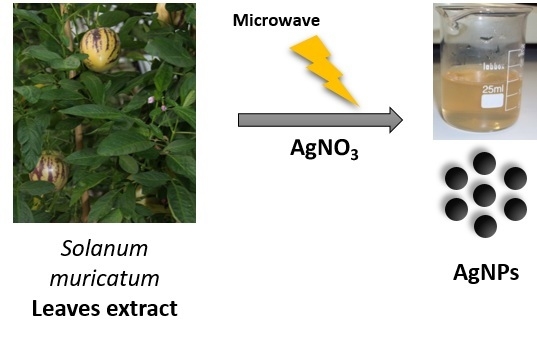
Rapid Biosynthesis of Silver Nanoparticles Using Pepino (Solanum muricatum) Leaf Extract and Their Cytotoxicity on HeLa Cells
Abstract
:1. Introduction
2. Results
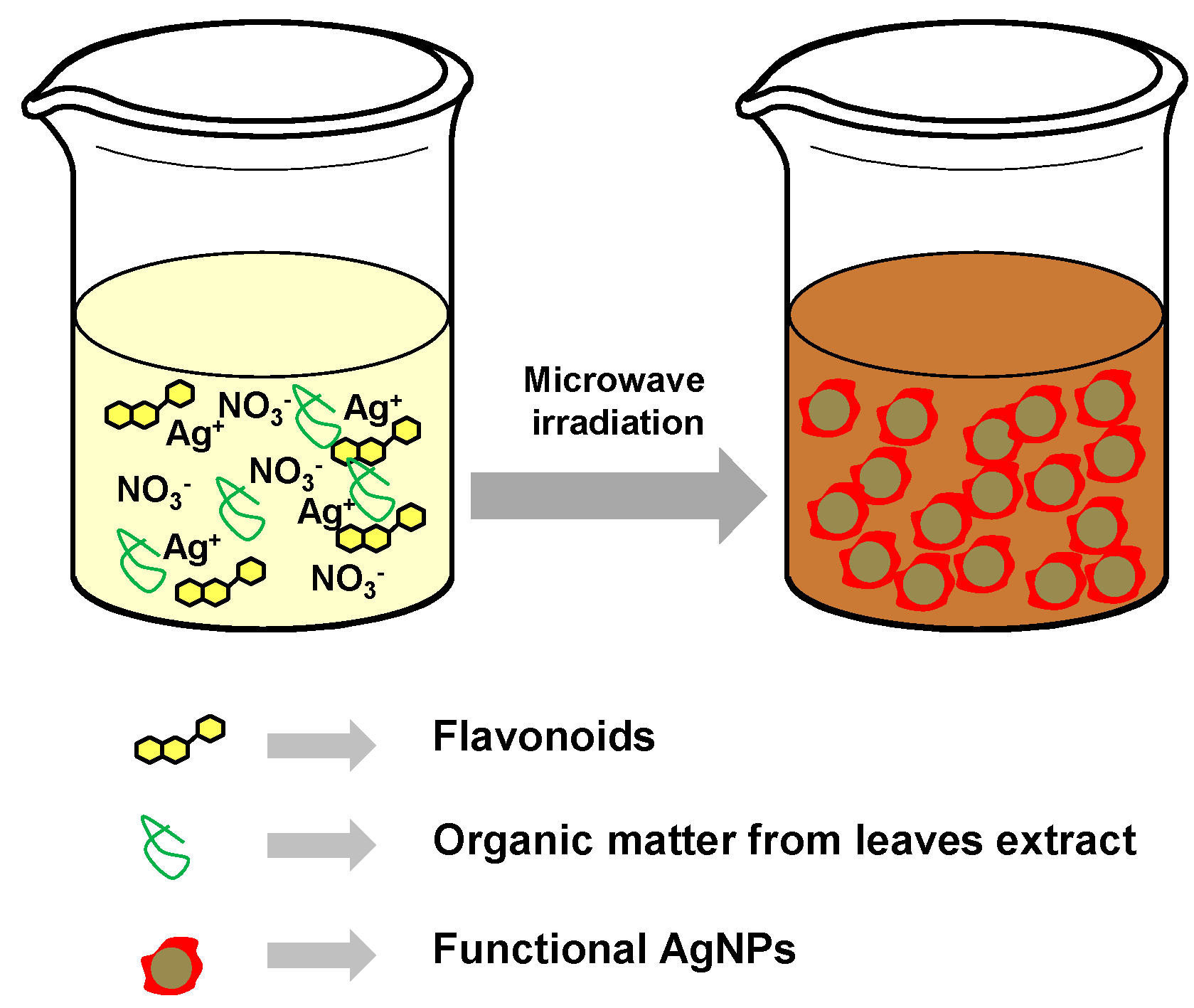
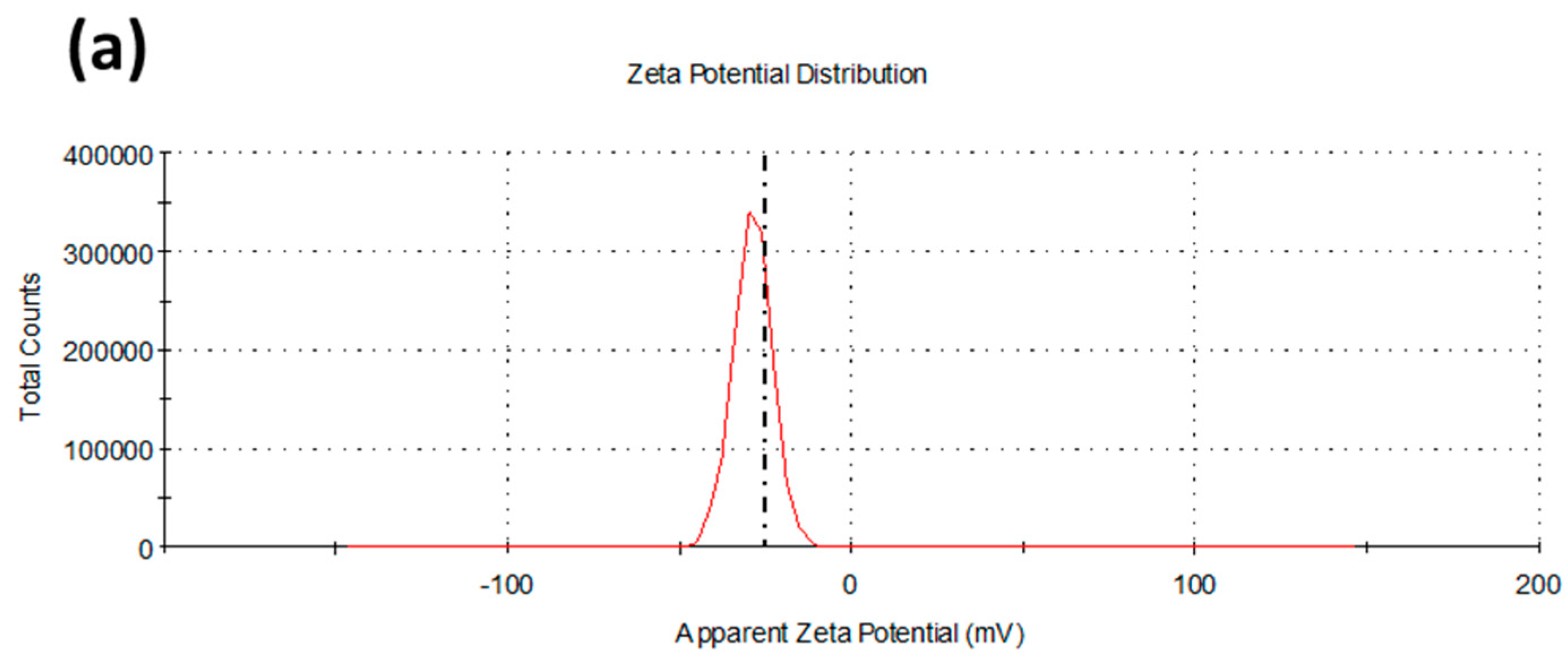
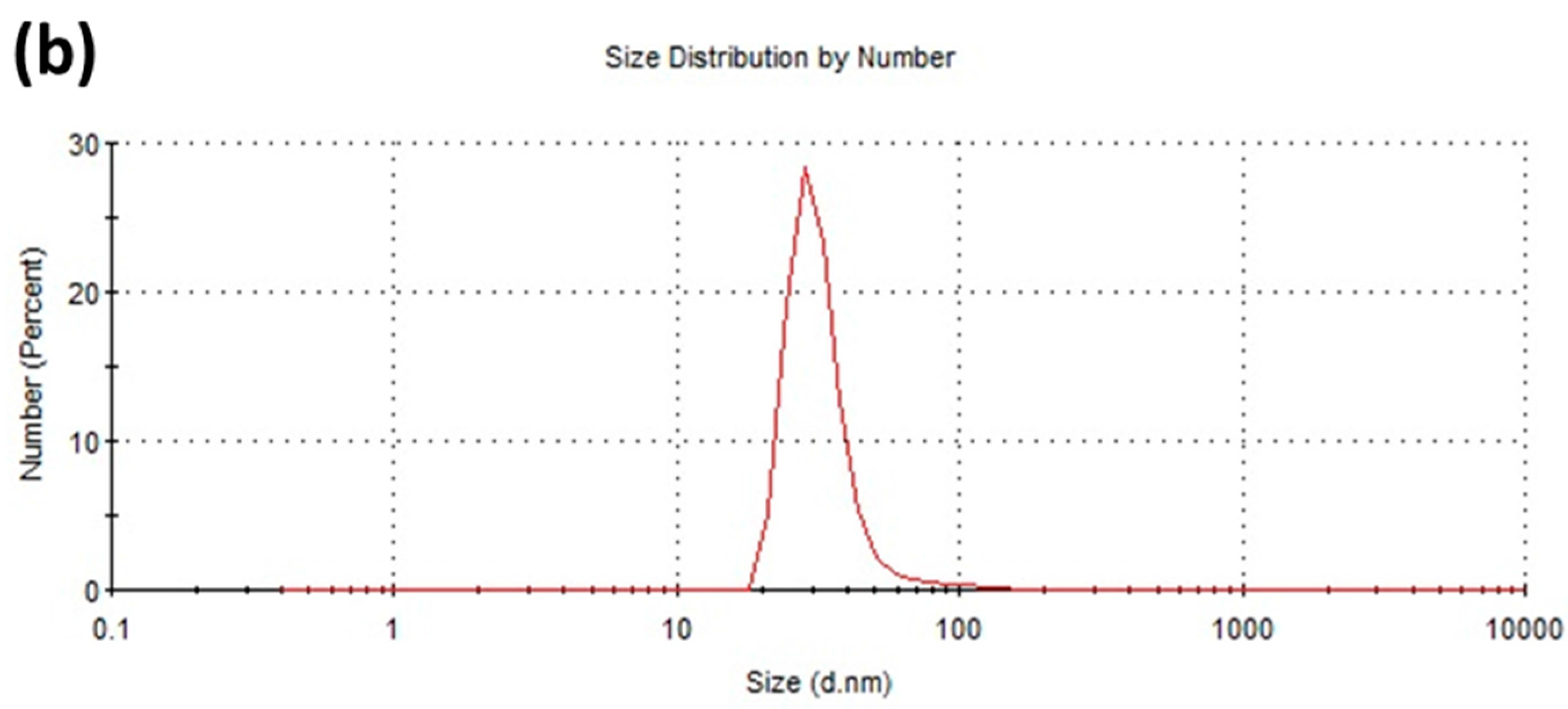
2.1. Synthesis and Characterization of Biofunctionalized Silver Nanoparticles (FAgNP)
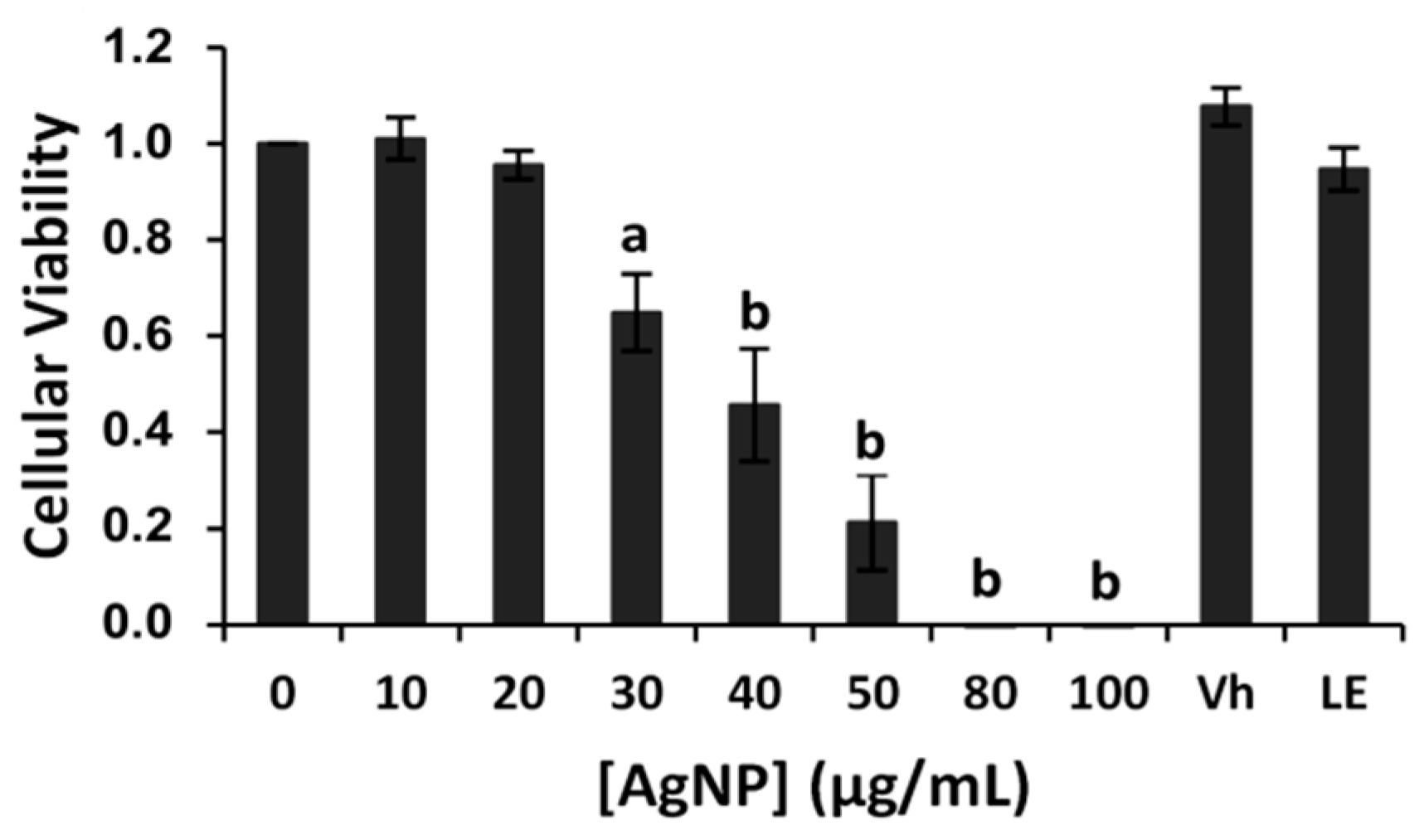
2.2. Cytotoxicity Studies
3. Materials and Methods
3.1. General Remarks
3.2. Green Synthesis of the Silver Nanoparticles
3.3. Characterization
3.4. Cell Lines and Culture Conditions
3.5. Effects on Cell Growth/Viability
3.6. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNP | Silver nanoparticles |
| DLS | Dynamic Light Scattering |
| FAgNP | Functionalized silver nanoparticles |
| FESEM | Field emission scanning electron microscopy |
| LE | Leaf extract |
| MNP | Metal Nanoparticles |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| S. muricatum | Solanum muricatum |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| Vh | Vehicle |
References
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Brauman, J.I. Room at the Bottom. Science 1991, 254, 1277. [Google Scholar] [CrossRef] [PubMed]
- Habouti, S.; Solterbeck, C.H.; Es-Souni, M. Synthesis of silver nano-fir-twigs and application to single molecules detection. J. Mater. Chem. 2010, 20, 5215–5219. [Google Scholar] [CrossRef]
- Hu, X.; Chan, C.T. Photonic crystals with silver nanowires as a near-infrared superlens. Appl. Phys. Lett. 2004, 85, 1520–1522. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced electrical conductivity of silver nanoparticles for high frequency electronic applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, J.; Lam, C.; Yu, Y. A novel green synthesis approach for polymer nanocomposites decorated with silver nanoparticles and their antibacterial activity. Analyst 2014, 139, 5793–5799. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.C.; Hutchison, J.E. Green nanoscience: An integrated approach to greener products, processes and applications. Chim. Oggi 2004, 22, 30–33. [Google Scholar]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [PubMed]
