Effect of Ionic Correlations on the Surface Forces in Thin Liquid Films: Influence of Multivalent Coions and Extended Theory
Abstract
:1. Introduction
2. Materials, Methods and Experimental Results
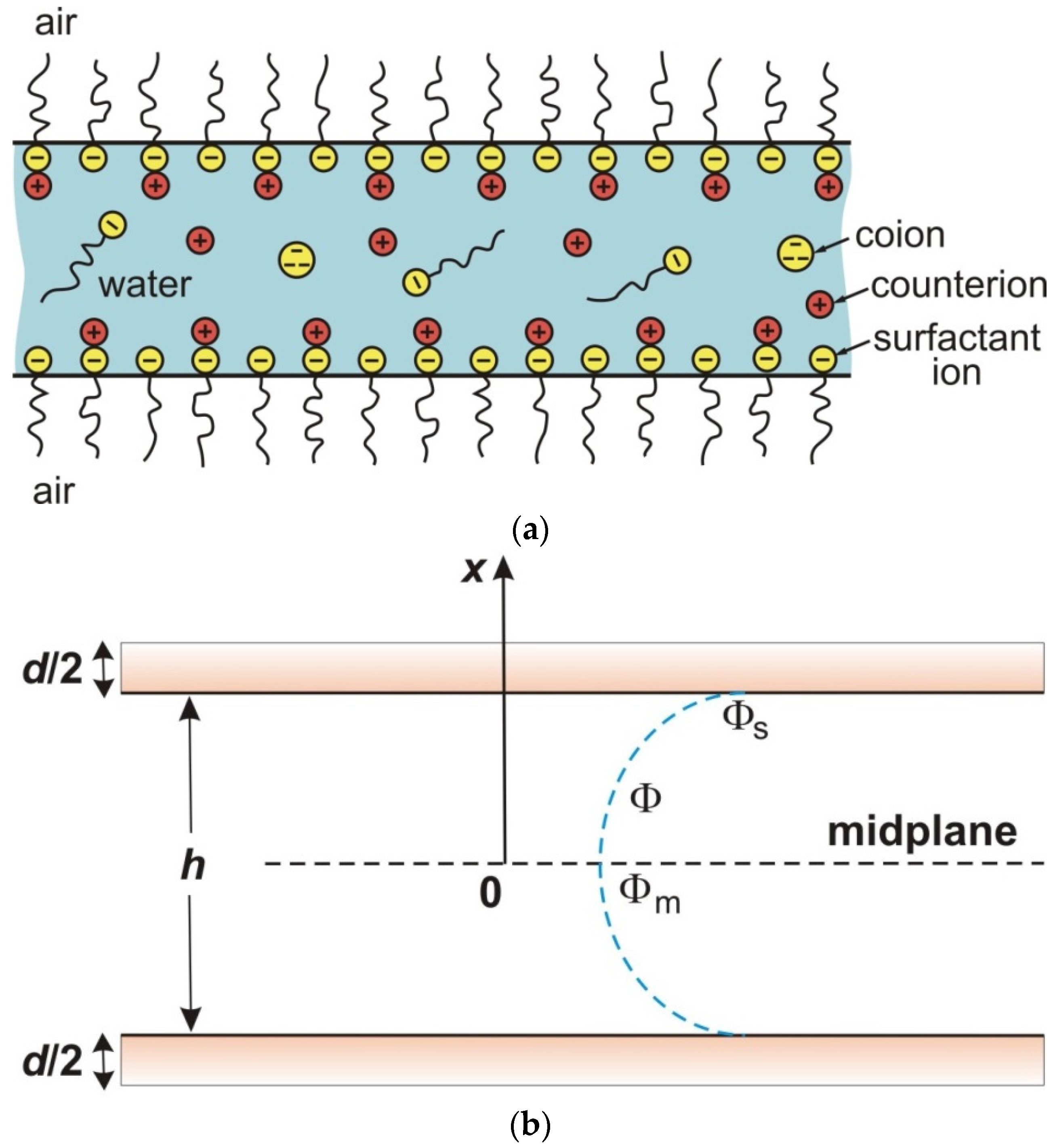
3. Generalized Theory of Electrostatic Surface Forces in Thin Liquid Films
3.1. Expression for Disjoining Pressure Extended to Account for Ionic Correlations
3.2. Calculation of the Electrostatic Disjoining Pressure with Ionic Correlations
- For a given value of Φm, from Equations (3.6) and (3.10) we determine the respective values of the concentrations and activity coefficients, ci = cim and γi = γim, by iterations. To start the iterations, first ci is calculated from Equation (3.10) with Φ = Φm and γi = γi∞. The obtained ci is substituted in Equations (3.6) and (3.7) to determine the first approximation for γi, which is then substituted in Equation (3.10) to find the next approximation for ci, etc. The iterations are fast convergent: less than 20 iterations are sufficient to determine the values of ci and γi with accuracy better than 14 significant digits.
- From Equations (3.7) and (3.8) with ci = cim, we determine p(Φm), and then from Equations (3.9) and (3.11) we calculate Πel(Φm).
