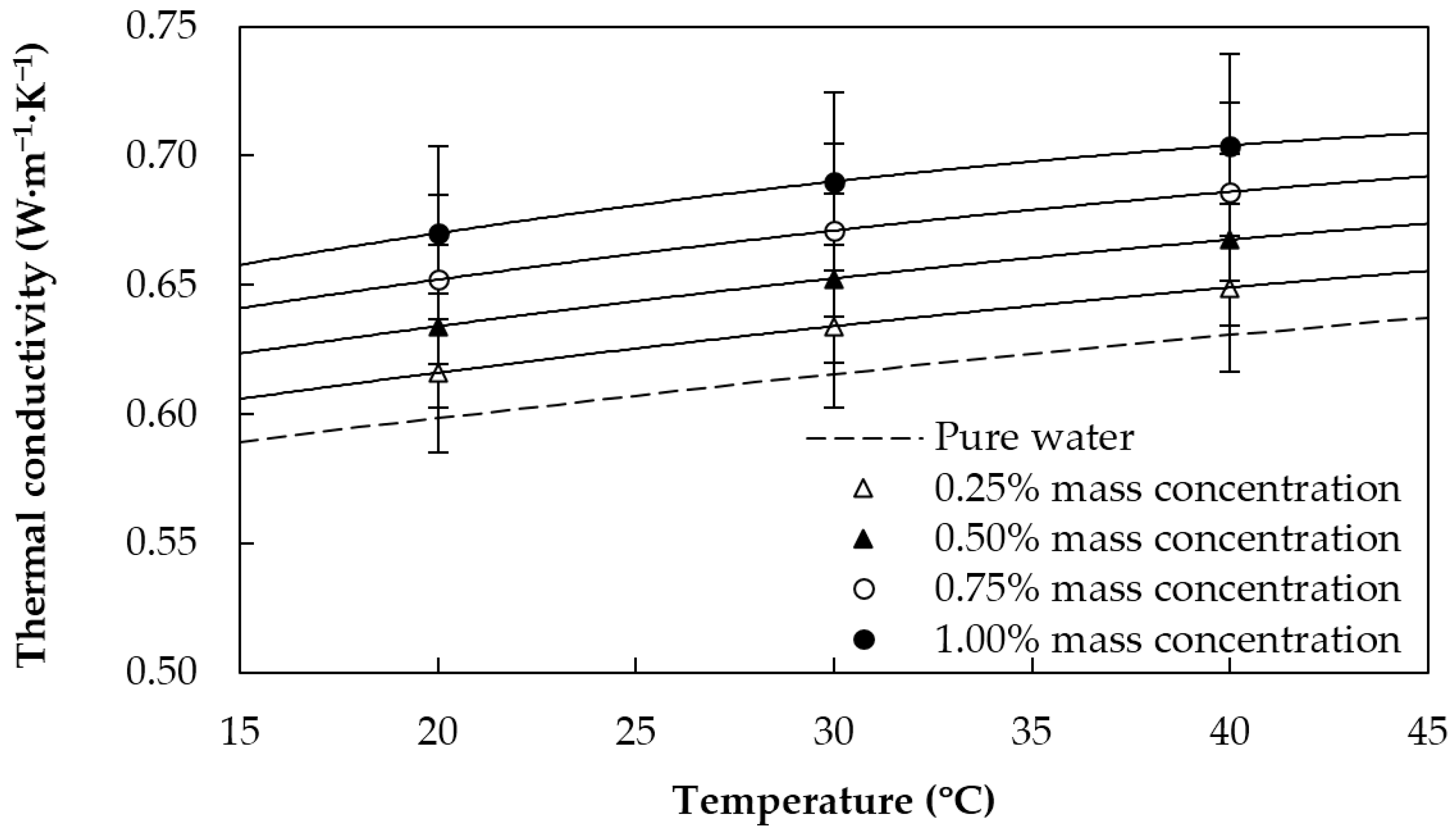
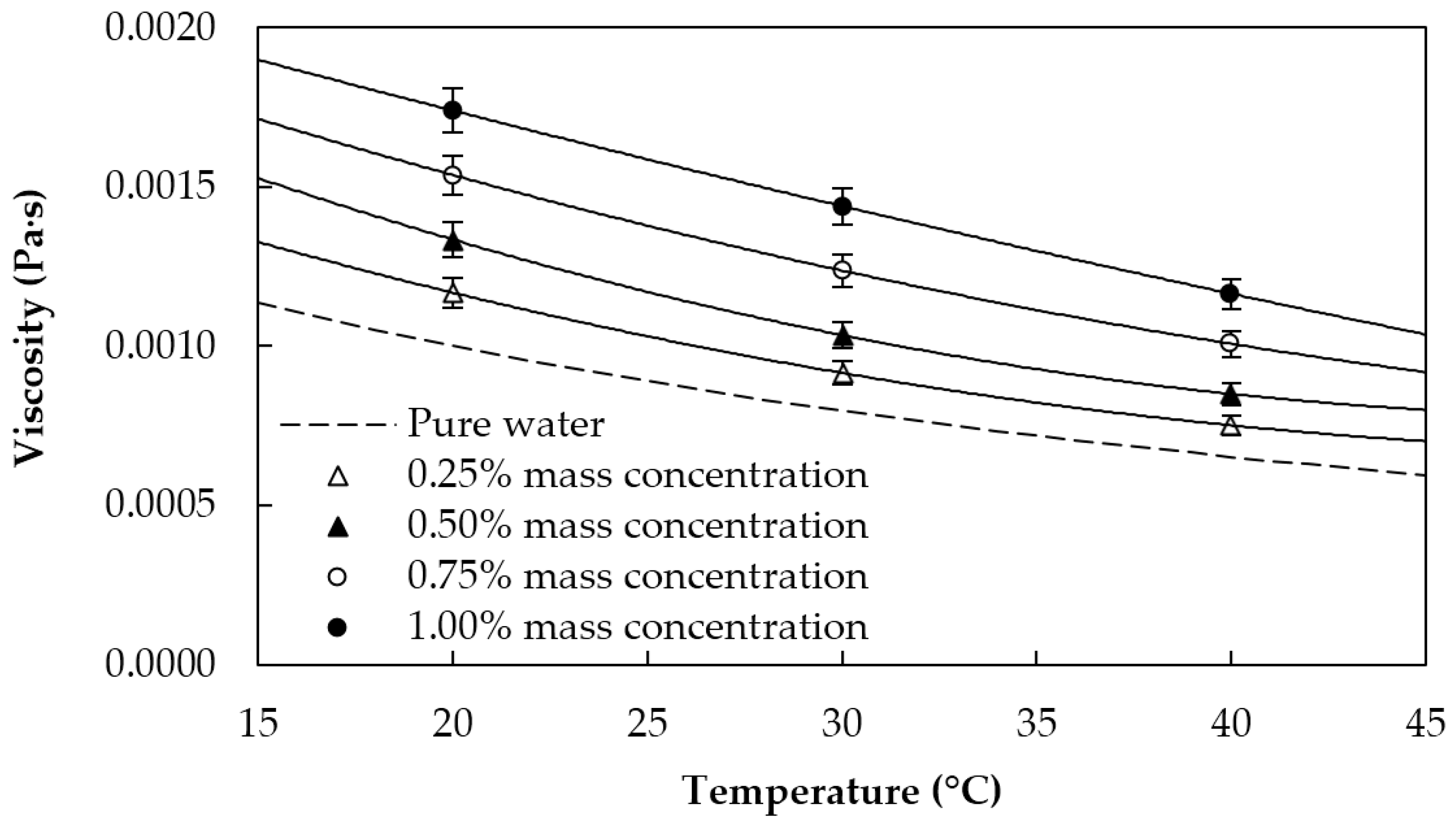
2.3. Thermophysical Characterization
3. Heat Transfer Coefficient Determination
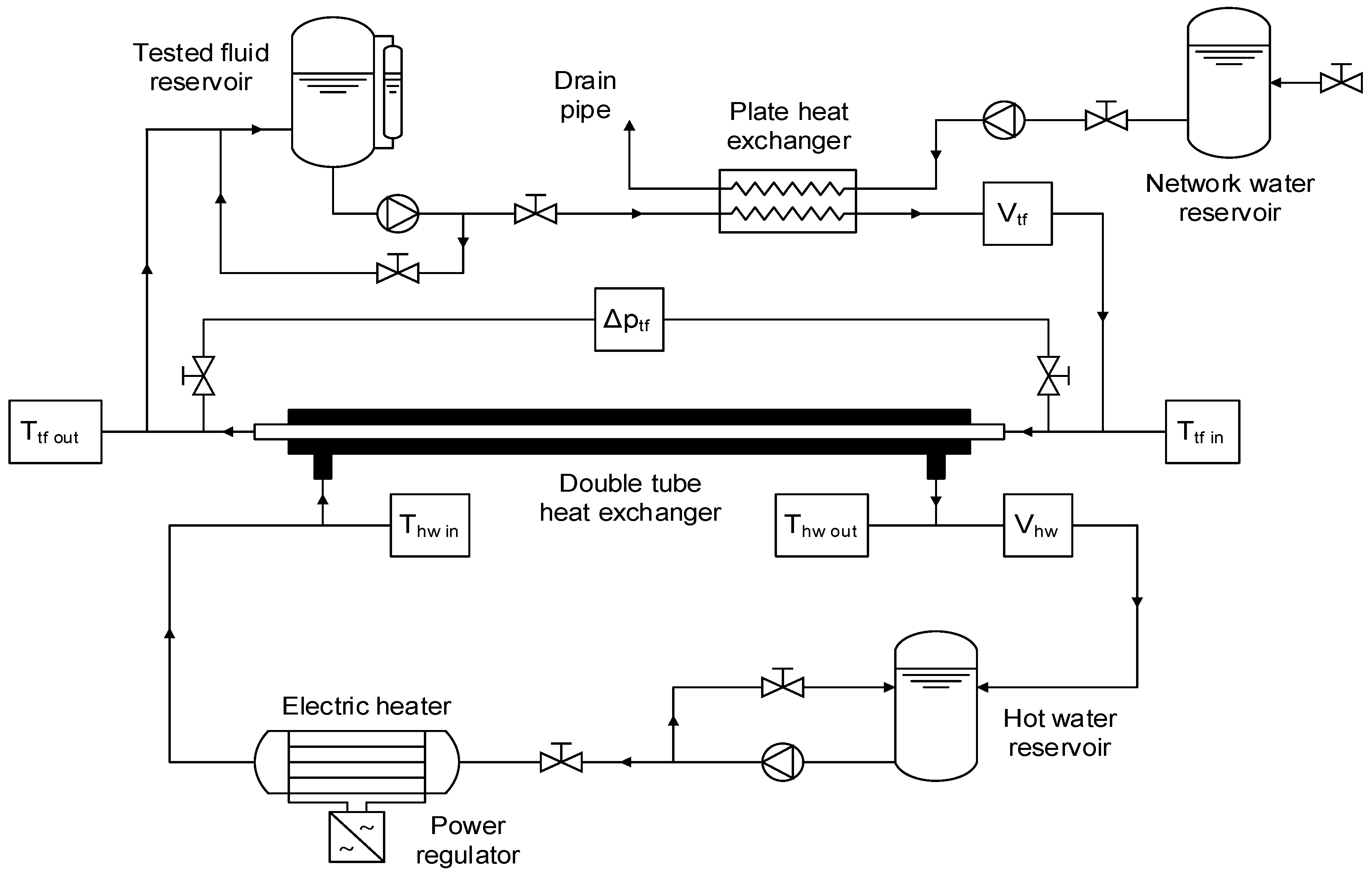
3.1. Experimental Equipment
3.2. Experimental Procedure
4. Data Analysis
5. Results
5.1. Heat Transfer Performance
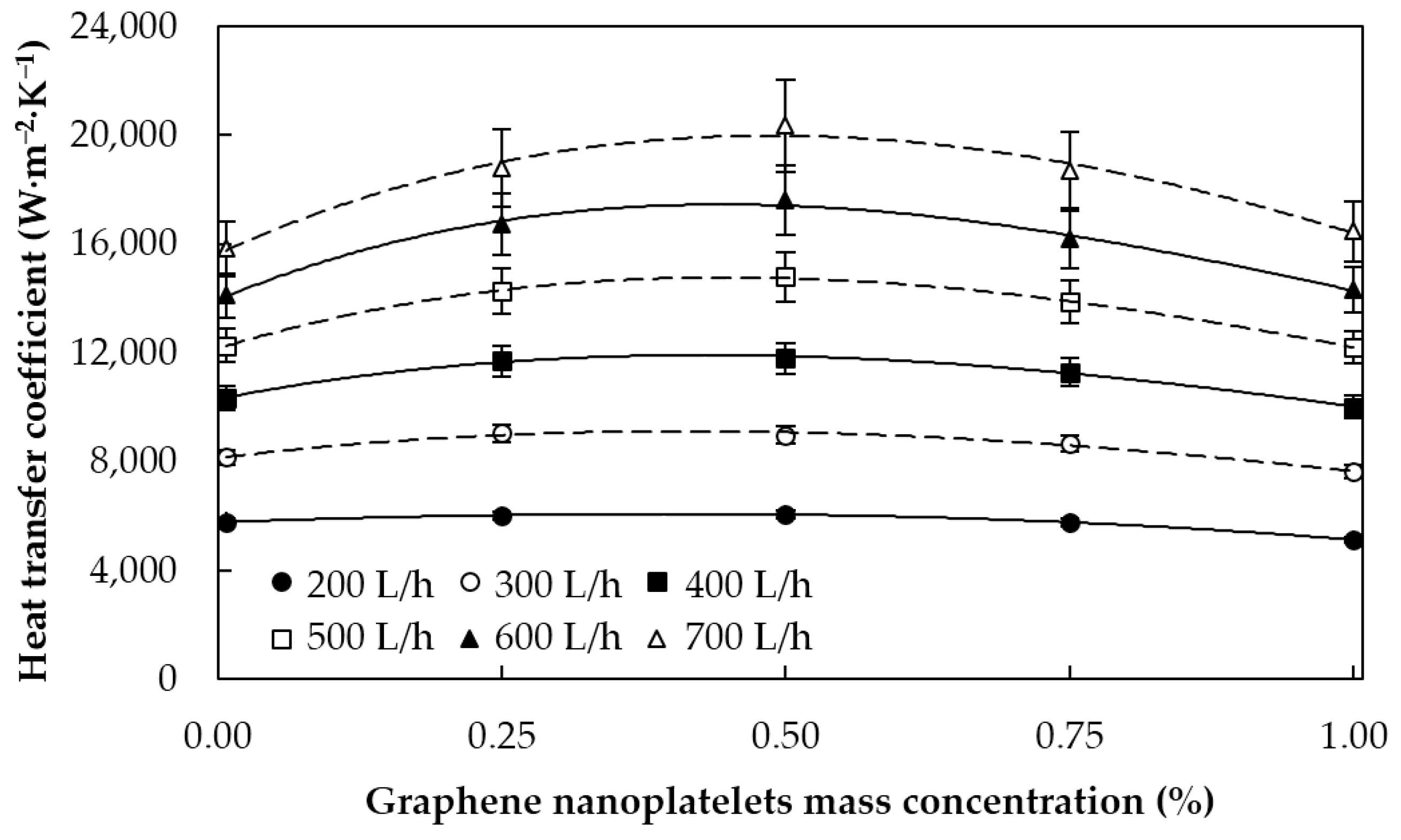
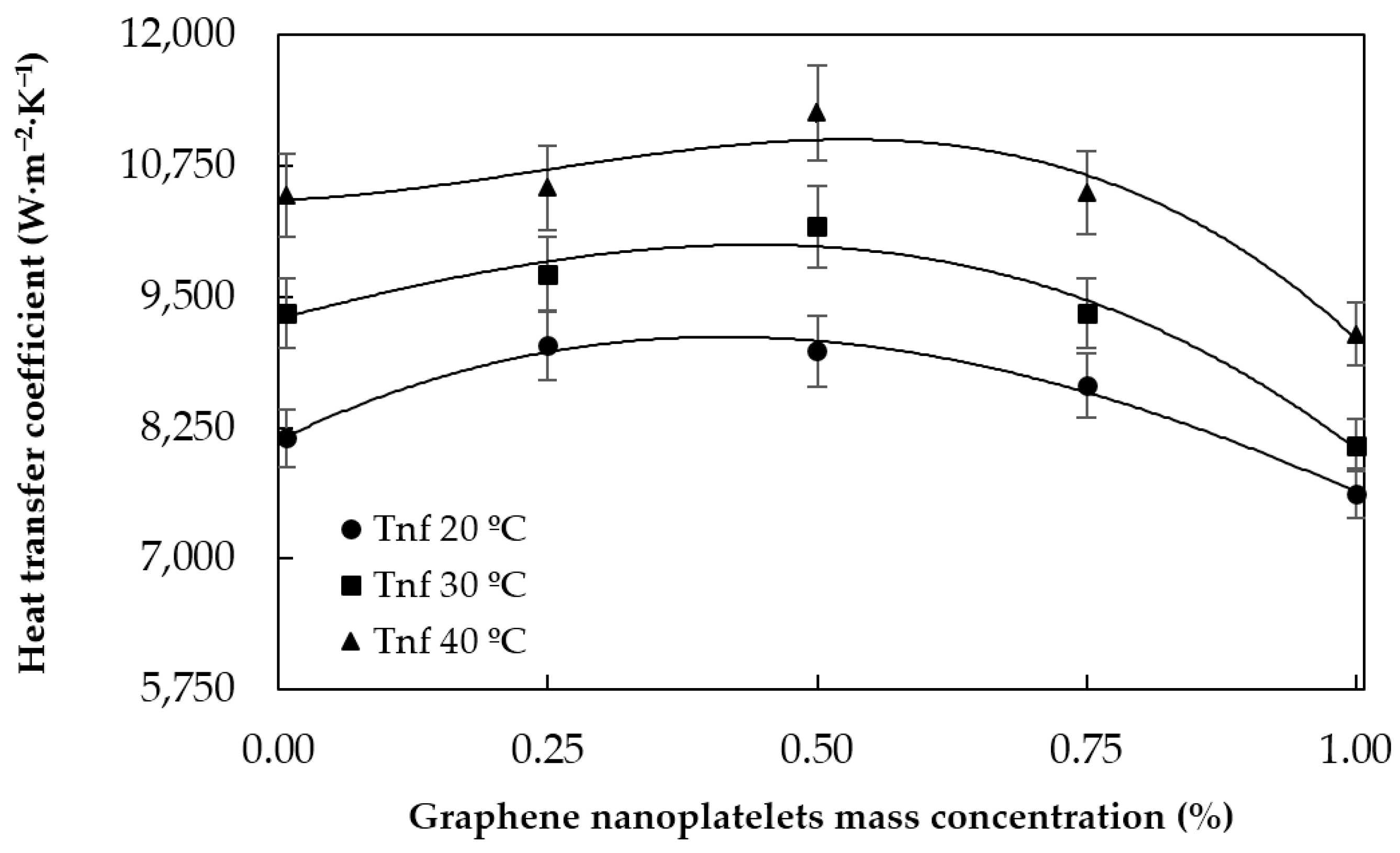
5.1.1. Heat Transfer Coefficients
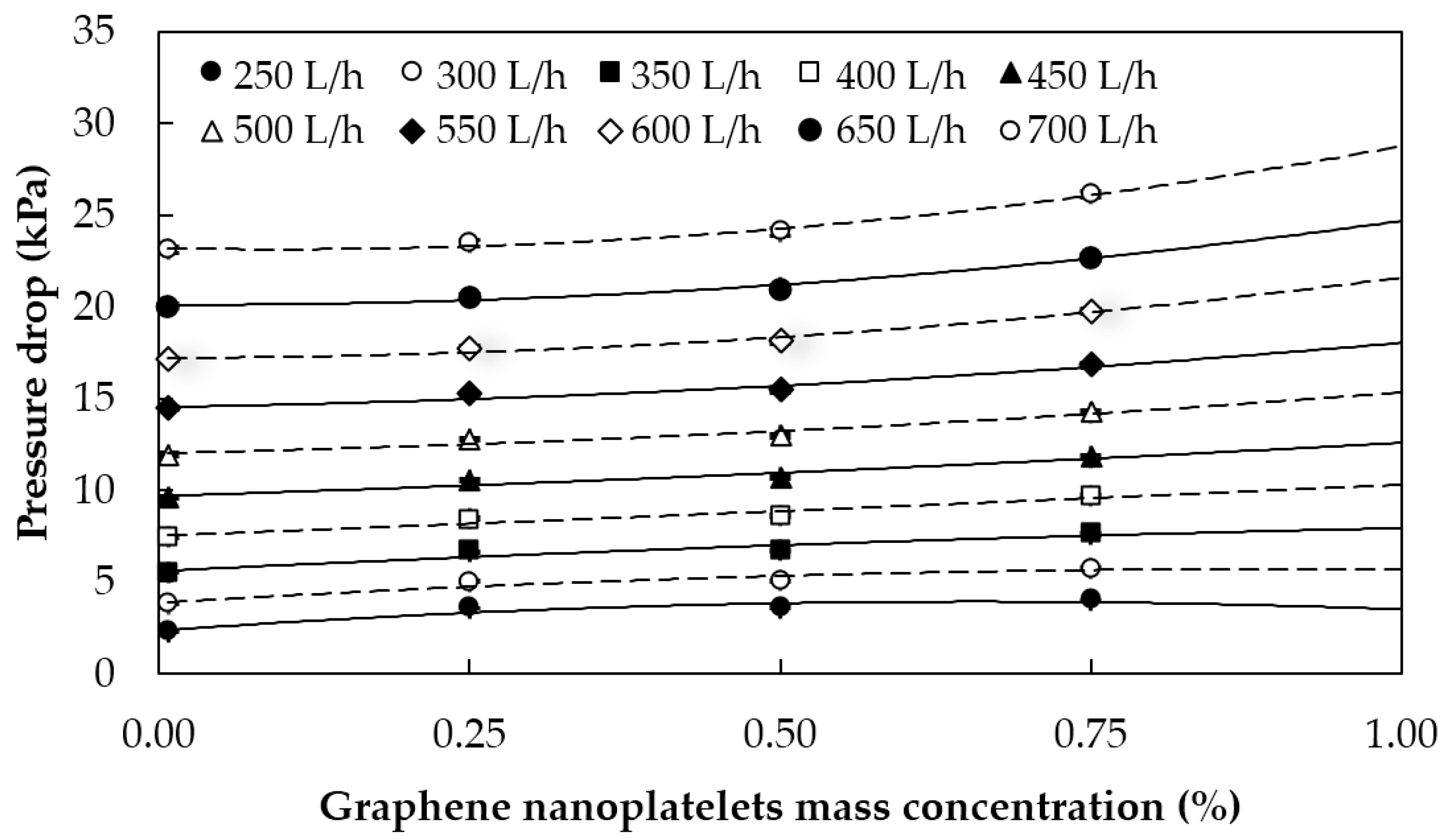
5.1.2. Pressure Losses
5.2. Dimensionless Analysis
5.2.1. Nusselt Number Correlation
5.2.2. Darcy Friction Factor Correlation
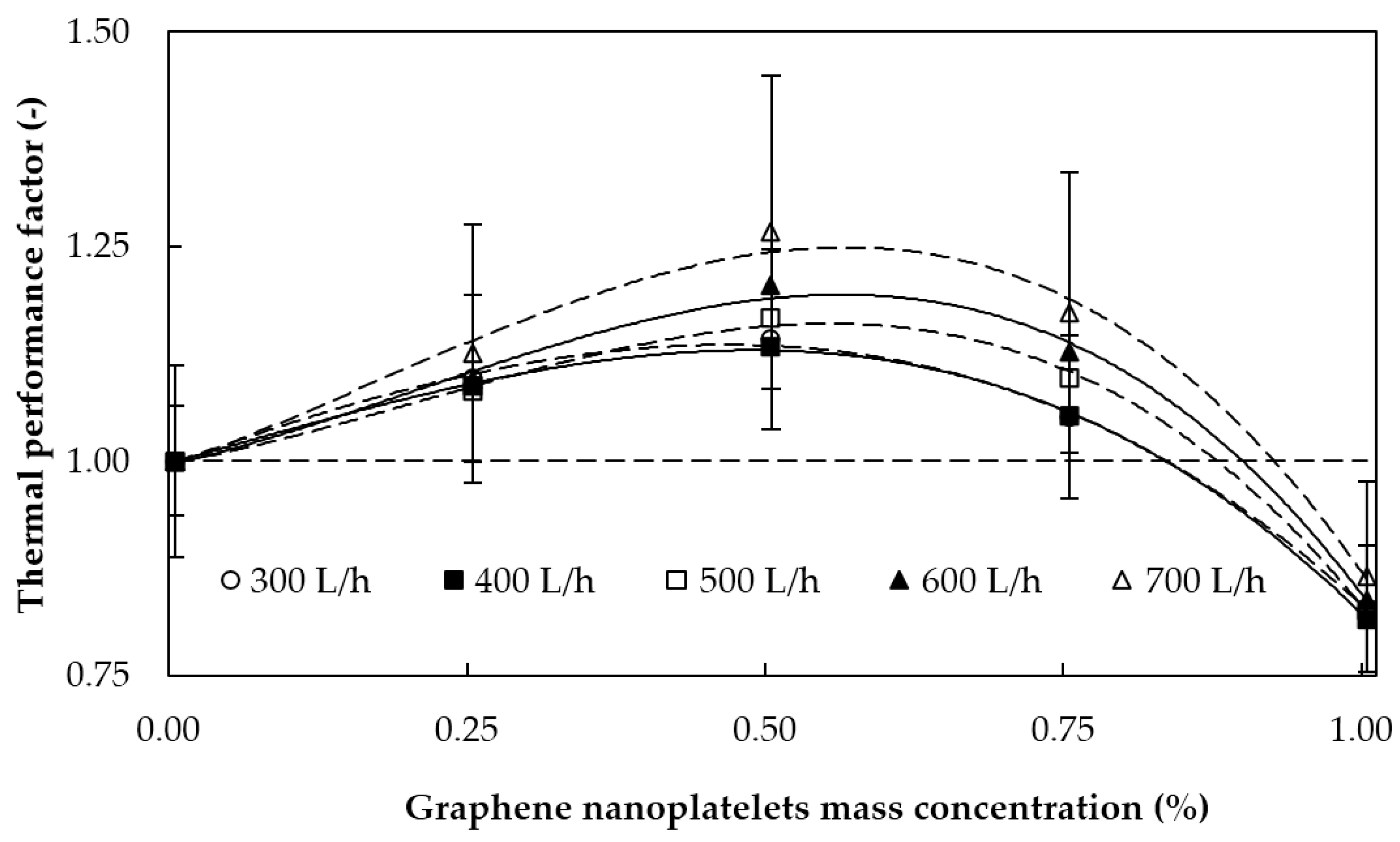
5.2.3. Thermal Performance Factor
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Nomenclature | |
| a | Annulus diameter ratio, dimensionless |
| ci | Correlation fitting constant, dimensionless |
| cp | Specific heat capacity, J·kg−1·K−1 |
| d1 | Inner diameter of the inner tube, m |
| d2 | Outer diameter of the inner tube, m |
| d3 | Inner diameter of the outer tube, m |
| dh | Hydraulic diameter, m |
| f | Darcy friction factor, dimensionless |
| h | Convection coefficient, W·m−2·K−1 |
| k | Thermal conductivity, W·m−1·K−1 |
| LMTD | Logarithmic mean temperature difference, °C |
| Lh | Effective heating length, m |
| Lp | Effective pressure drop length, m |
| Nu | Nusselt number, dimensionless |
| Pr | Prandtl number, dimensionless |
| Heat flow rate, W | |
| R | Thermal resistance, K·W−1 |
| Re | Reynolds number, dimensionless |
| T | Temperature, K, °C |