- Mahendra, R.; Avinash, P.I.; Indarchand, R.G.; Sonal, S.B.; Alka, P.Y.; Kamel, A.A. Potential role of biological systems in formation of nanoparticles: Mechanism of synthesis and biomedical applications. Curr. Nanosci. 2013, 9, 576–587. [Google Scholar]
- Joseph, S.; Mathew, B. Microwave assisted facile green synthesis of silver and gold nanocatalysts using the leaf extract of Aerva lanata. Spectrochim. Acta Mol. Biomol. Spectrosc. 2014, 136PC, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K.; et al. Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B 2013, 106, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Sharanabasava, V.G.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus florida and its anticancer evaluation. J. Photochem. Photobiol. B Biol. 2013, 125, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Ganachari, S.; Deshpande, R.; Ravindra, G.; Venkataraman, A. Rapid biosynthesis of silver nanoparticles using areca nut (areca catechu) extract under microwave-assistance. J. Cluster Sci. 2012, 24, 107–114. [Google Scholar] [CrossRef]
- Bhat, R.; Deshpande, R.; Ganachari, S.V.; Huh do, S.; Venkataraman, A. Photo-irradiated biosynthesis of silver nanoparticles using edible mushroom pleurotus Florida and their antibacterial activity studies. Bioinorg. Chem. Appl. 2011, 650979. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Saware, K.; Venkataraman, A. Biosynthesis and characterization of stable silver nanoparticles using ficus religiosa leaf extract: A mechanism perspective. J. Cluster Sci. 2014, 25, 1157–1171. [Google Scholar] [CrossRef]
