Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S
Abstract
:1. Introduction
2. Materials and Methods
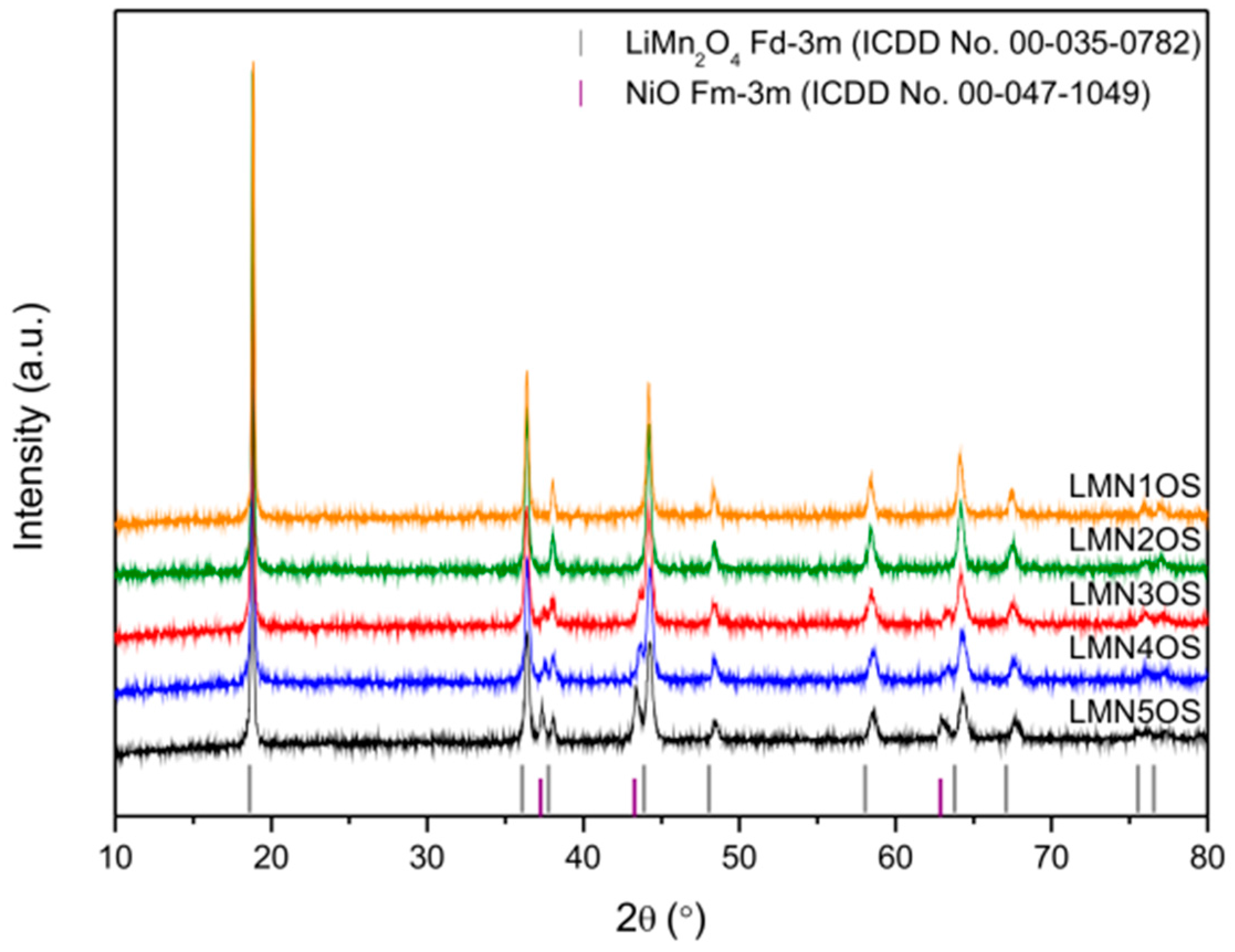
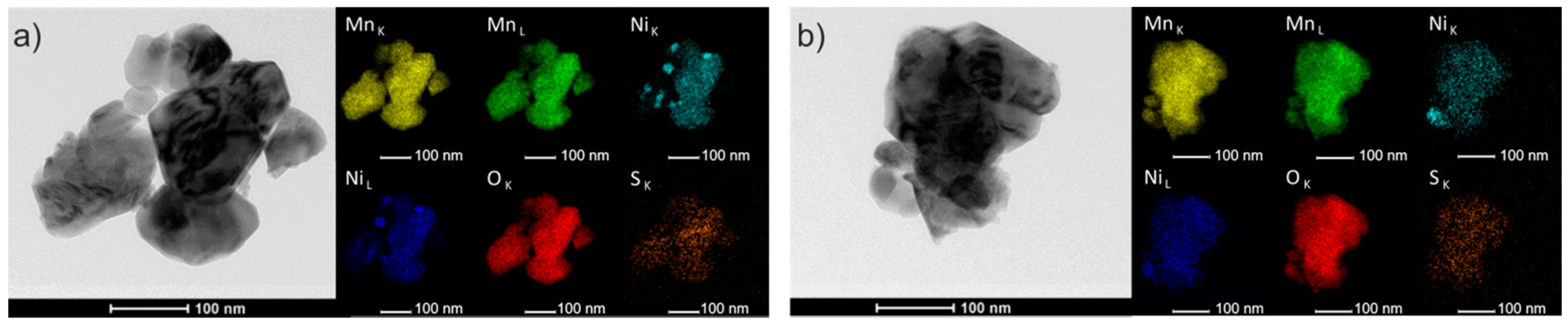
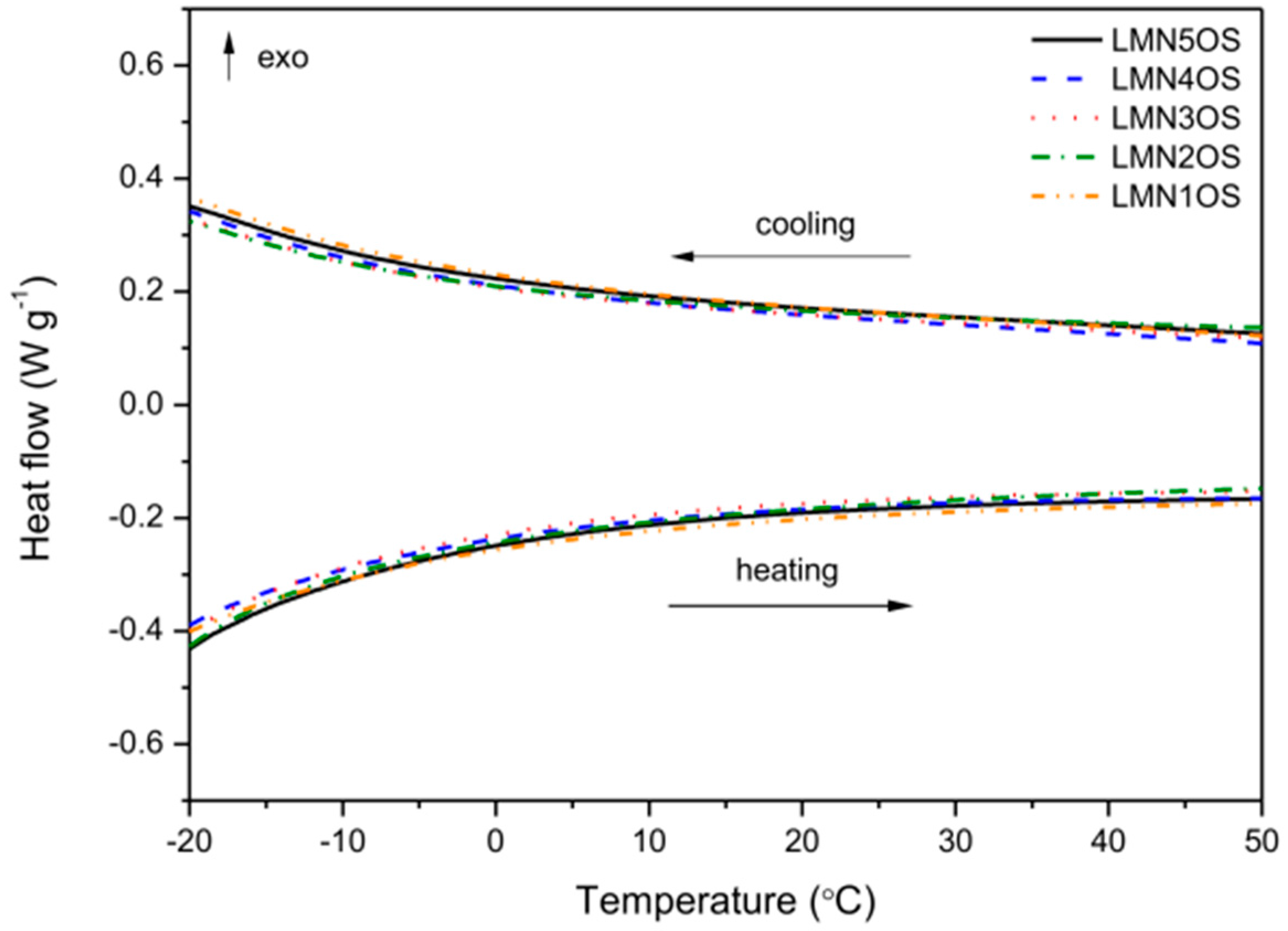
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Braun, P.V.; Cho, J.; Pikul, J.H.; King, W.P.; Zhang, H. High power rechargeable batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 186–198. [Google Scholar] [CrossRef]
- Amine, K.; Liu, J.; Belharouak, I.; Kang, S.H.; Bloom, I.; Vissers, D.; Henriksen, G. Advanced cathode materials for high-power applications. J. Power Sources 2005, 146, 111–115. [Google Scholar] [CrossRef]
- Ohzuku, T.; Brodd, R.J. An overview of positive-electrode materials for advanced lithium-ion batteries. J. Power Sources 2007, 174, 449–456. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Mukherjee, R.; Krishnan, R.; Lu, T.M.; Koratkar, N. Nanostructured electrodes for high-power lithium ion batteries. Nano Energy 2012, 1, 518–533. [Google Scholar] [CrossRef]
- Iwata, E.; Takahashi, K.; Maeda, K.; Mouri, T. Capacity failure on cycling or storage of lithium-ion batteries with Li–Mn–O ternary phases having spinel-framework structure and its possible solution. J. Power Sources 1999, 81–82, 430–433. [Google Scholar] [CrossRef]
- Yang, L.; Takahashi, M.; Wang, B. A study on capacity fading of lithium-ion battery with manganese spinel positive electrode during cycling. Electrochim. Acta 2006, 51, 3228–3234. [Google Scholar] [CrossRef]
- Yamada, A.; Tanaka, M.; Tanaka, K.; Sekai, K. Jahn–Teller instability in spinel Li–Mn–O. J. Power Sources 1999, 81–82, 73–78. [Google Scholar] [CrossRef]