| v | Average velocity of the fluid, m·s−1 |
| Volume flow rate, m3·s−1 | |
| vol % | Percentual graphene nanoplatelets volume concentration, dimensionless |
| wt % | Percentual graphene nanoplatelets mass concentration, dimensionless |
| Δp | Pressure drop, Pa |
| η | Thermal performance factor, dimensionless |
| ρ | Density, kg·m−3 |
| φm | Graphene nanoplatelets mass concentration, dimensionless |
| φv | Graphene nanoplatelets volume concentration, dimensionless |
| ψ | ω | Correction factors for the Nusselt number in the annulus, dimensionless |
| Subscripts | |
| bf | Base fluid |
| hw | Heating water |
| in | Inlet of the test section |
| nf | Nanofluid |
| out | Outlet of the test section |
| ov | Overall |
| s | Steel |
| tf | Tested fluid |
| w | Water |
| wall | Tube wall |
References
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. FED 1995, 231, 99–105. [Google Scholar]
- Cabaleiro, D.; Pastoriza-Gallego, M.J.; Gracia-Fernández, C.; Piñeiro, M.M.; Lugo, L. Rheological and volumetric properties of TiO2-ethylene glycol nanofluids. Nanoscale Res. Lett. 2013, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2016, 53, 779–791. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Application of nanofluids in heat exchangers: A review. Renew. Sustain. Energy Rev. 2012, 16, 5625–5638. [Google Scholar] [CrossRef]
- Trisaksri, V.; Wongwises, S. Critical review of heat transfer characteristics of nanofluids. Renew. Sustain. Energy Rev. 2007, 11, 512–523. [Google Scholar] [CrossRef]