- Manikprabhu, D.; Lingappa, K. Microwave assisted rapid and green synthesis of silver nanoparticles using a pigment produced by streptomyces coelicolor klmp33. Bioinorg. Chem. Appl. 2013, 341798. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, B.D.; Prabhakar, B.K.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Karman, S.B.; Diah, S.Z.M.; Gebeshuber, I.C. Raw materials synthesis from heavy metal industry effluents with bioremediation and phytomining: A biomimetic resource management approach. Adv. Mater. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Pavani, K.V.; Sunil Kumar, N.; Gayathramma, K. Plants as ecofriendly nanofactories. J. Bionanosci. 2012, 6, 1–6. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-mediated synthesis of nanoparticles: A newer and safer tool against mosquito-borne diseases? Asia Pacif. J. Trop. Biomed. 2015. [Google Scholar] [CrossRef]
- Mittal, A.K.; Tripathy, D.; Choudhary, A.; Aili, P.K.; Chatterjee, A.; Singh, I.P.; Banerjee, U.C. Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Mol. Med. 2016, 11, 715–728. [Google Scholar]
- Sathishkumar, P.; Vennila, K.; Jayakumar, R.; Yusoff, A.R.M.; Hadibarata, T.; Palvannan, T. Phyto-synthesis of silver nanoparticles using Alternanthera tenella leaf extract: An effective inhibitor for the migration of human breast adenocarcinoma (MCF-7) cells. Bioprocess Biosyst. Eng. 2016. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Kariuki, V.M.; Yazgan, I.; Jimenez, A.; Luther, D.; Schulte, J.; Sadik, O.A. Synthesis and antibacterial characterization of sustainable nanosilver using naturally-derived macromolecules. Sci. Total Environ. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, D.; Basavaraja, S.; Mahesh, B.; Balaji, S.; Manjunath, S.Y.; Venkataraman, A. Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. Nanobiotechnology 2009, 5, 34–41. [Google Scholar] [CrossRef]
- Raghunandan, D.; Basavaraja, S.; Mahesh, B.; Balaji, S.; Manjunath, S.Y.; Venkataraman, A. Microwave-assisted rapid extracellular synthesis of stable bio-functionalized silver nanoparticles from guava (Psidium guajava) leaf extract. J. Nanopart. Res. 2010, 13, 2021–2028. [Google Scholar] [CrossRef]