- Li, G.; Iijima, Y.; Kudo, Y.; Azuma, H. Structural changes of manganese spinel at elevated temperatures. Solid State Ion. 2002, 146, 55–63. [Google Scholar] [CrossRef]
- Raveendranath, K.; Ravi, J.; Tomy, R.M.; Jayalekshmi, S.; Mangalaraja, R.V.; Lee, S.T. Evidence of Jahn–Teller distortion in LixMn2O4 by thermal diffusivity measurements. Appl. Phys. A 2008, 90, 437–440. [Google Scholar] [CrossRef]
- Wang, L.F.; Ou, C.C.; Striebel, K.A.; Chen, J.S. Study of Mn dissolution from LiMn2O4 spinel electrodes using rotating ring-disk collection experiments. J. Electrochem. Soc. 2003, 150, A905–A911. [Google Scholar] [CrossRef]
- Chen, J.S.; Wang, L.F.; Fang, B.J.; Lee, S.Y.; Guo, R.Z. Rotating ring–disk electrode measurements on Mn dissolution and capacity losses of spinel electrodes in various organic electrolytes. J. Power Sources 2006, 157, 515–521. [Google Scholar] [CrossRef]
- Vogler, C.; Butz, A.; Dittrich, H.; Arnold, G.; Wohlfahrt-Mehrens, M. Electrochemical and structural comparison of doped lithium manganese spinels. J. Power Sources 1999, 84, 243–247. [Google Scholar] [CrossRef]
- Mandal, S.; Rojas, R.M.; Amarilla, J.M.; Calle, P.; Kosova, N.V.; Anufrienko, V.F.; Rojo, J.M. High temperature Co-doped LiMn2O4-based spinels. structural, electrical, and electrochemical characterization. Chem. Mater. 2002, 14, 1598–1605. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Tan, H.; Yang, Z.; Zeng, J. Preparation and doping mode of doped LiMn2O4 for Li-ion batteries. Energies 2013, 6, 1718–1730. [Google Scholar] [CrossRef]