- Lomascolo, M.; Colangelo, G.; Milanese, M.; de Risi, A. Review of heat transfer in nanofluids: Conductive, convective and radiative experimental results. Renew. Sustain. Energy Rev. 2015, 43, 1182–1198. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Gu, B.; Hou, B.; Lu, Z.; Wang, Z.; Chen, S. Thermal conductivity of nanofluids containing high aspect ratio fillers. Int. J. Heat Mass Transf. 2013, 64, 108–114. [Google Scholar] [CrossRef]
- Mintsa, H.A.; Roy, G.; Nguyen, C.T.; Doucet, D. New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 2009, 48, 363–371. [Google Scholar] [CrossRef]
- Ellahi, R.; Hassan, M.; Zeeshan, A. Shape effects of nanosize particles in Cu–H2O nanofluid on entropy generation. Int. J. Heat Mass Transf. 2015, 81, 449–456. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. Int. J. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Fard, M.H.; Esfahany, M.N.; Talaie, M. Numerical study of convective heat transfer of nanofluids in a circular tube two-phase model versus single-phase model. Int. Commun. Heat Mass Transf. 2010, 37, 91–97. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ellahi, R.; Nadeem, S.; Zia, Q.M.Z. Simultaneous effects of nanoparticles and slip on jeffrey fluid through tapered artery with mild stenosis. J. Mol. Liq. 2016, 218, 484–493. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; de Risi, A. Thermal conductivity, viscosity and stability of Al2O3-diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; de Risi, A.; Laforgia, D. Results of experimental investigations on the heat conductivity of nanofluids based on diathermic oil for high temperature applications. Appl. Energy 2012, 97, 828–833. [Google Scholar] [CrossRef]