- Raghunandan, D.; Bedre, M.D.; Basavaraja, S.; Sawle, B.; Manjunath, S.Y.; Venkataraman, A. Rapid biosynthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf. B Biointerfaces 2010, 79, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Borase, H.P.; Salunke, B.K.; Salunkhe, R.B.; Patil, C.D.; Hallsworth, J.E.; Kim, B.S.; Patil, S.V. Plant extract: A promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014, 173, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pasricha, R.; Sastry, M. Ultra-low level optical detection of mercuric ions using biogenic gold nanotriangles. Analyst 2012, 137, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, D.; Ravishankar, B.; Ganachari, S.; Mahesh, D.B.; Harsoor, V.; Yalagatti, M.S.; Bhagawanraju, M.; Venkataraman, A. Anti-cancer studies of noble metal nanoparticles synthesized using different plant extracts. Cancer Nanotechnol. 2011, 2, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Kavithaa, K.; Paulpandi, M.; Ponraj, T.; Alsalhi, M.S.; Devanesan, S.; Subramaniam, J.; Rajaganesh, R.; Wei, H.; et al. Hydrothermal synthesis of titanium dioxide nanoparticles: Mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7). Parasitol. Res. 2016, 115, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, A.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Dinesh, D.; Vadivalagan, C.; Aziz, A.T.; Chandramohan, B.; Suresh, U.; Rajaganesh, R.; et al. Earthworm-mediated synthesis of silver nanoparticles: A potent tool against hepatocellular carcinoma, pathogenic bacteria, Plasmodium parasites and malaria mosquitoes. Parasitol. Int. 2016, 65, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Hsieh, C.L.; Peng, C.C.; Hsieh-Li, H.M.; Chiang, H.S.; Huang, K.D.; Peng, R.Y. Brain derived metastatic prostate cancer DU-145 cells are effectively inhibited in vitro by guava (Psidium gujava L.) leaf extracts. Nutr. Cancer 2007, 58, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.; Reddy, R.C.; Maheswaran, T.; Asokan, G.S.; Dany, A.; Anand, B. Theranostics: A treasured tailor for tomorrow. J. Pharm. Bioall. Sci. 2014, 6, S6–S8. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Nakamura, T.; Hosokawa, T.; Suzuki-Yamamoto, T.; Yamashita, H.; Kimoto, M.; Tsuji, H.; Yoshida, H.; Hada, T.; Takahashi, Y. Antiproliferative activity of guava leaf extract via inhibition of prostaglandin endoperoxide H synthase isoforms. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Popenoe, H.; King, S.R.; León, J.; Kalinowski, L.S.; Vietmeyer, N.D.; Dafforn, M. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academy Press: Washington, DC, USA, 1989; pp. 297–305. [Google Scholar]
- Rodríguez-Burruezo, A.; Prohens, J.; Fita, A.M. Breeding strategies for improving the performance and fruit quality of the pepino (Solanum muricatum): A model for the enhancement of underutilized exotic fruits. Food Res. Intl. 2011, 44, 1927–1935. [Google Scholar] [CrossRef]
- Hsu, C.C.; Guo, Y.R.; Wang, Z.H.; Yin, M.C. Protective effects of an aqueous extract from pepino (Solanum muricatum Ait.) in diabetic mice. J. Sci. Food Agric. 2011, 91, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Tang, D.G. Extract of Solanum muricatum (Pepino/CSG) inhibits tumor growth by inducing apoptosis. Anticancer Res. 1999, 19, 403–408. [Google Scholar] [PubMed]
- Sudha, G.; Priya, M.S.; Shree, R.B.; Vadivukkarasi, S. Antioxidant activity of ripe and unripe pepino fruit (Solanum muricatum Aiton). J. Food Sci. 2012, 77, C1131–C1135. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; IARC Nonserial Publication: Lyon, France, 2014. [Google Scholar]
- Siegel, R.; Jemal, A. Cancer Facts & Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015. [Google Scholar]
