Electrospun Nafion®/Polyphenylsulfone Composite Membranes for Regenerative Hydrogen Bromine Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
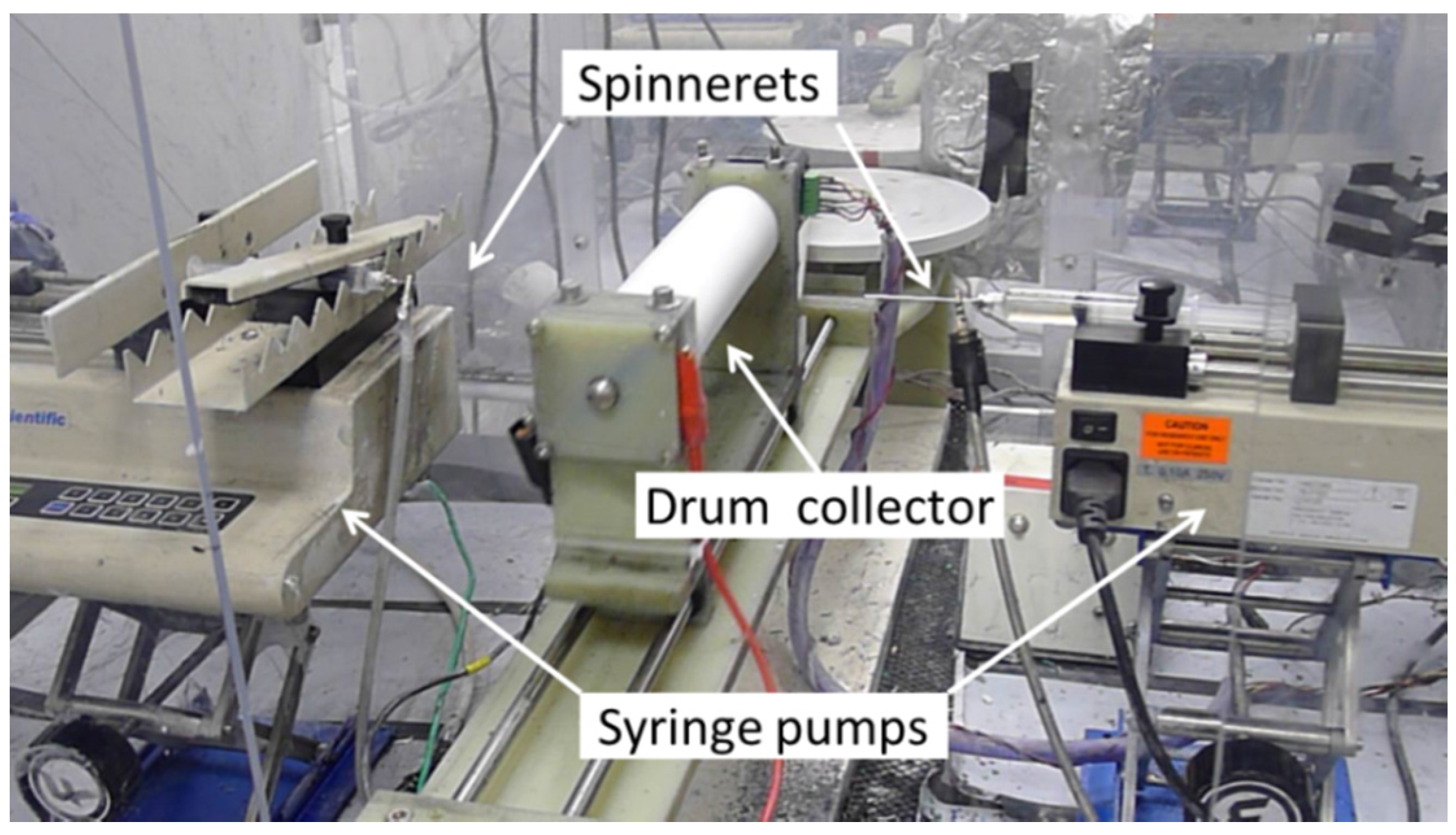
2.1. Electrospinning Nafion/PPSU
2.2. Dual Nanofiber Mat Processing
2.3. SEM Microscopy
2.4. Ion-Exchange Capacity
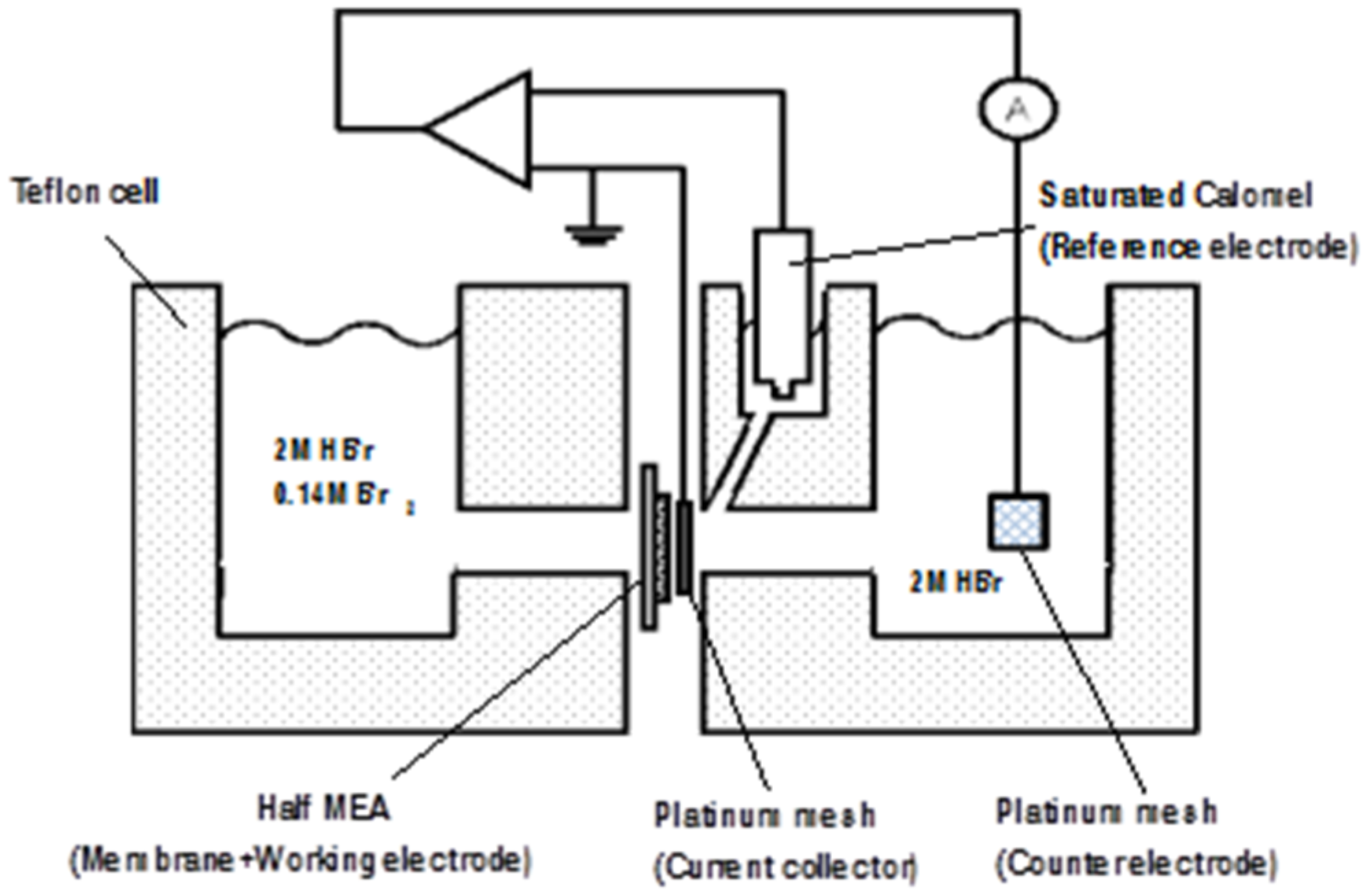
2.5. Conductivity Measurements
2.6. Membrane Swelling
2.7. Diffusivity Measurements
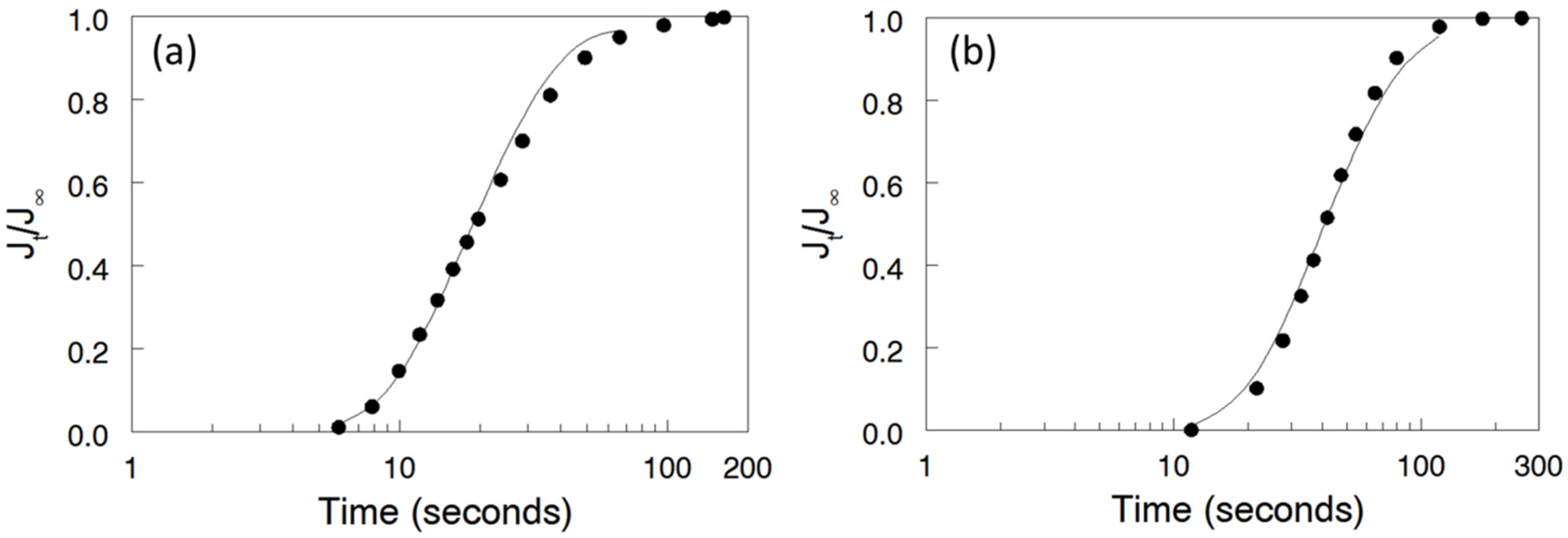
2.8. Analysis of Breakthrough Curves
2.9. Fuel Cell Performance
3. Results and Discussion
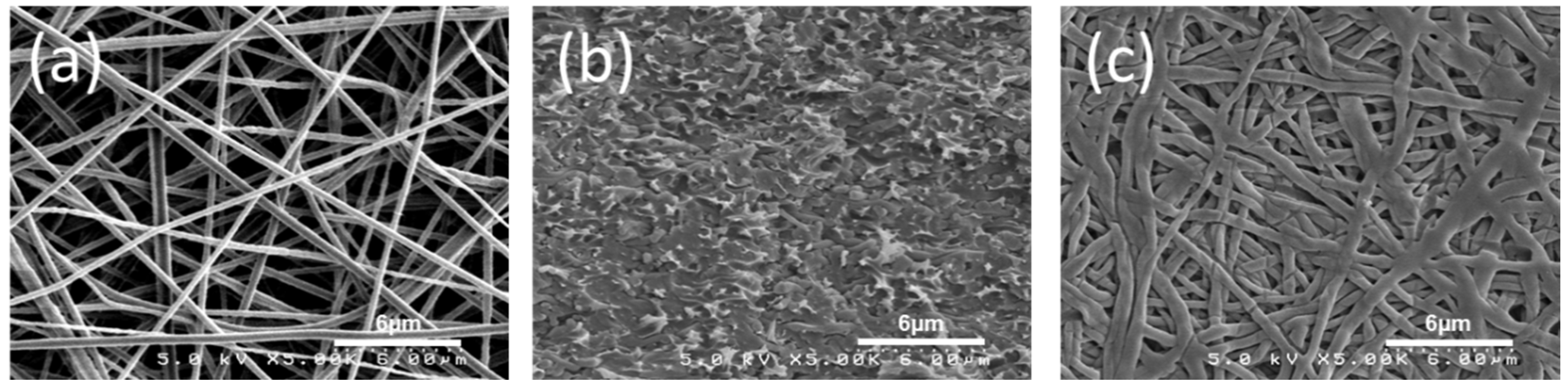
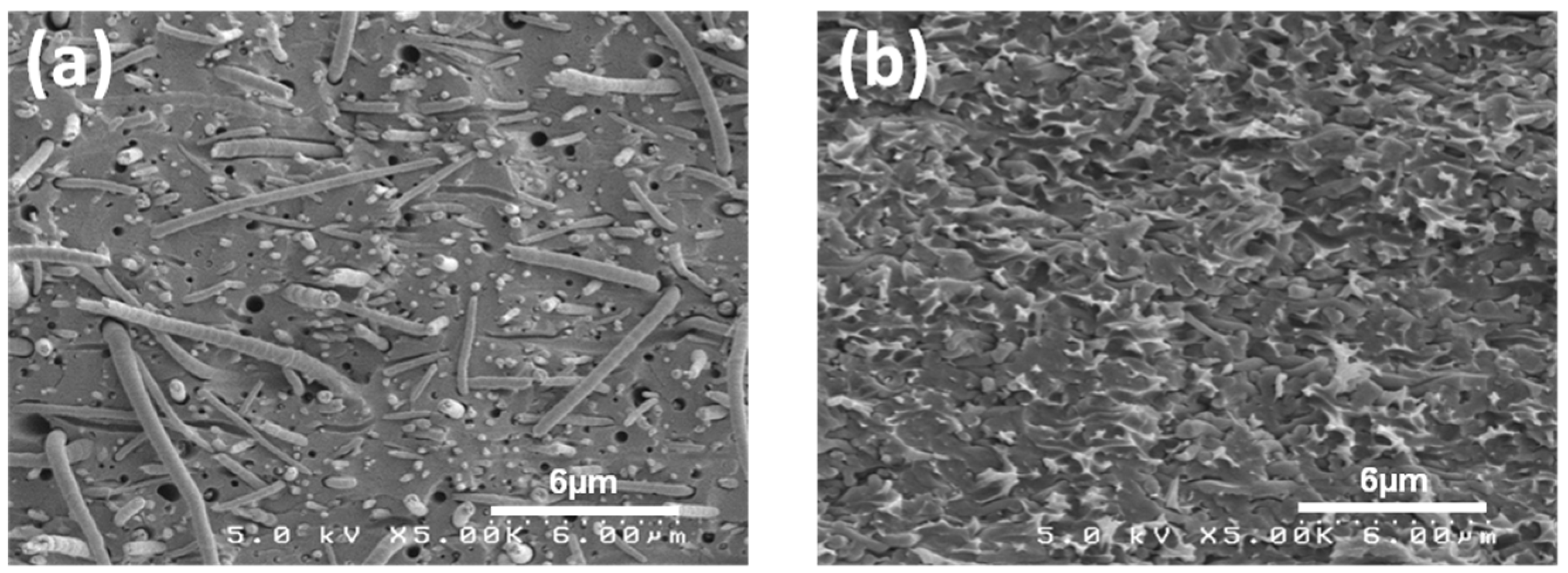
3.1. Membrane Morphology
3.2. Membrane Swelling
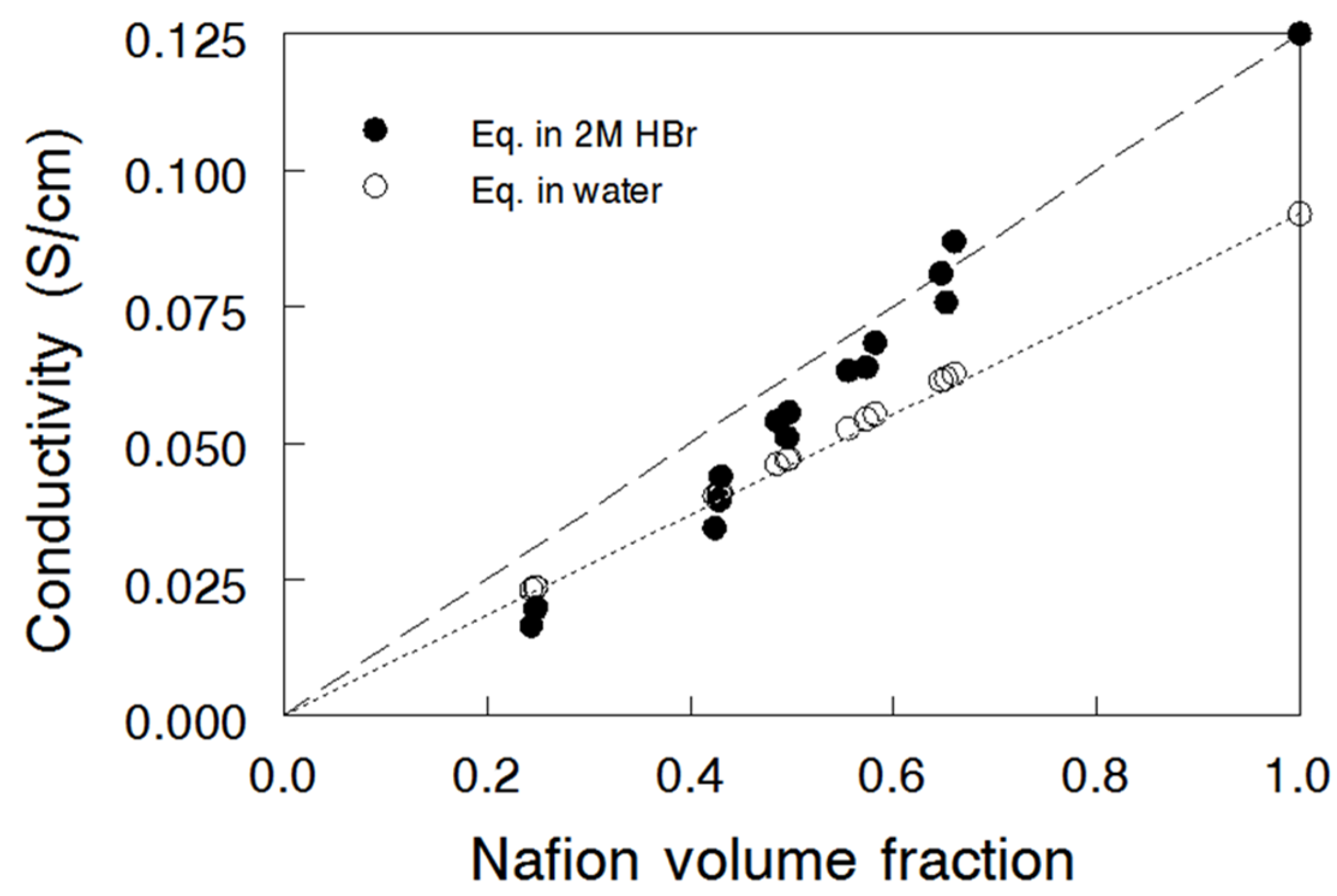
3.3. Ion Conductivity
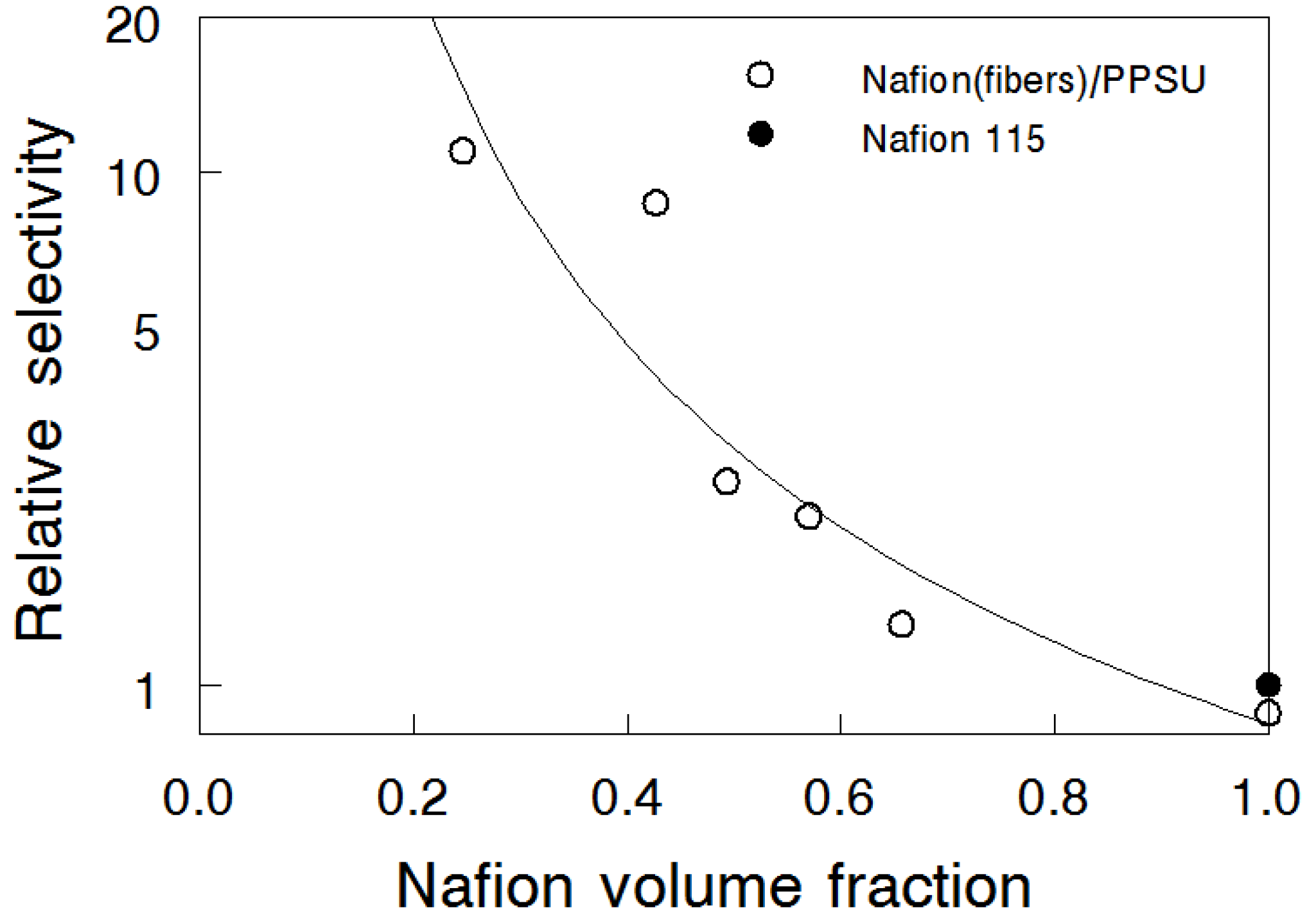
3.4. Bromine Species (Br2/Br3-) Permeation