- Huang, J.; Yang, F.; Guo, Y.; Peng, C.; Bai, H.; Peng, J.; Guo, J. LiMgxMn2−xO4 (x ≤ 0.10) cathode materials with high rate performance prepared by molten-salt combustion at low temperature. Ceram. Int. 2015, 41, 9662–9667. [Google Scholar] [CrossRef]
- Chen, R.; Knapp, M.; Yavuz, M.; Heinzmann, R.; Wang, D.; Ren, S.; Trouillet, V.; Lebedkin, S.; Doyle, S.; Hahn, H.; et al. Reversible Li+ storage in a LiMnTiO4 spinel and its structural transition mechanisms. J. Phys. Chem. C 2014, 118, 12608–12616. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, D.; Zhang, H.; Wang, S. Plasma-assisted sulfur doping of LiMn2O4 for high-performance lithium-ion batteries. J. Phys. Chem. C 2015, 119, 28776–28782. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Li, X.; Wang, Z.; Guo, H.; Xu, D.; Zhang, K. Sputtering graphite coating to improve the elevated-temperature cycling ability of the LiMn2O4 electrode. Phys. Chem. Chem. Phys. 2014, 16, 16021–16029. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, M.; Li, X.; Chen, C.; Xiong, D.; Dong, L.; Li, D.; Lushington, A.; Sun, X. A novel coating onto LiMn2O4 cathode with increased lithium ion battery performance. Appl. Surf. Sci. 2014, 317, 884–891. [Google Scholar] [CrossRef]
- Wang, J.; Yao, S.; Lin, W.; Wu, B.; He, X.; Li, J.; Zhao, J. Improving the electrochemical properties of high-voltage lithium nickel manganese oxide by surface coating with vanadium oxides for lithium ion batteries. J. Power Sources 2015, 280, 114–124. [Google Scholar] [CrossRef]