- Sakamoto, J.H.; van de Ven, A.L.; Godin, B.; Blanco, E.; Serda, R.E.; Grattoni, A.; Ziemys, A.; Bouamrani, A.; Hu, T.; Ranganathan, S.I.; et al. Enabling individualized therapy through nanotechnology. Pharmacol. Res. 2010, 62, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Seigneuric, R.; Markey, L.; Nuyten, D.S.; Dubernet, C.; Evelo, C.T.; Finot, E.; Garrido, C. From Nanotechnology to Nanomedicine: Applications to Cancer Research. Curr. Mol. Med. 2010, 10, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kiessling, F.; Gätjens, J. Advanced nanomaterials in multimodal imaging: Design, functionalization, and biomedical applications. J. Nanomater. 2010, 15. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.P.; Lahtinen, M.; Särkkä, H.; Sillanpää, M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B Biointerfaces 2010, 80, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Taneja, P.; Ayyub, P.; Chandra, R. Size dependence of the optical spectrum in nanocrystalline silver. Phys. Rev. B 2002, 65, 5412. [Google Scholar] [CrossRef]
- Duman, O.; Tunc, S. Electrokinetic and rheological properties of Na-bentonite in some electrolyte solutions. Microporous Mesoporous Mater. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomedicine 2014, 9, 2399–2407. [Google Scholar] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, P.; Sathishkumar, G.; Sankar, R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 138, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Balakumaran, M.D.; Ramachandran, R.; Kalaichelvan, P.T. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitrobiological activities. Microbiol. Res. 2015, 178. [Google Scholar] [CrossRef] [PubMed]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R.Br.) Asch. & Magnus mediated silver nanoparticles. J. Photochem. Photobiol. B Biol. 2015, 153, 145. [Google Scholar] [CrossRef]
- Sreekanth, T.V.; Ravikumar, S.; Eom, I.Y. Green synthesized silver nanoparticles using Nelumbonucifera root extract for efficient protein binding, antioxidant and cytotoxicity activities. J. Photochem. Photobiol. B 2014, 141, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Hang, Y.; et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Bala, N. Biomaterials Science: Processing, Properties and Applications II; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 249–259. [Google Scholar]