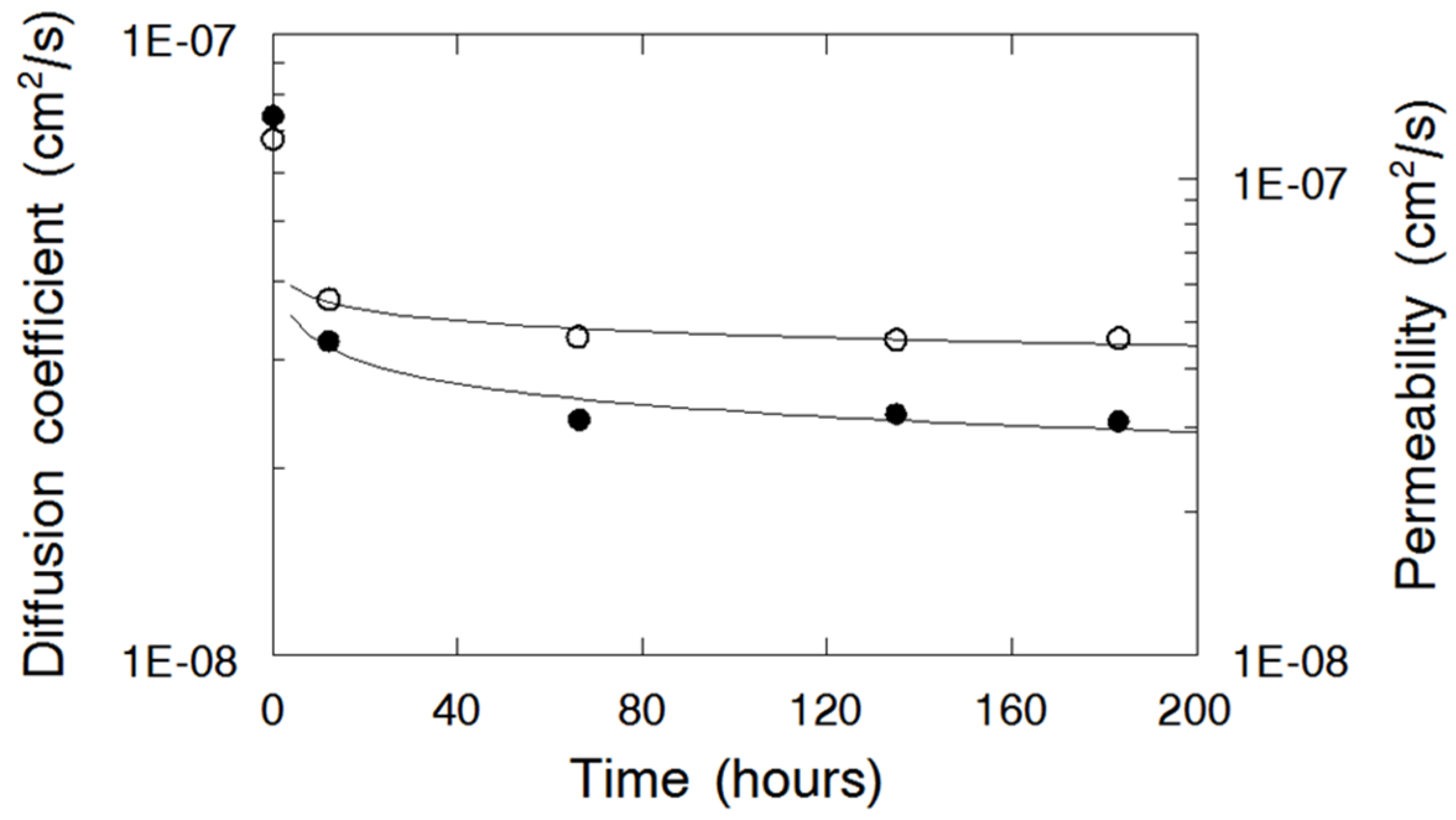
3.5. Nafion Nanofiber Composite Membrane Stability in 2 M HBr-0.14 M Br2
3.6. Bromine Species (Br2/Br3-) Permeation for the Two Complementary Nanofiber Composite Membrane Structures
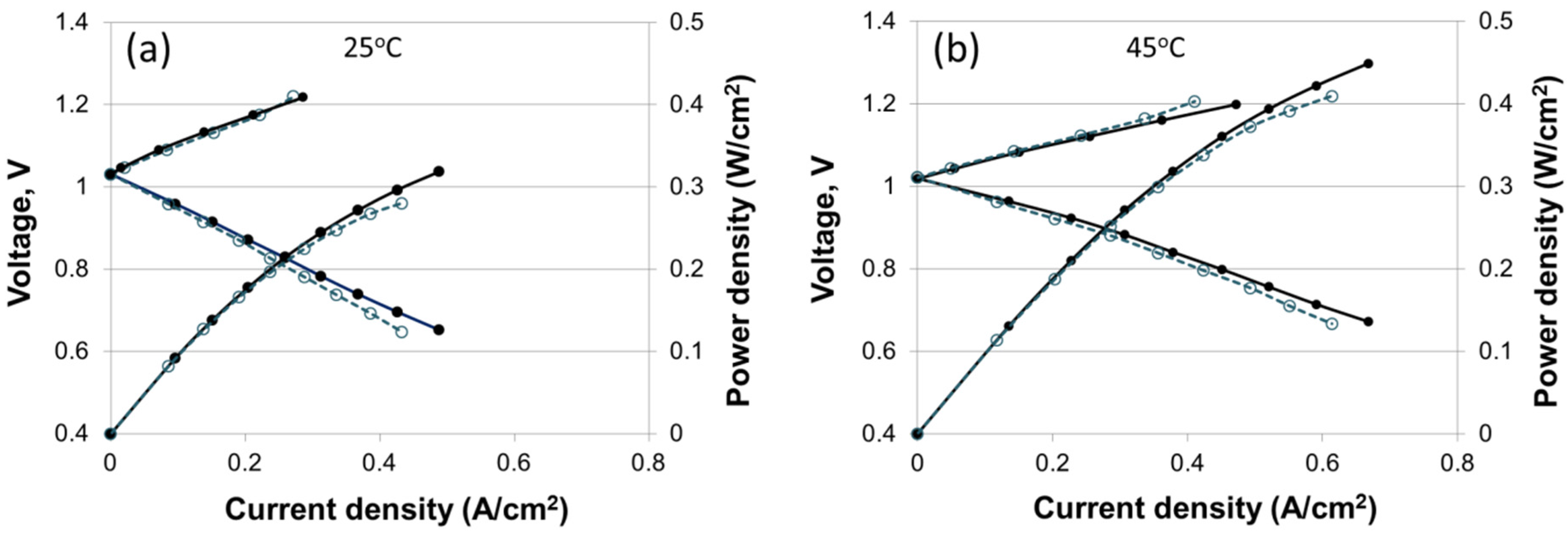
3.7. H2-Br2 Regenerative Fuel Cell Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ASR | Area specific resistance |
| MEA | Membrane electrode assembly |
| N | Nafion® |
| OCV | Open circuit voltage |
| PEO | Polyethylene oxide |
| PFSA | Perfluorosulfonic acid |
| PPSU | Polyphenylsulfone |
| PVDF | Polyvinylidene fluoride |
| SEM | Scanning electron microscopy |
References
- Livshits, V.; Ulus, A.; Peled, E. High-power H2/Br2 fuel cell. Electrochem. Commun. 2006, 8, 1358–1362. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Battery Technologies for Large-Scale Stationary Energy Storage. Annu. Rev. Chem. Biomol. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.T.; Albertus, P.; Battaglia, V.; Kojic, A.; Srinivasan, V.; Weber, A.Z. Optimization and analysis of high-power hydrogen/bromine-flow batteries for grid-scale energy storage. Energy Technol. 2013, 1, 596–608. [Google Scholar] [CrossRef]
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High performance hydrogen/bromine redox flow battery for grid-scale energy storage. J. Electrochem. Soc. 2012, 159, A1806–A1815. [Google Scholar] [CrossRef]
- Kreutzer, H.; Yarlagadda, V.; Nguyen, T.V. Performance evaluation of a regenerative hydrogen-bromine fuel cell. J. Electrochem. Soc. 2012, 159, F331–F337. [Google Scholar] [CrossRef]
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.V.; Wycisk, R.J.; Pintauro, P.N.; Bates, M.; Mukerjee, S.; Tucker, M.C.; Weber, A.Z. Advanced hydrogen-bromine flow batteries with improved efficiency, durability and cost. J. Electrochem. Soc. 2016, 163, A5049–A5056. [Google Scholar] [CrossRef]
- Yeo, R.S.; Chin, D.T. A Hydrogen-Bromine Cell for Energy Storage Applications. J. Electrochem. Soc. 1980, 127, 549–555. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nagao, M.; Yamamoto, Y.; Heo, P.; Hibinoa, T. Rechargeable PEM Fuel-Cell Batteries Using Porous Carbon Modified with Carbonyl Groups as Anode Materials. J. Electrochem. Soc. 2015, 162, F868–F877. [Google Scholar] [CrossRef]
- Nagao, M.; Kobayashi, K.; Yamamoto, Y.; Hibino, T. Rechargeable PEM Fuel-Cell Batteries Using Quinones as Hydrogen Carriers. J. Electrochem. Soc. 2015, 162, F410–F418. [Google Scholar] [CrossRef]