- Jiang, R.; Cui, C.; Ma, H.; Ma, H.; Chen, T. Study on the enhanced electrochemical performance of LiMn2O4 cathode material at 55 °C by the nano Ag-coating. J. Electroanal. Chem. 2015, 744, 69–76. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Zhu, D.; Huang, L.; Chen, Y. Improvement of the overall performances of LiMn2O4 via surface-modification by polypyrrole. Mater. Res. Bull. 2015, 71, 91–97. [Google Scholar] [CrossRef]
- Shang, Y.; Lin, X.; Lu, X.; Huang, T.; Yu, A. Nano-TiO2(B) coated LiMn2O4 as cathode materials for lithium-ion batteries at elevated temperatures. Electrochim. Acta 2015, 156, 121–126. [Google Scholar] [CrossRef]
- Molenda, M.; Dziembaj, R.; Podstawka, E.; Proniewicz, L.M.; Piwowarska, Z. An attempt to improve electrical conductivity of the pyrolised carbon-LiMn2O4−ySy (0 ≤ y ≤ 0.5) composites. J. Power Sources 2007, 174, 613–618. [Google Scholar] [CrossRef]
- Lin, H.B.; Hu, J.N.; Rong, H.B.; Zhang, Y.M.; Mai, S.W.; Xing, L.D.; Xu, M.Q.; Li, X.P.; Li, W.S. Porous LiMn2O4 cubes architectured with single-crystalline nanoparticles and exhibiting excellent cyclic stability and rate capability as the cathode of a lithium ion battery. J. Mater. Chem. A 2014, 2, 9272–9279. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, Y.; Wang, X.; Jia, D.; Pang, W.; Guo, Z.; Du, Y.; Tang, X. Facile synthesis of LiMn2O4 octahedral nanoparticles as cathode materials for high capacity lithium ion batteries with long cycle life. J. Power Sources 2015, 278, 574–581. [Google Scholar] [CrossRef]
- Gao, X.; Sha, Y.; Lin, Q.; Cai, R.; Tade, M.O.; Shao, Z. Combustion-derived nanocrystalline LiMn2O4 as a promising cathode material for lithium-ion batteries. J. Power Sources 2015, 275, 38–44. [Google Scholar] [CrossRef]