- Lee, G.J.; Rhee, C.K. Enhanced thermal conductivity of nanofluids containing graphene nanoplatelets prepared by ultrasound irradiation. J. Mater. Sci. 2014, 49, 1506–1511. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; McCarthy, J.E.; Baranov, A.; Gun’ko, Y.K. Development of graphene nano-platelet based counter electrodes for solar cells. Materials 2015, 8, 5953–5973. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Mo, S.; Jia, L.; Shao, X. Effect of surface modification on the stability and thermal conductivity of water-based SiO2-coated graphene nanofluid. Thermochim. Acta 2014, 595, 6–10. [Google Scholar] [CrossRef]
- Amiri, A.; Sadri, R.; Shanbedi, M.; Ahmadi, G.; Chew, B.T.; Kazi, S.N.; Dahari, M. Performance dependence of thermosyphon on the functionalization approaches: An experimental study on thermo-physical properties of graphene nanoplatelet-based water nanofluids. Energy Convers. Manag. 2015, 92, 322–330. [Google Scholar] [CrossRef]
- Ijam, A.; Moradi Golsheikh, A.; Saidur, R.; Ganesan, P. A glycerol-water-based nanofluid containing graphene oxide nanosheets. J. Mater. Sci. 2014, 49, 5934–5944. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Ahmadi, G.; Shirazi, S.F.S.; Baradaran, S.; Montazer, E.; Zubir, M.N.M.; Alehashem, M.S.; Kazi, S.; Dahari, M. Graphene nanoplatelets-silver hybrid nanofluids for enhanced heat transfer. Energy Convers. Manag. 