- For a given value of Φs, from Equations (3.6) and (3.10) we determine the respective values of the concentrations and activity coefficients, ci = cis and γi = γis, by iterations. The procedure is analogous to that in point 1 above.
- With the same value of Φs, Equation (3.16), is solved numerically (e.g., by the bisection method) to determine Γ1. Further, Γ2 is calculated from Equation (3.17).
- From Equations (3.7) and (3.8) with ci = cis, we determine p(Φs), and then we solve Equation (3.15) numerically to determine Φs.
- The integral in Equation (3.13) is solved numerically. For this goal, at each given Φ from Equations (3.6) and (3.10) we determine the respective values of the concentrations and activity coefficients, ci and γi, by iterations. The procedure is analogous to that in point 1 above. Next, from Equations (3.7) and (3.8) we calculate p(Φs). As a result of integration, we determine h(Φm).
- The value of Φm is varied to obtain the dependences Πel = Πel(Φm) and h = h(Φm).
3.3. Conventional DLVO Theory of Electrostatic Disjoining Pressure
3.4. Calculation of the Total Disjoining Pressure
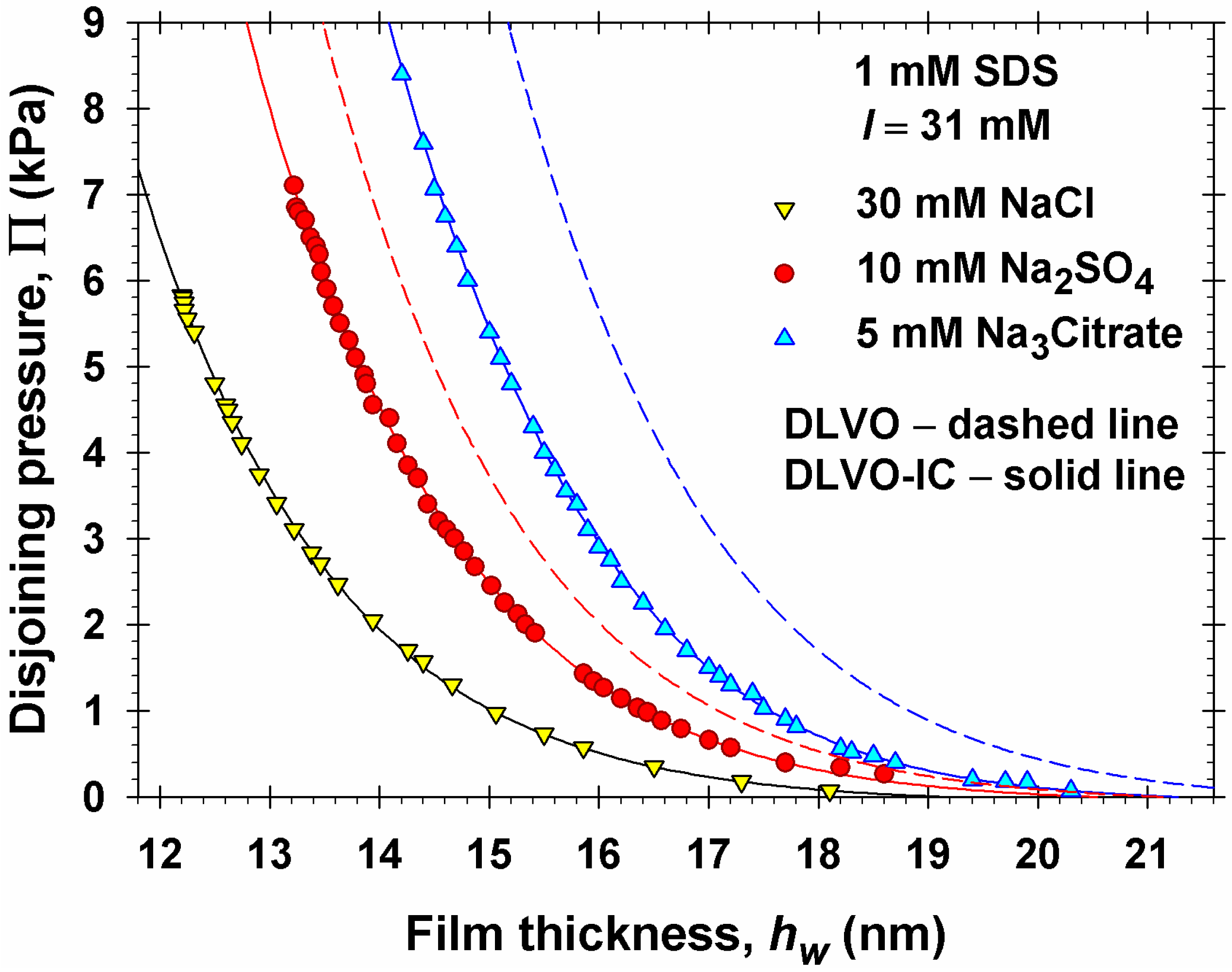
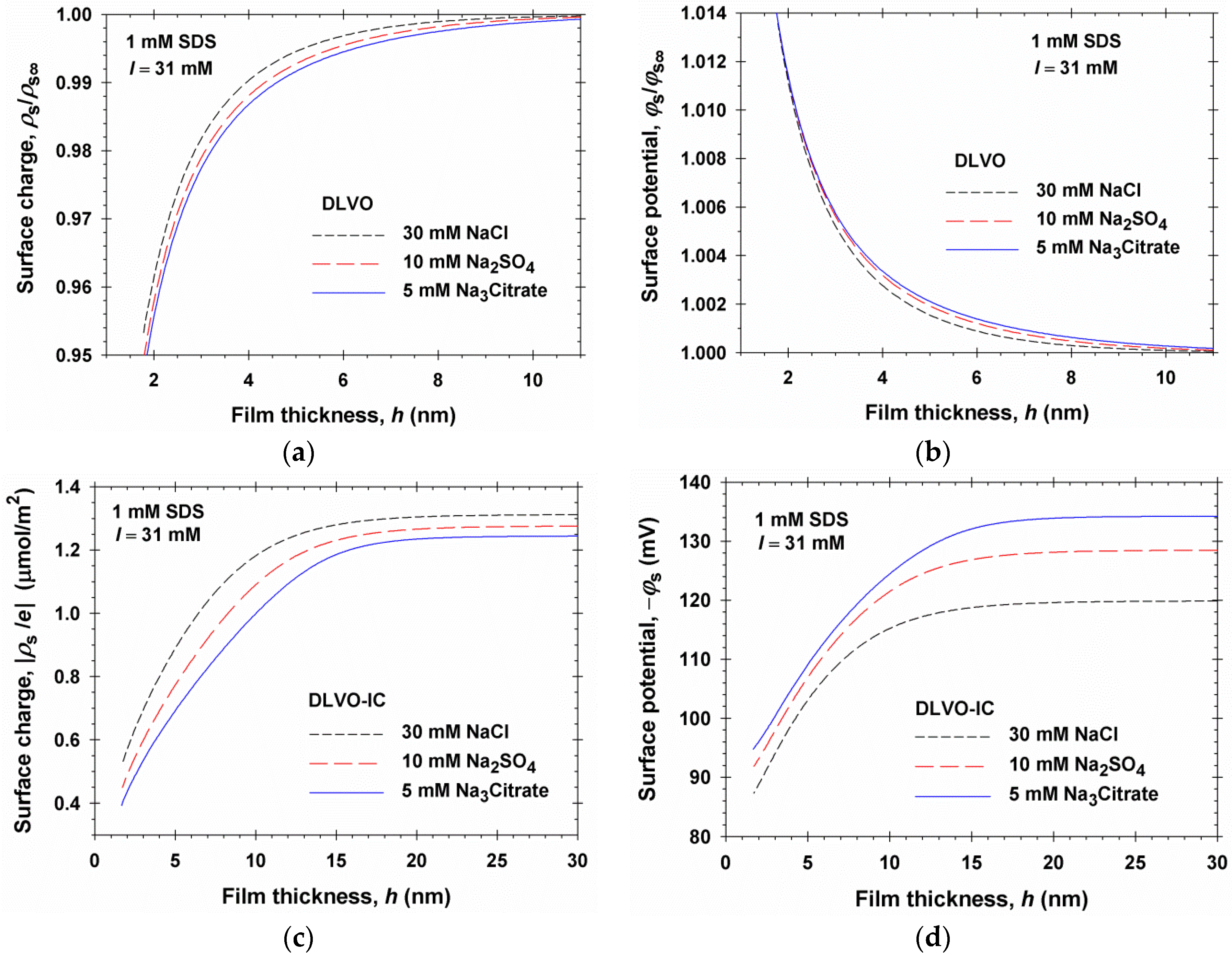
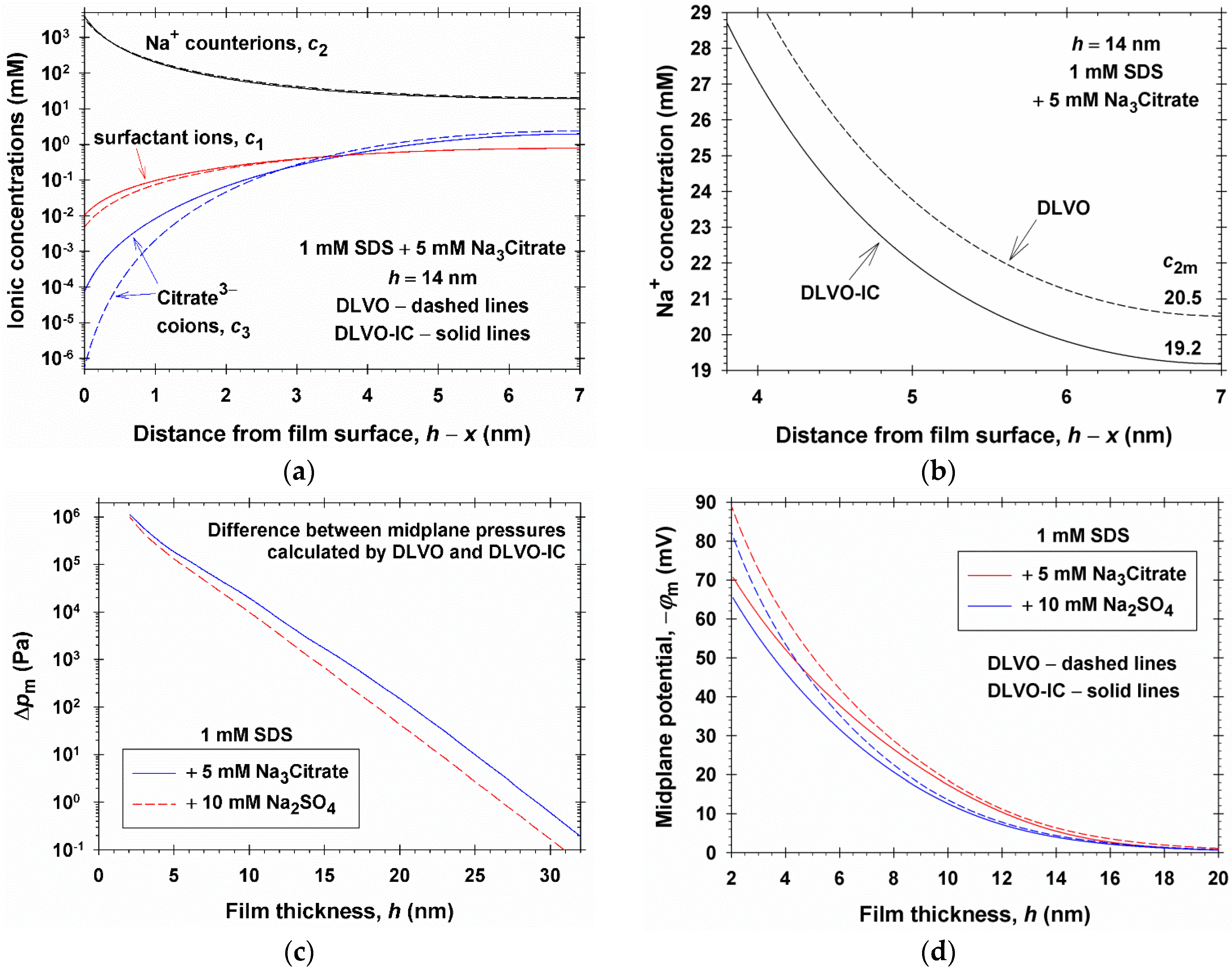
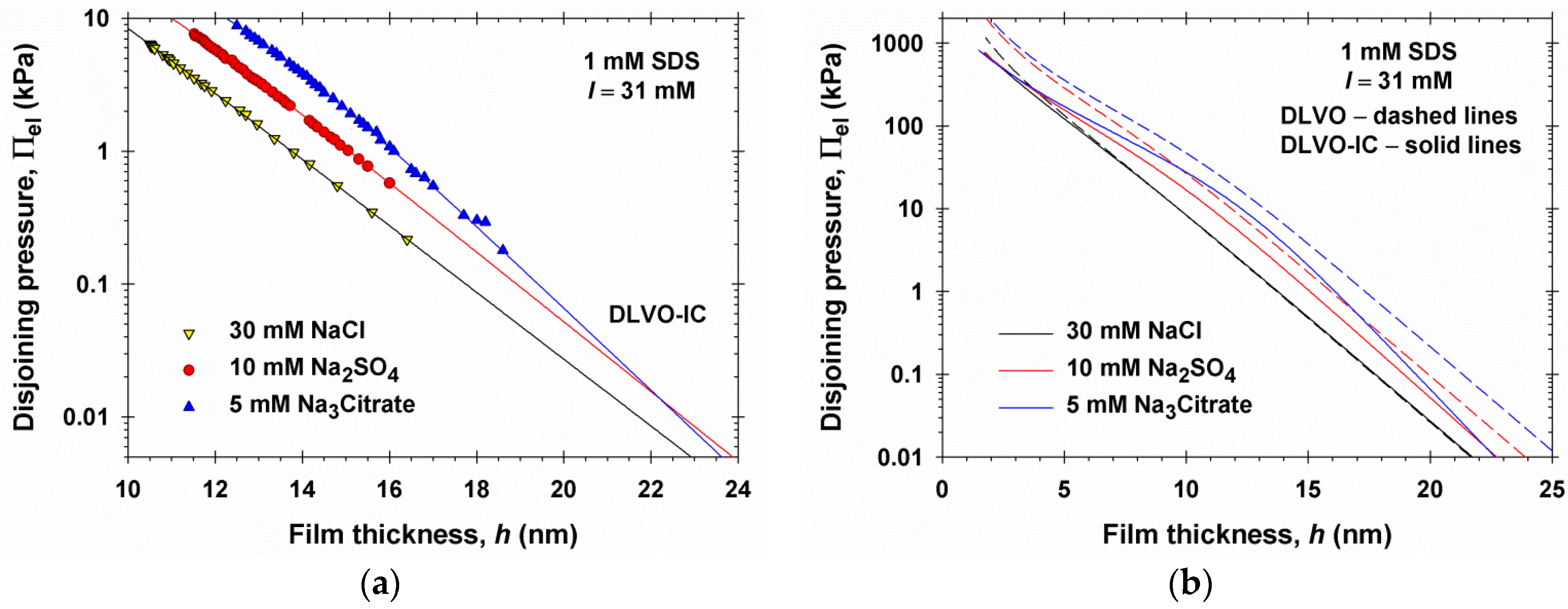
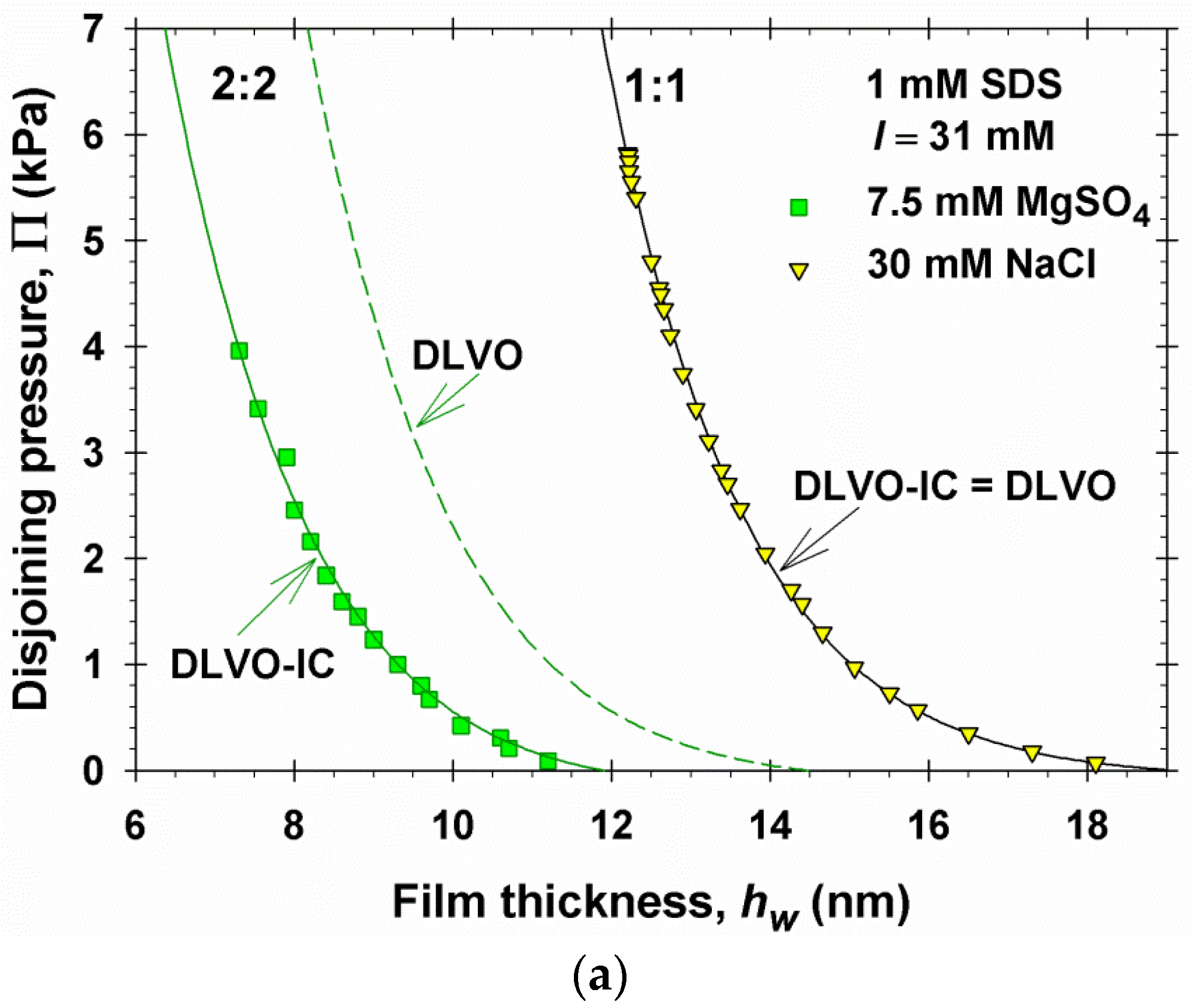
4. Numerical Results and Discussion
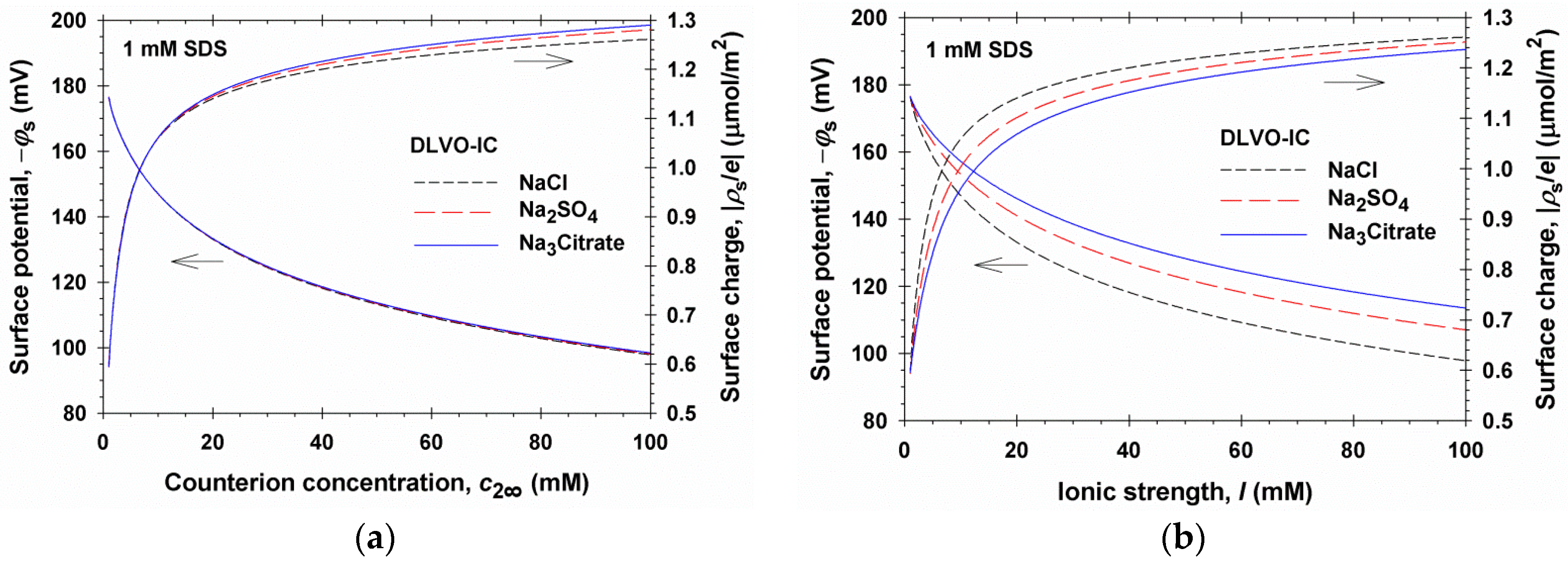