- Zhao, H.; Li, F.; Liu, X.; Xiong, W.; Chen, B.; Shao, H.; Que, D.; Zhang, Z.; Wu, Y. A simple, low-cost and eco-friendly approach to synthesize single-crystalline LiMn2O4 nanorods with high electrochemical performance for lithium-ion batteries. Electrochim. Acta 2015, 166, 124–133. [Google Scholar] [CrossRef]
- Sun, Y.K.; Oh, S.W.; Yoon, C.S.; Bang, H.J.; Prakash, J. Effect of sulfur and nickel doping on morphology and electrochemical performance of LiNi0.5Mn1.5O4−xSx spinel material in 3-V region. J. Power Sources 2006, 161, 19–26. [Google Scholar] [CrossRef]
- Raja, M.W.; Mahanty, S.; Basu, R.N. Influence of S and Ni co-doping on structure, band gap and electrochemical properties of lithium manganese oxide synthesized by soft chemical method. J. Power Sources 2009, 192, 618–626. [Google Scholar] [CrossRef]
- Molenda, M.; Bakierska, M.; Majda, D.; Świętosławski, M.; Dziembaj, R. Structural and electrochemical characterization of sulphur-doped lithium manganese spinel cathode materials for lithium ion batteries. Solid State Ion. 2015, 272, 127–132. [Google Scholar] [CrossRef]
- Hernán, L.; Morales, J.; Sánchez, L.; Rodríguez Castellón, E.; Aranda, M.A.G. Synthesis, characterization and comparative study of the electrochemical properties of doped lithium manganese spinels as cathodes for high voltage lithium batteries. J. Mater. Chem. 2002, 12, 734–741. [Google Scholar] [CrossRef]
- Markovsky, B.; Talyossef, Y.; Salitra, G.; Aurbach, D.; Kim, H.-J.; Choi, S. Cycling and storage performance at elevated temperatures of LiNi0.5Mn1.:5O4 positive electrodes for advanced 5 V Li-ion batteries. Electrochem. Commun. 2004, 6, 821–826. [Google Scholar] [CrossRef]