2015, 100, 419–428. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Rudyak, V.Y. Viscosity of nanofluids—Why it is not described by the classical theories. Adv. Nanopart. 2013, 2, 266–279. [Google Scholar] [CrossRef]
- Gupta, S.S.; Siva, M.V.; Krishnan, S.; Sreeprasad, T.S.; Singh, P.K.; Pradeep, T.; Das, S.K. Thermal conductivity enhancement of nanofluids containing graphene nanosheets. J. Appl. Phys. 2011, 110. [Google Scholar] [CrossRef]
- Hajjar, Z.; Rashidi, A.M.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, W. Experimental investigation on thermal conductivity of nanofluids containing graphene oxide nanosheets. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Togun, H.; Mehrali, M.; Nejad, P.S.; Latibari, S.T.; Abdulrazzaq, T.; Kazi, S.; Metselaar, H.S.C. An experimental and numerical investigation of heat transfer enhancement for graphene nanoplatelets nanofluids in turbulent flow conditions. Int. J. Heat Mass Transf. 2015, 81, 41–51. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Solangi, K.; Amiri, A.; Luhur, M.; Ghavimi, S.A.A.; Zubir, M.N.M.; Kazi, S.; Badarudin, A. Experimental investigation of the propylene glycol-treated graphene nanoplatelets for the enhancement of closed conduit turbulent convective heat transfer. Int. Commun. Heat Mass Transf. 2016, 73, 43–53. [Google Scholar] [CrossRef]