4.1. Results for 1:1, 1:2, and 1:3 Electrolytes
4.2. Results for a 2:2 Electrolyte
5. Analytical Expression for the Asymptotic Screening Parameter
- (i)
- The screening parameter κa depends not only on the sum that enters the expression for the conventional Debye parameter κ∞, but also on the sums and . This leads to a considerable effect of ions of higher valence, including coions, on the Debye screening.
- (ii)
- Equation (5.12) shows that κa ≥ κ∞, which leads to a stronger screening and weaker electrostatic repulsion as a result of the ionic correlation effect: compare the DLVO-IC with the DLVO curves in Figure 7b.
- (iii)
- The effect of ionic correlations is a long-range effect—it influences the long-range asymptotics of Πel and can be significant when the film thickness is tens of nanometers, not only in films a few nanometers thick.
- (iv)
- If all dissolved electrolytes are symmetric, then = 0 and Equation (5.12) yields κa = κ∞, i.e., the ionic correlation effect on the asymptotic decay length disappears for symmetric electrolytes, supposedly the electric potential Φm in the film’s midplane is low. This fact is related to the coincidence of the predictions of DLVO and DLVO-IC theories for NaCl in Figure 2.
- (v)
- The ionic radius, rm, essentially affects the value of the screening parameter κa. If we formally set rm = 0 in Equation (5.12), the calculated κa will be markedly different from the asymptotic slope of the exact numerical solution in Figure 7b.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Derivation of the Expression for the Activity Coefficient
A.1. Interaction Energy of the Individual Ions
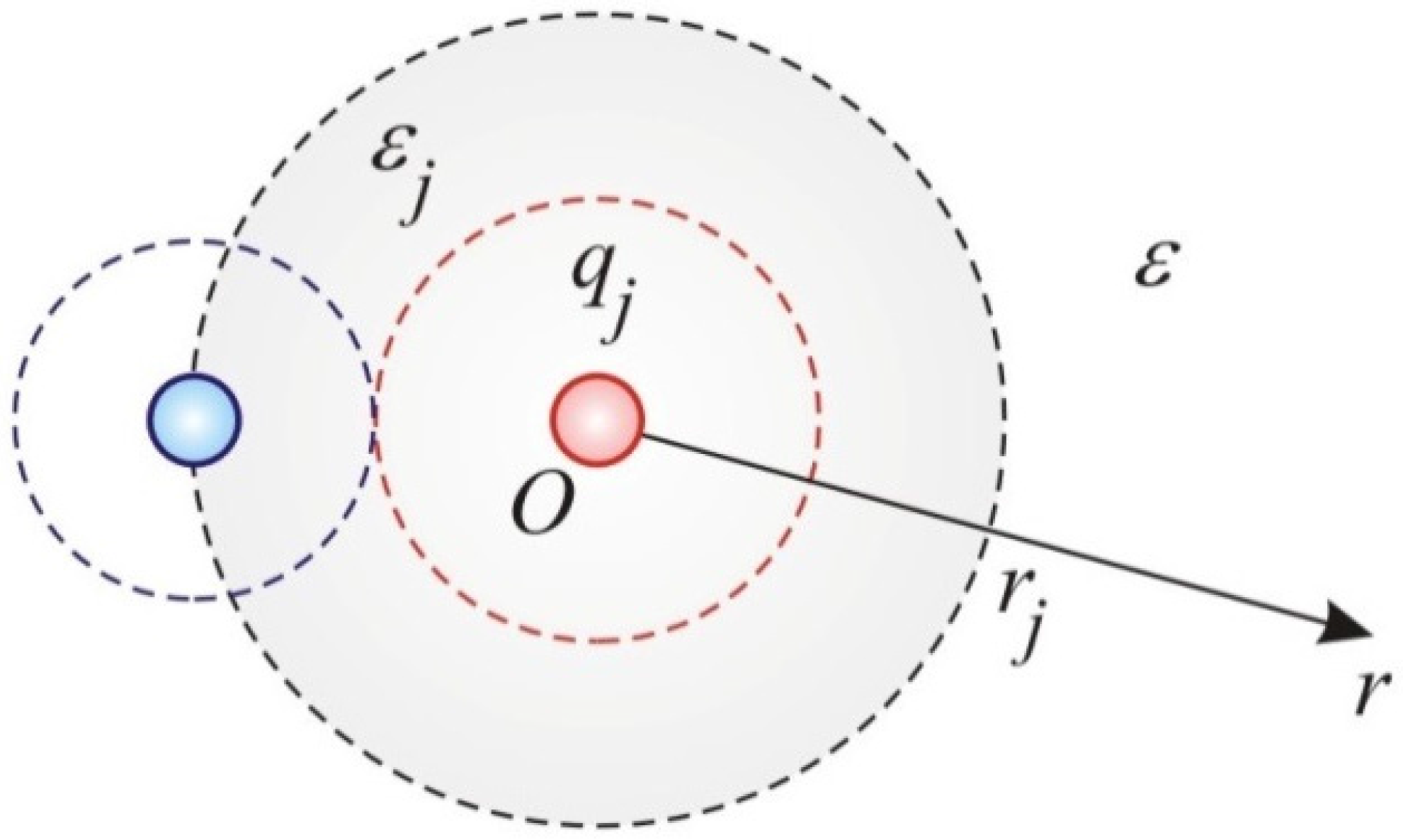
A.2. Free Energy and Electrochemical Potentials
References
- Derjaguin, B.V.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. URS 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Langmuir, I. The role of attractive and repulsive forces in the formation of tactoids, thixotropic gels, protein crystals and coacervates. J. Chem. Phys. 1938, 6, 873–895. [Google Scholar] [CrossRef]
- Debye, P.; Hückel, E. The theory of electrolytes. I. Lowering of freezing point and related phenomena. Phys. Z. 1923, 24, 185–206. [Google Scholar]
- Landau, L.D.; Lifshitz, E.M. Statistical Physics. Course of Theoretical Physics; Elsevier: Amstredam, The Netherlands, 1980; Volume 5. [Google Scholar]
- Derjaguin, B.V. Theory of Stability of Colloids and Thin Films; Springer: Berlin, Germany, 1989. [Google Scholar]
- Outhwaite, C.W.; Bhuiyan, L.B. An improved modified Poisson-Boltzmann equation in electric-double-layer theory. J. Chem. Soc. Faraday Trans. 1983, 79, 707–718. [Google Scholar] [CrossRef]