- Patoux, S.; Sannier, L.; Lignier, H.; Reynier, Y.; Bourbon, C.; Jouanneau, S.; Cras, F.; Martinet, S. High voltage nickel manganese spinel oxides for Li-ion batteries. Electrochim. Acta 2008, 53, 4137–4145. [Google Scholar] [CrossRef]
- Dziembaj, R.; Molenda, M.; Majda, D.; Walas, S. Synthesis, thermal and electrical properties of Li1+δMn2−δO4 prepared by a sol-gel method. Solid State Ion. 2003, 157, 81–87. [Google Scholar] [CrossRef]
- Dziembaj, R.; Molenda, M. Stabilization of the spinel structure in Li1+δMn2−δO4 obtained by sol-gel method. J. Power Sources 2003, 119, 121–124. [Google Scholar] [CrossRef]
- Park, D.H.; Lim, S.T.; Hwang, S.J.; Choy, J.H.; Choi, J.H.; Choo, J. Influence of nickel content on the chemical bonding character of LiMn2−xNixO4 spinel oxides. J. Power Sources 2006, 159, 1346–1352. [Google Scholar] [CrossRef]
- Oikawa, K.; Kamiyama, T.; Izumi, F.; Chakoumakos, B.C.; Ikuta, H.; Wakihara, M.; Li, J.; Matsui, Y. Structural phase transition of the spinel-type oxide LiMn2O4. Solid State Ion. 1998, 109, 35–41. [Google Scholar] [CrossRef]
- Chitra, S.; Kalyani, P.; Mohan, T.; Massot, M.; Ziolkiewicz, S.; Gangandharan, R.; Eddrief, M.; Julien, C. Physical properties of LiMn2O4 spinel prepared at moderate temperature. Ionics 1998, 4, 8–15. [Google Scholar] [CrossRef]
- Molenda, J.; Świerczek, K.; Kucza, W.; Marzec, J.; Stoklosa, A. Electrical properties of LiMn2O4-δ at temperatures 220–1100 K. Solid State Ion. 1999, 123, 155–163. [Google Scholar] [CrossRef]
