Synthesis of Monodispersed Ag-Doped Bioactive Glass Nanoparticles via Surface Modification
Abstract
:1. Introduction
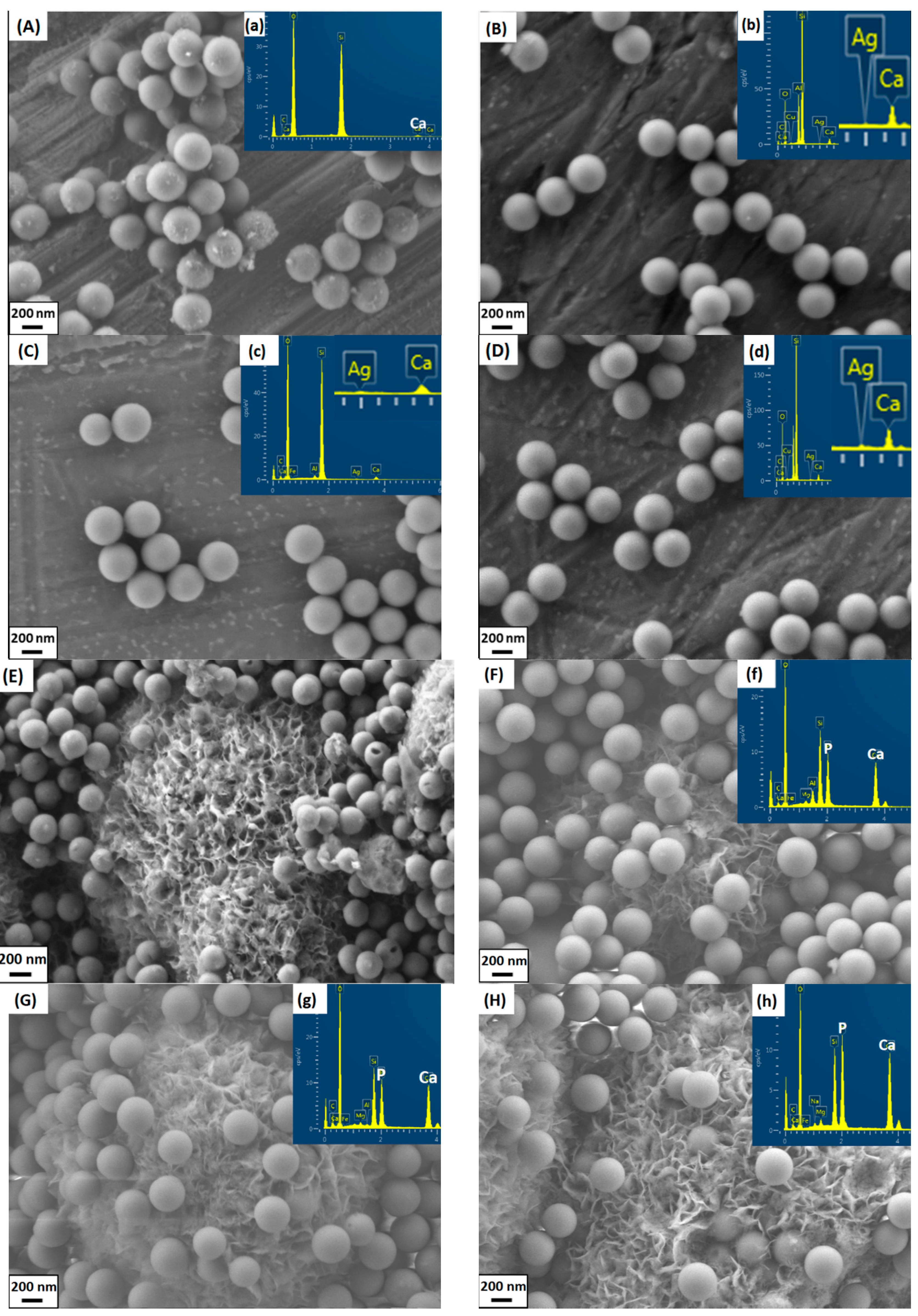
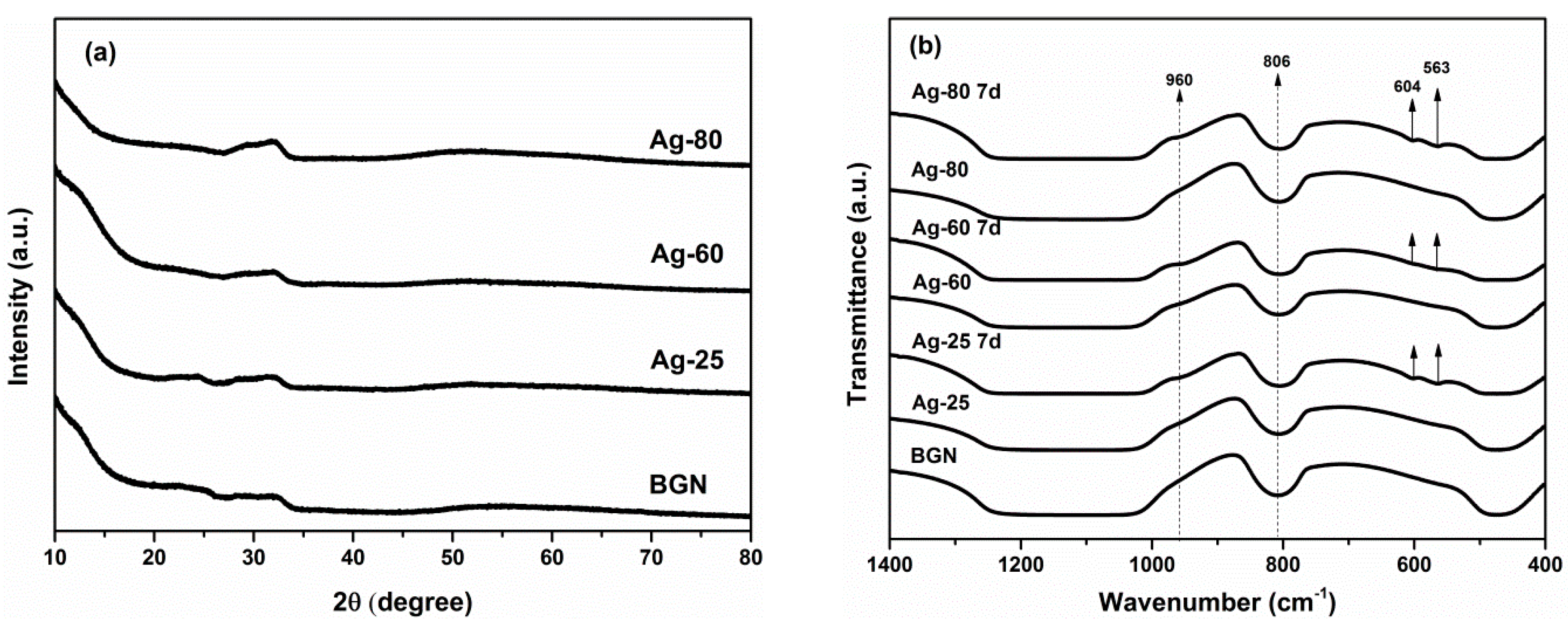
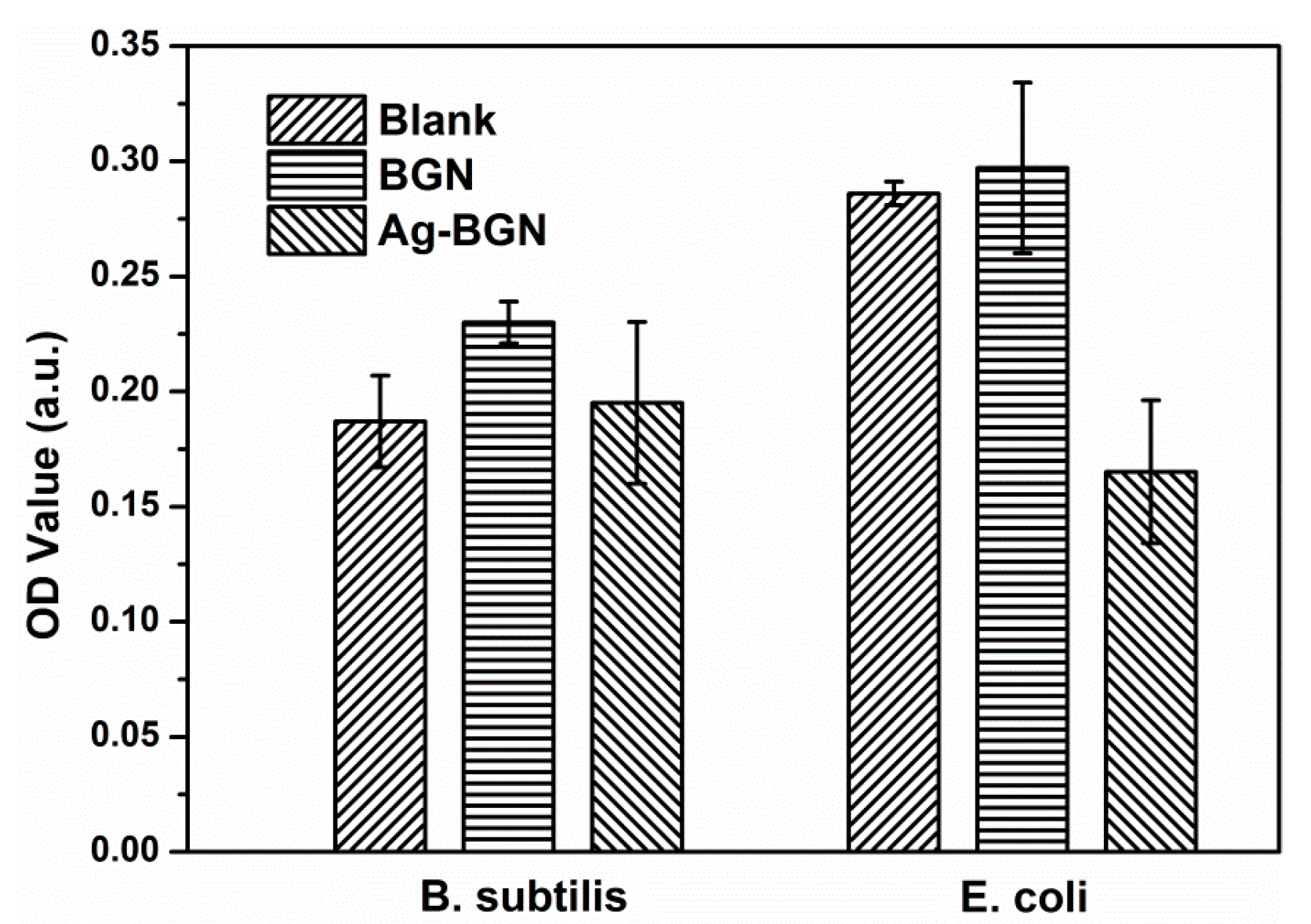
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of BGNs
3.2. Surface Modification
3.3. Characterization
3.4. In Vitro Mineralization
3.5. Antibacterial Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L. Opening paper 2015—Some comments on bioglass: Four eras of discovery and development. Biomed. Glas. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Erol-Taygun, M.; Zheng, K.; Boccaccini, A.R. Nanoscale bioactive glasses in medical applications. Int. J. Appl. Glas. Sci. 2013, 4, 136–148. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Tsigkou, O.; Labbaf, S.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Monodispersed bioactive glass submicron particles and their effect on bone marrow and adipose tissue-derived stem cells. Adv. Healthc. Mater. 2014, 3, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Labbaf, S.; Tsigkou, O.; Müller, K.H.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Spherical bioactive glass particles and their interaction with human mesenchymal stem cells in vitro. Biomaterials 2011, 32, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.B.; Kretlow, J.D.; Mikos, A.G.; Kasper, F.K. Infection and tissue engineering in segmental bone defects—A mini review. Curr. Opin. Biotechnol. 2011, 22, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.E.; Bender, G.; Marquis, R.E. Inhibitory and cidal antimicrobial actions of electrically generated silver ions. J. Oral Maxillofac. Surg. 1987, 45, 779–784. [Google Scholar] [CrossRef]