- Attard, P.; Mitchell, D.J.; Ninham, B.W. Beyond Poisson-Boltzmann: Images and correlations in the electric double layer. II. Symmetric electrolyte. J. Chem. Phys. 1988, 89, 4358–4367. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Ninham, B.W. Range of the screened Coulomb interaction in electrolytes and double layer problems. Chem. Phys. Lett. 1978, 53, 397–399. [Google Scholar] [CrossRef]
- Knackstedt, M.A.; Ninham, B.W. Correlations and thermodynamic coefficients in dilute asymmetric electrolyte solutions. J. Phys. Chem. 1996, 100, 1330–1335. [Google Scholar] [CrossRef]
- Kohonen, M.M.; Karaman, M.E.; Pashley, R.M. Debye length in multivalent electrolyte solutions. Langmuir 2000, 16, 5749–5753. [Google Scholar] [CrossRef]
- Ruiz-Cabello, F.J.M.; Moazzami-Gudarzi, M.; Elzbieciak-Wodka, M.; Maroni, P.; Labbez, C.; Borkovec, M.; Gregor Trefalt, G. Long-ranged and soft interactions between charged colloidal particles induced by multivalent coions. Soft Matter 2015, 11, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Bratko, D.; Blanch, H.W.; Prausnitz, J.M. Monte Carlo simulation for the potential of mean force between ionic colloids in solutions of asymmetric salts. J. Chem. Phys. 1999, 111, 7084–7094. [Google Scholar] [CrossRef]
- Ulander, J.; Greberg, H.; Kjellander, R. Primary and secondary effective charges for electrical double layer systems with asymmetric electrolytes. J. Chem. Phys. 2001, 115, 7144–7160. [Google Scholar] [CrossRef]