- Zhuang, Q.C.; Wei, T.; Du, L.L.; Cui, Y.L.; Fang, L.; Sun, S.G. An electrochemical impedance spectroscopic study of the electronic and ionic transport properties of spinel LiMn2O4. J. Phys. Chem. C 2010, 114, 8614–8621. [Google Scholar] [CrossRef]
- Wu, P.; Zeng, X.L.; Zhou, C.; Gu, G.F.; Tong, D.G. Improved electrochemical performance of LiNi0.5−xRhxMn1.5O4 cathode materials for 5 V lithium ion batteries via Rh-doping. Mater. Chem. Phys. 2013, 138, 716–723. [Google Scholar] [CrossRef]
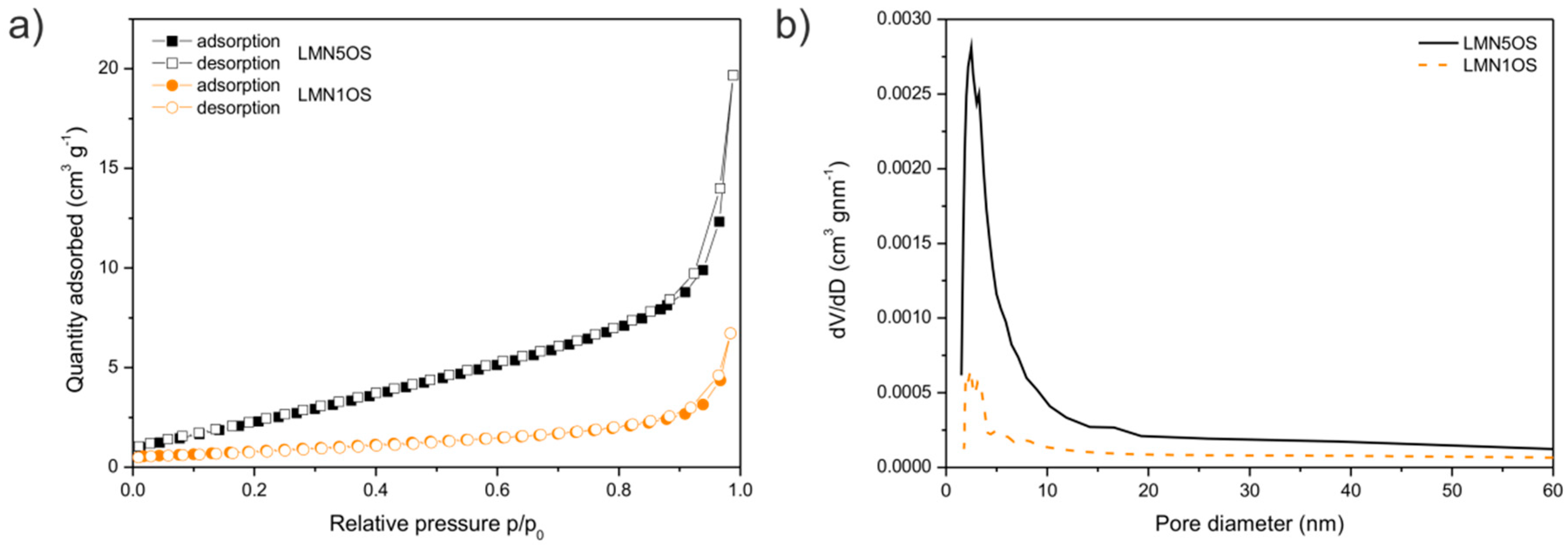
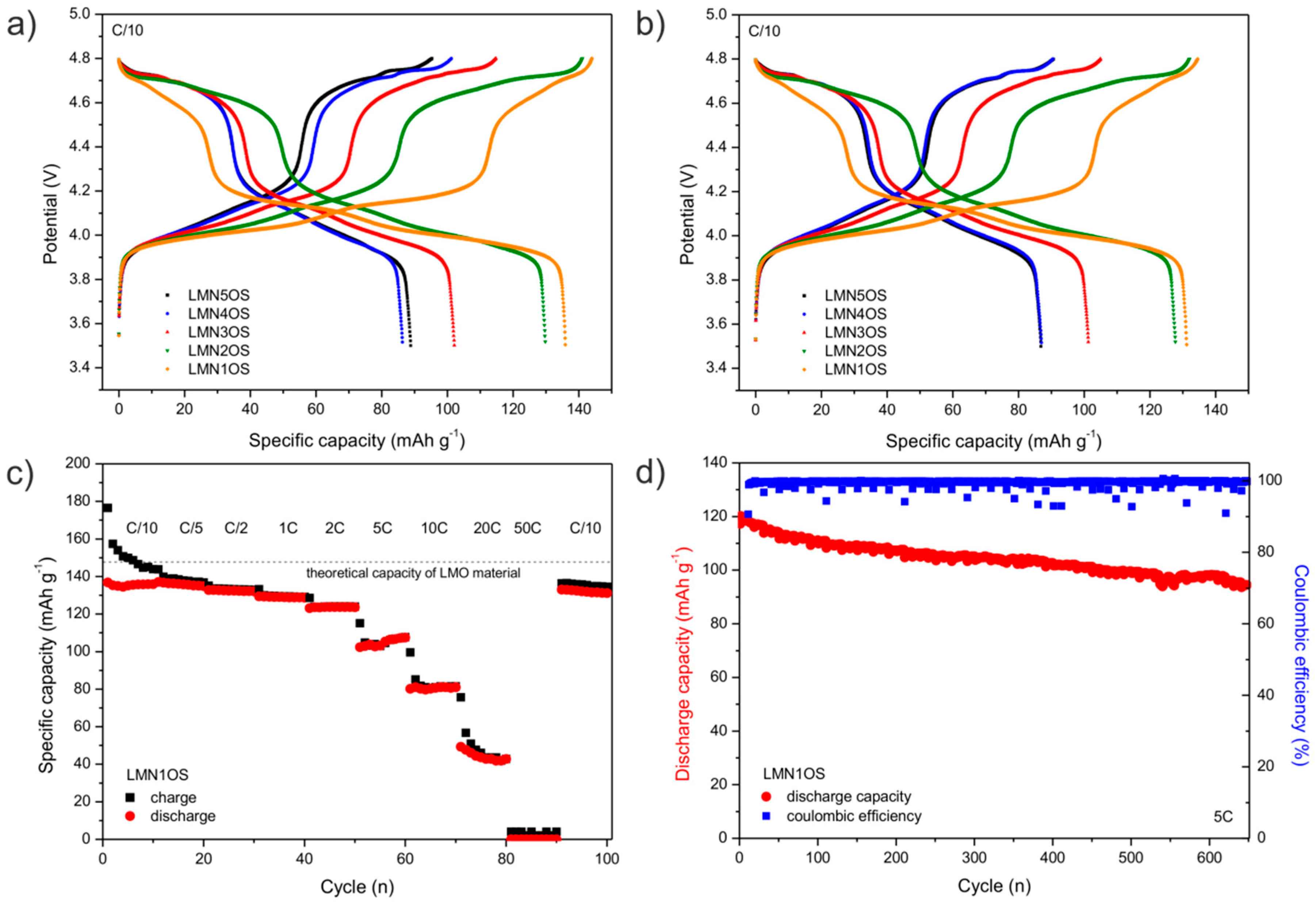
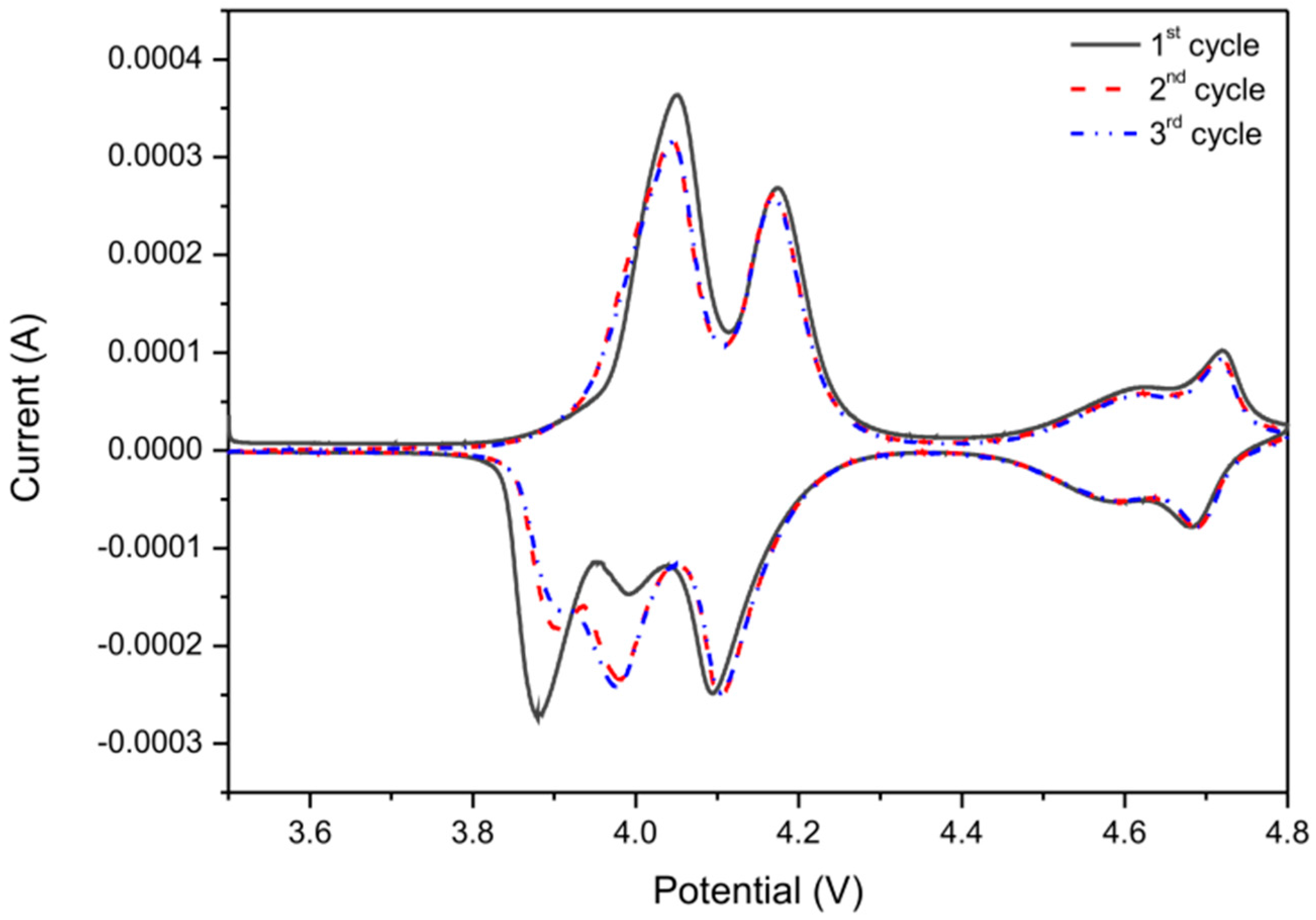
| Sample | Nominal Composition | Lattice Constant (nm) | Average Crystallites Size (nm) | Surface BET Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|---|
| LMN5OS | LiMn1.5Ni0.5O3.99S0.01 | 0.8181 | 42 | 10.9 | 0.030 | 11 |
| LMN4OS | LiMn1.6Ni0.4O3.99S0.01 | 0.8169 | 40 | 8.7 | 0.041 | 19 |
| LMN3OS | LiMn1.7Ni0.3O3.99S0.01 | 0.8183 | 36 | 7.9 | 0.030 | 19 |
| LMN2OS | LiMn1.8Ni0.2O3.99S0.01 | 0.8172 | 47 | 3.5 | 0.012 | 13 |
| LMN1OS | LiMn1.9Ni0.1O3.99S0.01 | 0.8149 | 48 | 3.1 | 0.010 | 13 |
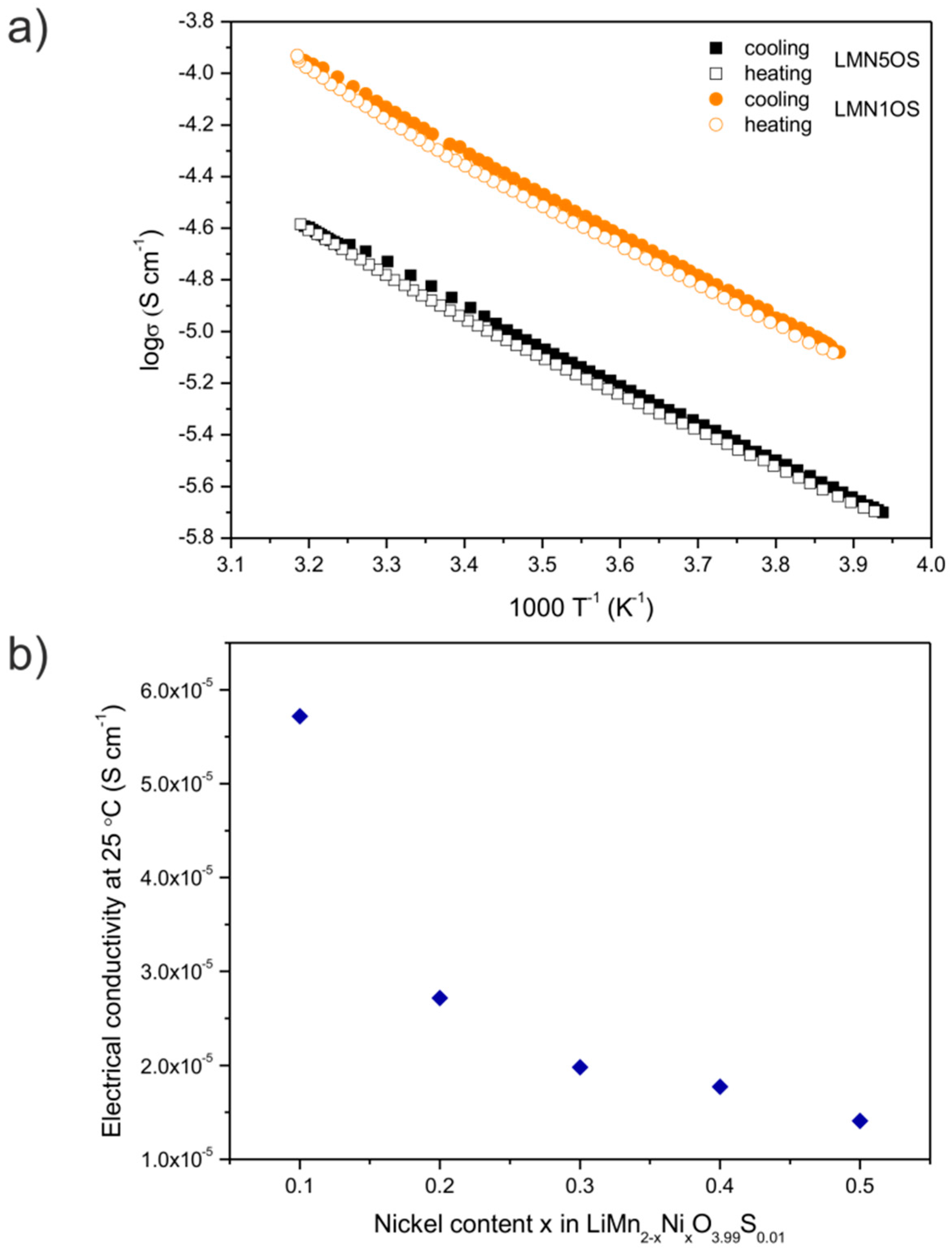
| Sample | Activation Energy (Cooling) (eV) | Activation Energy (Heating) (eV) | Electrical Conductivity at Around 25 °C (Cooling) (10−5·S·cm−1) | Electrical Conductivity at Around 25 °C (Heating) (10−5·S·cm−1) |
|---|---|---|---|---|
| LMN5OS | 0.30 | 0.30 | 1.46 | 1.35 |
| LMN4OS | 0.30 | 0.30 | 1.84 | 1.71 |
| LMN3OS | 0.30 | 0.30 | 2.12 | 1.84 |
| LMN2OS | 0.32 | 0.32 | 2.84 | 2.59 |
| LMN1OS | 0.32 | 0.32 | 5.97 | 5.46 |
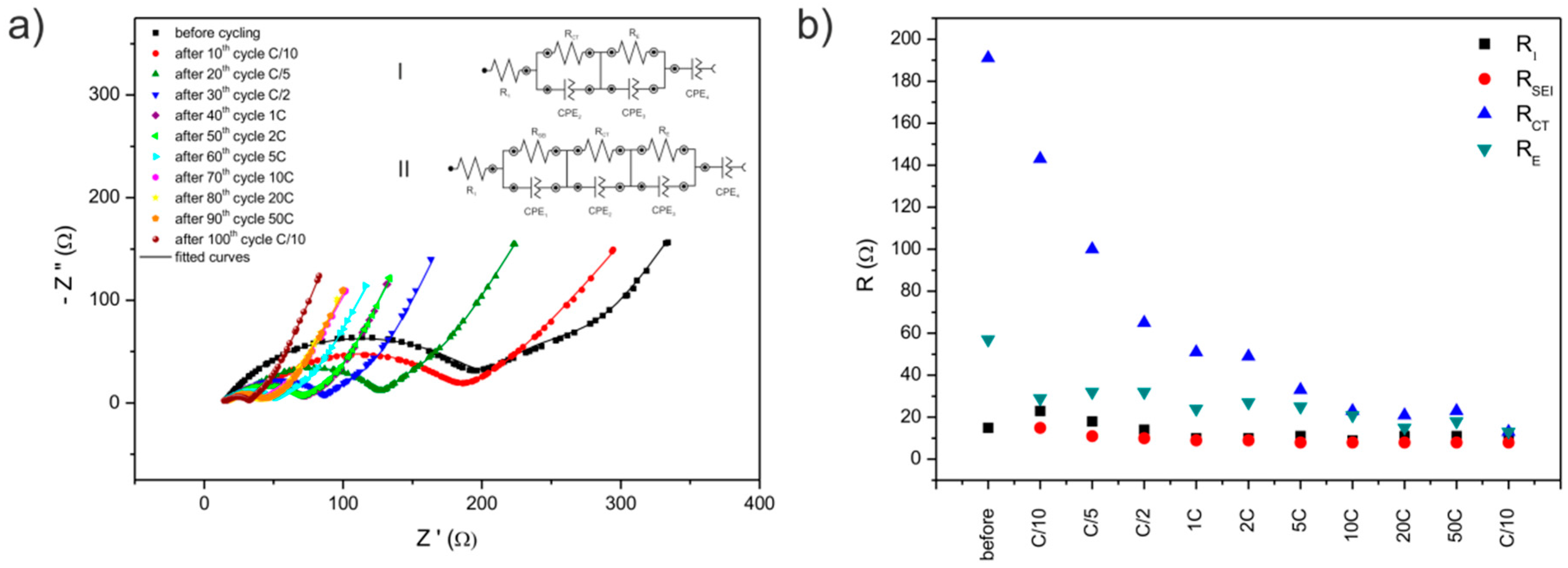
| R1 (Ω) | RSEI (Ω) | RCT (Ω) | RE (Ω) | |