- Fan, W.; Wu, D.; Tay, F.R.; Wu, Y.; Fan, B. Effects of adsorbed and templated nanosilver in mesoporous calcium-silicate nanoparticles on inhibition on bacteria colonization of dentin. Int. J. Nanomed. 2014, 9, 5217–5230. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.F.; Alshemary, A.Z.; Akram, M.; Abdul Kadir, M.R.; Hussain, R. Bioactive glass: An in-vitro comparative study of doping with nanoscale copper and silver particles. Int. J. Appl. Glas. Sci. 2014, 266, 255–266. [Google Scholar] [CrossRef]
- Delben, J.R.J.; Pimentel, O.M.; Coelho, M.B.; Candelorio, P.D.; Furini, L.N.; Alencar dos Santos, F.; de Vicente, F.S.; Delben, A.A.S.T. Synthesis and thermal properties of nanoparticles of bioactive glasses containing silver. J. Therm. Anal. Calorim. 2009, 433–436. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.F.; Rizk, R.A.; Ahmed, M.M. Synthesis, characterization and microbiological response of silver-doped bioactive glass nanoparticles. Ceram. Int. 2012, 38, 177–188. [Google Scholar] [CrossRef]
- Borrelli, N.F.; Senaratne, W.; Wei, Y.; Petzold, O. Physics and chemistry of antimicrobial behavior of ion-exchanged silver in glass. ACS Appl. Mater. Interfaces 2015, 7, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yuan, Y.; Liu, C.; Wei, J.; Hong, H.; Li, X.; Pan, X. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials 2009, 30, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanzadeh, M.; Azadegan, M.; Liu, G.Q. Bioactive delivery systems for the slow release of antibiotics: incorporation of Ag+ ions into micro-porous hydroxyapatite coatings. Mater. Lett. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Bonici, A.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Piva, A. Synthesis and characterization of bioactive glasses functionalized with Cu nanoparticles and organic molecules. J. Eur. Ceram. Soc. 2012, 32, 2777–2783. [Google Scholar] [CrossRef]
- Plumeré, N.; Ruff, A.; Speiser, B.; Feldmann, V.; Mayer, H.A. Stöber silica particles as basis for redox modifications: Particle shape, size, polydispersity, and porosity. J. Colloid Interface Sci. 2012, 368, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol-gel-derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non Cryst. Solids 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol-gel silicate glasses by IR and Raman spectroscopies. J. Non Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Zheng, K.; Solodovnyk, A.; Li, W.; Goudouri, O.-M.; Stähli, C.; Nazhat, S.N.; Boccaccini, A.R. Aging time and temperature effects on the structure and bioactivity of gel-derived 45S5 glass-ceramics. J. Am. Ceram. Soc. 2015, 98, 30–38. [Google Scholar] [CrossRef]
- Chen, S.; Osaka, A.; Hayakawa, S.; Tsuru, K.; Fujii, E.; Kawabata, K. Microstructure evolution in Stöber-type silica nanoparticles and their in vitro apatite deposition. J. Sol Gel Sci. Technol. 2008, 48, 322–335. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Chang, J. Functional mesoporous bioactive glass nanospheres: Synthesis, high loading efficiency, controllable delivery of doxorubicin and inhibitory effect on bone cancer cells. J. Mater. Chem. B 2013, 1, 2710–2718. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Vernè, E.; Di Nunzio, S.; Bosetti, M.; Appendino, P.; Vitale Broyarone, C.; Maina, G.; Cannas, M. Surface characterization of silver-doped bioactive glass. Biomaterials 2005, 26, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Chen, S.; Yu, H.; Ge, Z.; Zhang, X.; Zhang, P.; Tang, J. Facile preparation and synergistic antibacterial effect of three-component Cu/TiO2/CS nanoparticles. J. Mater. Chem. 2012, 22, 9092–9099. [Google Scholar] [CrossRef]
| Name | Composition (mol%) | Particles Size (nm) |
|---|---|---|
| BGN | 96.60SiO2-3.40CaO | 370 ± 35 |
| Ag-25 | 95.61SiO2-4.26CaO-0.12Ag2O | 365 ± 23 |
| Ag-60 | 95.46SiO2-4.35CaO-0.19Ag2O | 373 ± 25 |
| Ag-80 | 95.69SiO2-4.07CaO-0.24Ag2O | 367 ± 24 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozon, D.; Zheng, K.; Boccardi, E.; Liu, Y.; Liverani, L.; Boccaccini, A.R. Synthesis of Monodispersed Ag-Doped Bioactive Glass Nanoparticles via Surface Modification. Materials 2016, 9, 225. https://doi.org/10.3390/ma9040225
Kozon D, Zheng K, Boccardi E, Liu Y, Liverani L, Boccaccini AR. Synthesis of Monodispersed Ag-Doped Bioactive Glass Nanoparticles via Surface Modification. Materials. 2016; 9(4):225. https://doi.org/10.3390/ma9040225
Chicago/Turabian StyleKozon, Dominika, Kai Zheng, Elena Boccardi, Yufang Liu, Liliana Liverani, and Aldo R. Boccaccini. 2016. "Synthesis of Monodispersed Ag-Doped Bioactive Glass Nanoparticles via Surface Modification" Materials 9, no. 4: 225. https://doi.org/10.3390/ma9040225
APA StyleKozon, D., Zheng, K., Boccardi, E., Liu, Y., Liverani, L., & Boccaccini, A. R. (2016). Synthesis of Monodispersed Ag-Doped Bioactive Glass Nanoparticles via Surface Modification. Materials, 9(4), 225. https://doi.org/10.3390/ma9040225