- Terao, T.; Nakayama, T. Charge inversion of colloidal particles in an aqueous solution: Screening by multivalent ions. Phys. Rev. E 2001, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Ma, Y.-Q. Insights from Monte Carlo simulations on charge inversion of planar electric double layers in mixtures of asymmetric electrolytes. J. Chem. Phys. 2010, 133. [Google Scholar] [CrossRef] [PubMed]
- Ederth, T.; Claesson, P.M. Forces between carboxylic acid surfaces in divalent electrolyte solutions. J. Colloid Interface Sci. 2000, 229, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, I.; Sadeghpour, A.; Borkovec, M. Destabilization of colloidal suspensions by multivalent ions and polyelectrolytes: From screening to overcharging. Langmuir 2012, 28, 6211–6215. [Google Scholar] [CrossRef] [PubMed]
- Tiraferri, A.; Maroni, P.; Borkovec, M. Adsorption of polyelectrolytes to like-charged substrates induced by multivalent counterions as exemplified by poly(styrene sulfonate) and silica. Phys. Chem. Chem. Phys. 2015, 17, 10348–10352. [Google Scholar] [CrossRef] [PubMed]
- Moazzami-Gudarzi, M.; Trefalt, G.; Szilagyi, I.; Maroni, P.; Borkovec, M. Forces between negatively charged interfaces in the presence of cationic multivalent oligoamines measured with the atomic force microscope. J. Phys. Chem. C 2015, 119, 15482–15490. [Google Scholar] [CrossRef]