- Shena, Y.B.; Harada, T.; Teranishib, S.; Hibinoa, T. A solid-state particulate matter sensor based on electrochemical oxidation of carbon by active oxygen. Sensor Actuat. B-Chem. 2012, 162, 159–165. [Google Scholar] [CrossRef]
- Tsuneyama, K.; Tetanishi, S.; Hibino, T.; Nagao, S.; Hirata, H.; Matsumoto, S. Low-temperature hydrocarbon combustion over proton conductor/metal–mixed catalysts. J. Catal. 2010, 273, 59–65. [Google Scholar] [CrossRef]
- Baldwin, R.S. Electrochemical Performance and Transport Properties of a Nafion Membrane in a Hydrogen-Bromine Cell Environment; NASA: Washington, DC, USA, 1987; p. 89862.
- Savinell, R.F.; Fritts, S. Theoretical performance of a hydrogen-bromine rechargeable SPE fuel cell. J. Power Sources 1988, 22, 423–440. [Google Scholar] [CrossRef]
- Grot, W.; Rajendran, G. Membranes containing inorganic fillers and membrane and electrode assemblies and electrochemical cells employing same. U.S. Patent 5,919,583, 6 July 1999. [Google Scholar]
- Glass, W. Hydrogen-Bromine Fuel Cell; Ionics Inc.: Cambridge, MA, USA, 1964. [Google Scholar]
- Kerres, J.A. Blended and cross-linked ionomer membranes for application in membrane fuel cells. Fuel Cells 2005, 5, 230–247. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Pintauro, P.N. Composite Fuel Cell Membranes from Dual-Nanofiber Electrospun Mats. Macromolecules 2011, 44, 7307–7314. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.M.; Wycisk, R.; Pintauro, P.N.; Mather, P.T. Nanofiber network ion-exchange membranes. Macromolecules 2008, 41, 4569–4572. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.M.; Wycisk, R.; Pintauro, P.N.; Mather, P.T. Nanofiber composite membranes with low equivalent weight perfluorosulfonic acid polymers. J. Mater. Chem. 2010, 20, 6282–6290. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.M.; Wycisk, R.; Pintauro, P.N.; Mather, P.T.J. Electrochem. Sulfonated polysulfone/POSS nanofiber composite membranes for PEM fuel cells. J. Eletrochem. Soc. 2010, 157, B914–B919. [Google Scholar] [CrossRef]
- Park, A.M.; Pintauro, P.N. Alkaline Fuel Cell Membranes from Electrospun Fiber Mats. Electrochem. Solid State 2012, 15, B27–B30. [Google Scholar] [CrossRef]
- Park, J.W.; Wycisk, R.; Pintauro, P.N. Nafion/PVDF Nanofiber Composite Membranes for Regenerative Hydrogen/Bromine Fuel Cells. J. Membrane Sci. 2015, 490, 103–112. [Google Scholar] [CrossRef]
- Image J. Available online: http://rsbweb.nih.gov/ij/index.html (accessed on 8 January 2016).
