1. Introduction
Chlorophenols and other phenol-based compounds have come to represent some of the most dangerous and persistent organic pollutants because of their various industrial applications [
1]. Many of these compounds are byproducts of industrial processes including pharmaceutical, pesticide, paint and solvent production, and wood, paper, and pulp processing. Additionally, as a result of the widespread agricultural use of these compounds as herbicides, insecticides, and fungicides, phenolic pollutants have been detected in many sources of wastewater and drinking water [
2,
3]. Chlorophenols are easily absorbed after ingestion [
4], and while they do not tend to bioaccumulate, the toxicity of these chemicals is well known [
5]. Many phenolic compounds have been identified as possible carcinogens; long-term exposure has been found to cause liver, kidney, and neurological defects [
5].
For obvious reasons, the removal of chlorophenols, and the precursor phenols, from polluted areas and water sources is of utmost importance. Many methods for their removal have been investigated, including biological treatment with microorganisms [
6,
7,
8] and enzymes [
9,
10,
11]; and via adsorption using organobentonites [
12], zeolite [
13], fly ash [
14], and many sources of activated carbons [
15,
16]. Additionally, various catalytic methods have been employed, such as those involving photocatalytic degradation [
17,
18] and electrochemical oxidation [
19].
Activated carbon has proven to be a very effective adsorbent for many types of inorganic and organic compounds because of its high surface area and unique chemical properties, including the polarity and nature of surface functional groups. The surface chemistry of these carbons and the chemical characteristics of adsorbate, such as polarity, ionic nature, functional groups, and solubility, determine the nature of the adsorption mechanism, as well as the extent and strength of adsorption. Moreover, the porous structure of activated carbon consists of a network of interconnected macropores, mesopores, and micropores that provide a good capacity for the adsorption of organic molecules. Thus, it is expected that various mechanisms and forces, such as ion exchange, covalent bonding, van der Waals forces, H-binding, dipole-dipole interactions, and cation- and water-bridging, can be responsible for adsorption of organic compounds in activated carbon [
20,
21,
22,
23,
24]. Despite its many advantages, though, the production and regeneration of activated carbon is very expensive, with higher grades commanding even higher costs. In an attempt to find alternative, more economical, techniques many researchers have explored the use of other sorbents [
25,
26,
27], with varying degrees of success [
24]. Alternatively, activated carbons prepared from inexpensive and plentiful raw materials could prove to be an affordable, yet still very effective, solution [
20,
21,
22,
23]. Activated carbon from agricultural sources, usually wastes, can be prepared either by physical activation, which involves primary carbonization (below 700 °C) followed by controlled gasification under the action of oxidizing gases at high temperature (up to 1100 °C), or chemical activation where the precursor is mixed with a chemical that restricts the formation of tars (e.g., nitric acid, phosphoric acid, hydrogen peroxide,
etc.). After kneading, the precursor is carbonized and washed to produce the final AC. The chemical incorporated into the interior of the precursor particles reacts with the thermal decomposition products reducing the evolution of volatiles and inhibiting the shrinkage of the particles. This increases the conversion of the precursor to carbon and produces carbons with large internal porosity. After the heat treatment, the chemical is removed by copious washing [
20]. In the physical activation, the active carbon is prepared by activating the carbonized intermediate products with gaseous agents. The carbonized intermediate product is prepared by carbonization of the carbonaceous material at higher temperatures. Steam, carbon dioxide, and oxygen are the most common activating agents used. During the activation of the carbonized intermediate product first the disorganized carbon is removed, and by this the surface of the elementary crystallites becomes exposed to the action of the activating agent. The burning out of the crystallites must proceed at different rates on different parts of the surface exposed to reaction; otherwise new pores could not be formed [
28]. A possible explanation of the mechanism is that the velocity with which the crystallites burn is larger in the direction parallel with the plane of the carbon layers than in the direction perpendicular to this plane [
29]. The crystallites orientated perpendicularly to the surface exposed to the action of the activation agents can, therefore, be more easily attacked and burn out more quickly [
28].
Moreno-Castilla [
30] investigated in a comprehensive study the adsorption of organic molecules from dilute aqueous solutions on carbon materials. He concluded that the adsorption process is a complex interplay between non-electrostatic and electrostatic interactions. He showed that “Non-electrostatic interactions are essentially due to dispersion and hydrophobic interactions, whereas the electrostatic or coulombic interactions appear with electrolytes when they are ionized at the experimental conditions used. Both interactions depend on the characteristics of the adsorbent and the adsorptive and the solution chemistry.” A multitude of studies has investigated the surface properties and porous structure characteristics of activated carbons derived from natural residues using phosphoric acid [
31,
32,
33,
34,
35]. Preparation methods comprised chemical and/or physical activation mechanisms. Tailored textural properties and surficial characteristics were possible by applying the appropriate conditions [
32].