- Singh, R.P.; Ramarao, P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y.T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2−• versus 1O2. ACS Symp. Ser. 2003, 125, 12803–12809. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low, K.M.G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chem. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Sukirthaa, R.; Priyankaa, K.M.; Antonya, J.J.; Kamalakkannana, S.; Thangamb, R.; Gunasekaranb, P.; Krishnana, M.; Achiramana, S. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbe, M.; Bhat, R.; Aznar, E.; Sancenón, F.; Marcos, M.D.; Herraiz, F.J.; Prohens, J.; Venkataraman, A.; Martínez-Máñez, R. Rapid Biosynthesis of Silver Nanoparticles Using Pepino (Solanum muricatum) Leaf Extract and Their Cytotoxicity on HeLa Cells. Materials 2016, 9, 325. https://doi.org/10.3390/ma9050325
Gorbe M, Bhat R, Aznar E, Sancenón F, Marcos MD, Herraiz FJ, Prohens J, Venkataraman A, Martínez-Máñez R. Rapid Biosynthesis of Silver Nanoparticles Using Pepino (Solanum muricatum) Leaf Extract and Their Cytotoxicity on HeLa Cells. Materials. 2016; 9(5):325. https://doi.org/10.3390/ma9050325
Chicago/Turabian StyleGorbe, Mónica, Ravishankar Bhat, Elena Aznar, Félix Sancenón, M. Dolores Marcos, F. Javier Herraiz, Jaime Prohens, Abbaraju Venkataraman, and Ramón Martínez-Máñez. 2016. "Rapid Biosynthesis of Silver Nanoparticles Using Pepino (Solanum muricatum) Leaf Extract and Their Cytotoxicity on HeLa Cells" Materials 9, no. 5: 325. https://doi.org/10.3390/ma9050325
APA StyleGorbe, M., Bhat, R., Aznar, E., Sancenón, F., Marcos, M. D., Herraiz, F. J., Prohens, J., Venkataraman, A., & Martínez-Máñez, R. (2016). Rapid Biosynthesis of Silver Nanoparticles Using Pepino (Solanum muricatum) Leaf Extract and Their Cytotoxicity on HeLa Cells. Materials, 9(5), 325. https://doi.org/10.3390/ma9050325