- Ting, H.H.; Hou, S.S. Investigation of laminar convective heat transfer for Al2O3-water nanofluids flowing through a square cross-section duct with a constant heat flux. Materials 2015, 8, 5321–5335. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ellahi, R. Electrohydrodynamic nanofluid hydrothermal treatment in an enclosure with sinusoidal upper wall. Appl. Sci. 2015, 5, 294–306. [Google Scholar] [CrossRef]
- Rashidi, S.; Dehghan, M.; Ellahi, R.; Riaz, M.; Jamal-Abad, M.T. Study of stream wise transverse magnetic fluid flow with heat transfer around an obstacle embedded in a porous medium. J. Magn. Magn. Mater. 2015, 378, 128–137. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ellahi, R. Three dimensional mesoscopic simulation of magnetic field effect on natural convection of nanofluid. Int. J. Heat Mass Transf. 2015, 89, 799–808. [Google Scholar] [CrossRef]
- Sher Akbar, N.; Raza, M.; Ellahi, R. Influence of induced magnetic field and heat flux with the suspension of carbon nanotubes for the peristaltic flow in a permeable channel. J. Magn. Magn. Mater. 2015, 381, 405–415. [Google Scholar] [CrossRef]
- Calvo-Bravo, J.; Cabaleiro, D.; Piñeiro, M.M.; Lugo, L. Magnetorheological behaviour of propylene glycol-based hematite nanofluids. Rheol. Acta 2015, 54, 757–769. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. Reference Fluid Thermodynamic and Transport Properties (REFPROP); NIST Standard Reference Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Cabaleiro, D.; Gracia-Fernández, C.; Legido, J.L.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transf. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Nimo, J.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Legido, J.L.; Lugo, L. Thermal conductivity of dry anatase and rutile nano-powders and ethylene and propylene glycol-based TiO2 nanofluids. J. Chem. Thermodyn. 2015, 83, 67–76. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Legido, J.L.; Lugo, L. Thermophysical properties of (diphenyl ether + biphenyl) mixtures for their use as heat transfer fluids. J. Chem. Thermodyn. 2012, 50, 80–88. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Lugo, L. Characterization and measurements of thermal conductivity, density and rheological properties of zinc oxide nanoparticles dispersed in (ethane-1,2-diol + water) mixture. J. Chem. Thermodyn. 2013, 58, 405–415. [Google Scholar] [CrossRef]
- Gnielinski, V. Heat Transfer in Concentric Annular and Parallel Plate Ducts. VDI Heat Atlas; Springer: Heidelberg, Germany, 2010; pp. 701–708. [Google Scholar]
- Kim, C.S. Thermophysical Properties of Stainless Steels; Technical Report; Argonne National Lab.: Argonne, IL, USA, 1975. [Google Scholar]
- Bianco, V.; Manca, O.; Nardini, S.; Vafai, K. Heat Transfer Enhancement with Nanofluids; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Mehrali, M.; Sadeghinezhad, E.; Rosen, M.A.; Akhiani, A.R.; Tahan Latibari, S.; Mehrali, M.; Metselaar, H.S.C. Heat transfer and entropy generation for laminar forced convection flow of graphene nanoplatelets nanofluids in a horizontal tube. Int. Commun. Heat Mass Transf. 2015, 66, 23–31. [Google Scholar] [CrossRef]