|---|---|---|---|---|
| before cycling | 15 | - | 191 | 57 |
| after 10th cycle C/10 | 23 | 15 | 143 | 29 |
| after 20th cycle C/5 | 18 | 11 | 100 | 32 |
| after 30th cycle C/2 | 14 | 10 | 65 | 32 |
| after 40th cycle 1C | 10 | 9 | 51 | 24 |
| after 50th cycle 2C | 10 | 9 | 49 | 27 |
| after 60th cycle 5C | 11 | 8 | 33 | 25 |
| after 70th cycle 10C | 9 | 8 | 23 | 21 |
| after 80th cycle 20C | 11 | 8 | 21 | 15 |
| after 90th cycle 50C | 11 | 8 | 23 | 18 |
| after 100th cycle C/10 | 11 | 8 | 13 | 13 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakierska, M.; Świętosławski, M.; Gajewska, M.; Kowalczyk, A.; Piwowarska, Z.; Chmielarz, L.; Dziembaj, R.; Molenda, M. Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S. Materials 2016, 9, 366. https://doi.org/10.3390/ma9050366
Bakierska M, Świętosławski M, Gajewska M, Kowalczyk A, Piwowarska Z, Chmielarz L, Dziembaj R, Molenda M. Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S. Materials. 2016; 9(5):366. https://doi.org/10.3390/ma9050366
Chicago/Turabian StyleBakierska, Monika, Michał Świętosławski, Marta Gajewska, Andrzej Kowalczyk, Zofia Piwowarska, Lucjan Chmielarz, Roman Dziembaj, and Marcin Molenda. 2016. "Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S" Materials 9, no. 5: 366. https://doi.org/10.3390/ma9050366
APA StyleBakierska, M., Świętosławski, M., Gajewska, M., Kowalczyk, A., Piwowarska, Z., Chmielarz, L., Dziembaj, R., & Molenda, M. (2016). Enhancement of Electrochemical Performance of LiMn2O4 Spinel Cathode Material by Synergetic Substitution with Ni and S. Materials, 9(5), 366. https://doi.org/10.3390/ma9050366