Palm dates grow abundantly in tropical regions and the gulf countries of the Middle East; after processing the fruits, the residual stones, which constitute approximately 10% of the weight of the dried fruit [
36], represent a significant agricultural byproduct in these countries. As an economical solution to the problem associated with this waste, activated carbon with high surface areas can be prepared from date pits [
37]. Studies have shown that date pit-derived activated carbon is effective as an adsorbent for phenol and heavy metal contaminants [
38,
39]; here, we investigate its use in the sorption of 4-chlorophenol (4-CP) from aqueous solutions.
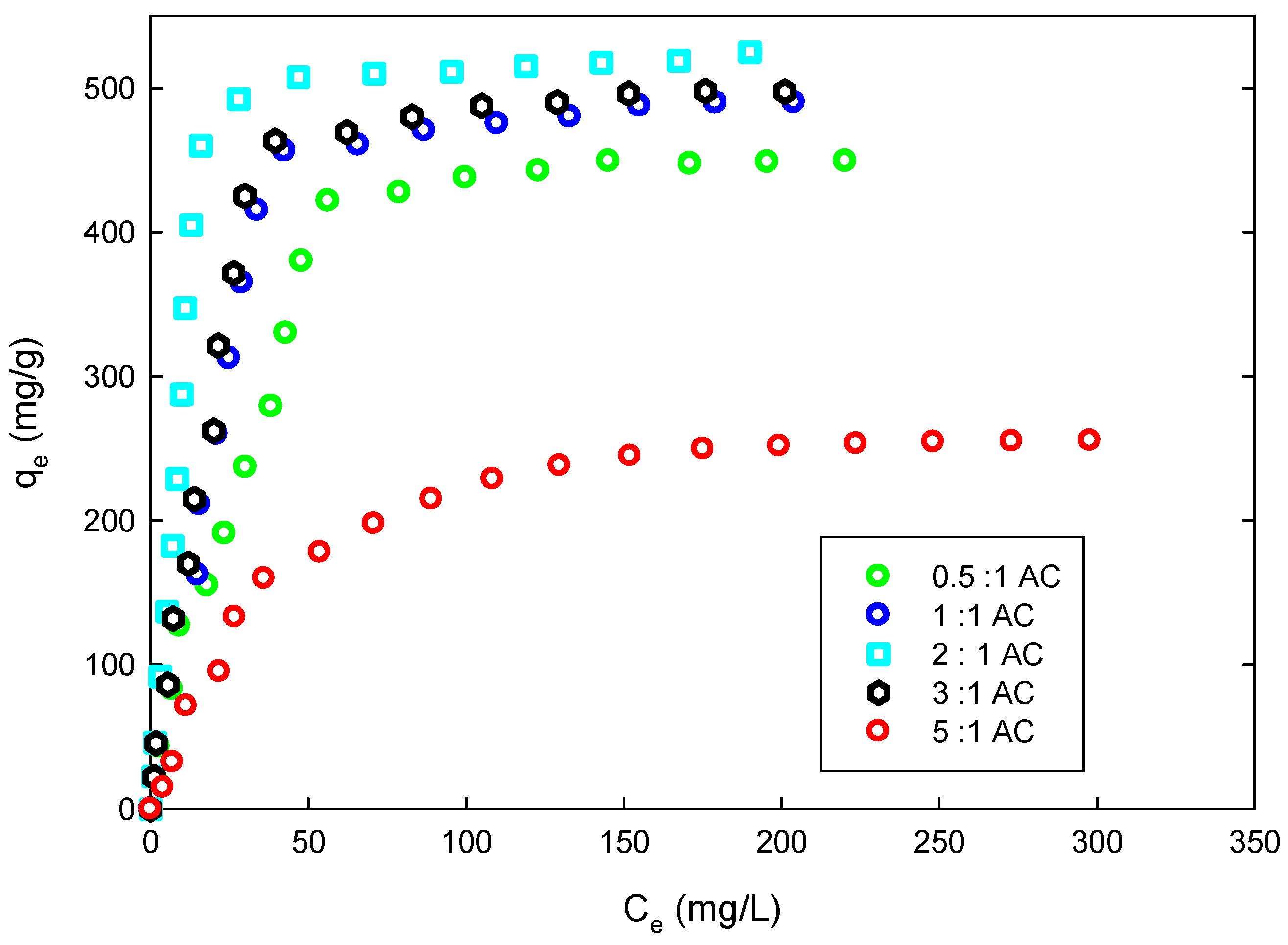
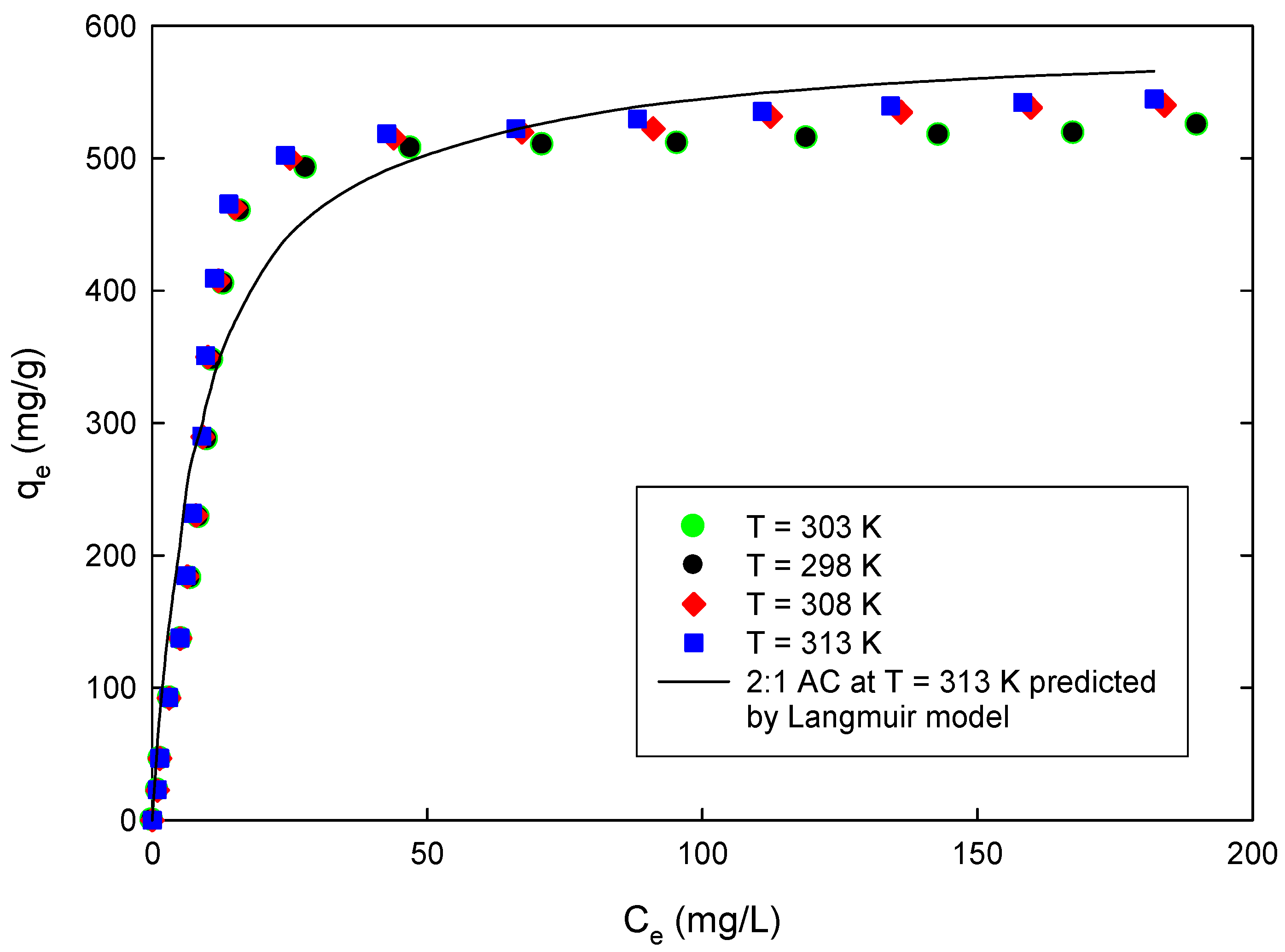
In our previous study, we used the activated carbon prepared from date pits to remove phenol from aqueous solution. The study involved dynamics and equilibrium analysis only. For this study, raw date pits were carbonized and activated by air and phosphoric acid; the resulting activated carbon was evaluated for its 4-CP sorbent capacity. The effects of solution pH on adsorbent capacity were investigated and the Langmuir and Freundlich models were utilized to describe the adsorption behavior. Parameters for each of these models were determined by non-linear error estimation using the derivative of Marquarrdt’s Percent Standard Deviation (MPSD). The pseudo second-order kinetics and Elovich equation, in addition to the Weber and Morris equation, and diffusion models were used to analyze the dynamics of sorption of the 4-CP on the prepared AC. Thermodynamics analysis of the 4-CP adsorption was carried out using both the Arrhenius and van’t Hoff equations.