- Joint Committee for Guides in Metrology. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurements; JCGM 100: Sèvres, France, 2008. [Google Scholar]

| Nanofluid | Minimum | Average | Maximum |
|---|---|---|---|
| φm = 0.25% | −1.7% | 6.5% | 18.6% |
| φm = 0.50% | 0.94% | 15.0% | 32.4% |
| φm = 0.75% | −2.3% | 7.0% | 22.8% |
| φm = 1.00% | −19.6% | –10.1% | 4.0% |
| Nanofluid | Minimum | Average | Maximum |
|---|---|---|---|
| φm = 0.25% | 0.92% | 14.5% | 55.4% |
| φm = 0.50% | 4.11% | 17.1% | 56.9% |
| φm = 0.75% | 13.0% | 28.0% | 73.4% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agromayor, R.; Cabaleiro, D.; Pardinas, A.A.; Vallejo, J.P.; Fernandez-Seara, J.; Lugo, L. Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids. Materials 2016, 9, 455. https://doi.org/10.3390/ma9060455
Agromayor R, Cabaleiro D, Pardinas AA, Vallejo JP, Fernandez-Seara J, Lugo L. Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids. Materials. 2016; 9(6):455. https://doi.org/10.3390/ma9060455
Chicago/Turabian StyleAgromayor, Roberto, David Cabaleiro, Angel A. Pardinas, Javier P. Vallejo, Jose Fernandez-Seara, and Luis Lugo. 2016. "Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids" Materials 9, no. 6: 455. https://doi.org/10.3390/ma9060455
APA StyleAgromayor, R., Cabaleiro, D., Pardinas, A. A., Vallejo, J. P., Fernandez-Seara, J., & Lugo, L. (2016). Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids. Materials, 9(6), 455. https://doi.org/10.3390/ma9060455