- Yeo, R.S.; Mcbreen, J. Transport properties of Nafion membranes in electrochemically regenerative hydrogen/halogen cells. J. Electrochem. Soc. 1979, 126, 1682–1687. [Google Scholar] [CrossRef]
- Kimble, M.C.; White, R.E.; Tsou, Y.M.; Beaver, R.N. Estimation of the Diffusion Coefficient and Solubility for a Ga s Diffusing Through a Membrane. J. Electrochem. Soc. 1990, 137, 2510–2514. [Google Scholar] [CrossRef]
- Yeo, S.C.; Eisenberg, A. Physical properties and supermolecular structure of perfluorinated ion-containing (Nafion) polymers. J. Appl. Polym. Sci. 1977, 21, 875–898. [Google Scholar] [CrossRef]
- Kusoglu, A.; Cho, K.T.; Prato, R.A.; Weber, A.Z. Structural and Transport Properties of Nafion in Hydrobromic-Acid Solutions. Solid State Ionics 2013, 252, 68–74. [Google Scholar] [CrossRef]
- Park, J.W.; Wycisk, R.; Pintauro, P.N. Membranes for a Regenerative H2/Br2 Fuel Cell. ECS Transactions 2012, 50, 1217–1231. [Google Scholar] [CrossRef]
- Libby, B.; Smyrl, W.H.; Cussler, E.L. Composite membranes for direct methanol fuel cells. Electrochem. Solid State 2001, 4, A197–A199. [Google Scholar] [CrossRef]
- Lin, J.; Lee, J.; Kellner, M.; Wycisk, R.; Pintauro, P. Nafion-fluorinated ethylene-propylene resin membrane blends for direct methanol fuel cells. J. Electrochem. Soc. 2006, 153, A1325–A1331. [Google Scholar] [CrossRef]
- Morrissey, P.; Vesely, D.; Cooley, G. Stability of sulphonate type membranes in aqueous bromine/bromide environments. J. Membr. Sci. 2005, 247, 169–178. [Google Scholar] [CrossRef]
- Tucker, M.C.; Cho, K.T.; Spingler, F.B.; Weber, A.Z.; Lin, G. Impact of membrane characteristics on the performance and cycling of the Br2-H2 redox flow cell. J. Power Sources 2015, 284, 212–221. [Google Scholar] [CrossRef]
- Cho, K.T.; Tucker, M.C.; Ding, M.; Ridgway, P.; Battaglia, V.S.; Srinivasan, V.; Weber, A.Z. Cyclic Performance Analysis of Hydrogen/Bromine Flow Batteries for Grid-Scale Energy Storage. Chem. Plus. Chem. 2015, 80, 402–411. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Dowd, R.P.; Park, J.W.; Pintauro, P.N.; Nguyen, T.V. A comprehensive study of an acid-based reversible H2-Br2 fuel cell system. J. Electrochem. Soc. 2015, 162, F919–F926. [Google Scholar] [CrossRef]

| Membrane | Mass Swelling (%) | Volume Swelling (%) | ||
|---|---|---|---|---|
| Water | 2 M HBr | Water | 2 M HBr | |
| Nafion® 115 | 28 | 20 | 52 | 35 |
| N (fibers)/PPSU | – | – | – | – |
| 57 vol% Nafion | 14 | 13 | 20 | 15 |
| 50 vol% Nafion | 11 | 12 | 18 | 13 |
| 44 vol% Nafion | 8 | 8 | 11 | 12 |
| 25 vol% Nafion | 4 | 4 | 7 | 7 |
| Structure | Ion Conductivity (mS/cm) | Diffusion Coefficient (cm2/s) | Permeability (cm2/s) | Relative Selectivity | |
|---|---|---|---|---|---|
| N/PPSU | – | – | – | – | |
| 57 vol% Nafion | Nafion fibers | 65 | 7.36 × 10−8 | 1.22 × 10−7 | 2.1 |
| PPSU fibers | 65 | 1.41 × 10−7 | 2.11 × 10−7 | 1.2 | |
| Nafion 115 | 107 | 1.45 × 10−6 | 4.26 × 10−7 | 1.0 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Wycisk, R.; Pintauro, P.N.; Yarlagadda, V.; Van Nguyen, T. Electrospun Nafion®/Polyphenylsulfone Composite Membranes for Regenerative Hydrogen Bromine Fuel Cells. Materials 2016, 9, 143. https://doi.org/10.3390/ma9030143
Park JW, Wycisk R, Pintauro PN, Yarlagadda V, Van Nguyen T. Electrospun Nafion®/Polyphenylsulfone Composite Membranes for Regenerative Hydrogen Bromine Fuel Cells. Materials. 2016; 9(3):143. https://doi.org/10.3390/ma9030143
Chicago/Turabian StylePark, Jun Woo, Ryszard Wycisk, Peter N. Pintauro, Venkata Yarlagadda, and Trung Van Nguyen. 2016. "Electrospun Nafion®/Polyphenylsulfone Composite Membranes for Regenerative Hydrogen Bromine Fuel Cells" Materials 9, no. 3: 143. https://doi.org/10.3390/ma9030143
APA StylePark, J. W., Wycisk, R., Pintauro, P. N., Yarlagadda, V., & Van Nguyen, T. (2016). Electrospun Nafion®/Polyphenylsulfone Composite Membranes for Regenerative Hydrogen Bromine Fuel Cells. Materials, 9(3), 143. https://doi.org/10.3390/ma9030143