- Besteman, K.; Zevenbergen, M.A.G.; Heering, H.A.; Lemay, S.G. Direct observation of charge inversion by multivalent ions as a universal electrostatic phenomenon. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Besteman, K.; Zevenbergen, M.A.G.; Lemay, S.G. Charge inversion by multivalent ions: Dependence on dielectric constant and surface-charge density. Phys. Rev. E 2005, 72. [Google Scholar] [CrossRef] [PubMed]
- Trulsson, M.; Jönsson, B.; Åkesson, T.; Forsman, J.; Labbez, C. Repulsion between oppositely charged surfaces in multivalent electrolytes. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.D.; Crozier, P.S.; Anderson, J.A.; Travesset, A. Molecular dynamics of ionic transport and electrokinetic effects in realistic silica channels. J. Phys. Chem. C 2008, 112, 10222–10232. [Google Scholar] [CrossRef]
- Wernersson, E.; Kjellander, R.; Lyklema, J. Charge inversion and ion-ion correlation effects at the mercury/aqueous MgSO4 interface: Toward the solution of a long-standing issue. J. Phys. Chem. C 2010, 114, 1849–1866. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Danov, K.D.; Broze, G.; Mehreteab, A. Thermodynamics of ionic surfactant adsorption with account for the counterion binding: Effect of salts of various valency. Langmuir 1999, 15, 2351–2365. [Google Scholar] [CrossRef]
- Kolev, V.L.; Danov, K.D.; Kralchevsky, P.A.; Broze, G.; Mehreteab, A. Comparison of the van der Waals and Frumkin adsorption isotherms for sodium dodecyl sulfate at various salt concentrations. Langmuir 2002, 18, 9106–9109. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Danov, K.D.; Kolev, V.L.; Broze, G.; Mehreteab, A. Effect of nonionic admixtures on the adsorption of ionic surfactants at fluid interfaces. Part 1. Sodium dodecyl sulfate and dodecanol. Langmuir 2003, 19, 5004–5018. [Google Scholar] [CrossRef]
- Christov, N.C.; Danov, K.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P.; Lips, A. The maximum bubble pressure method: Universal surface age and transport mechanisms in surfactant solutions. Langmuir 2006, 22, 7528–7542. [Google Scholar] [CrossRef] [PubMed]
- Mysels, K.J.; Jones, M.N. Direct measurement of the variation of double-layer repulsion with distance. Discuss. Faraday Soc. 1966, 42, 42–50. [Google Scholar] [CrossRef]
- Exerowa, D.; Kolarov, T.; Khristov, K. Direct measurement of disjoining pressure in black foam films. I. Films from an ionic surfactant. Colloids Surf. 1987, 22, 171–185. [Google Scholar] [CrossRef]
- Schelero, N.; Hedicke, G.; Linse, P.; von Klitzing, R. Effects of counterions and co-ions on foam films stabilized by anionic dodecyl sulfate. J. Phys. Chem. B 2010, 114, 15523–15529. [Google Scholar] [CrossRef] [PubMed]
- Vašíček, A. Optics of Thin Films; North-Holland: Amsterdam, The Netherlands, 1960; p. 123. [Google Scholar]
- Sheludko, A. Thin liquid films. Adv. Colloid Interface Sci. 1967, 1, 391–464. [Google Scholar] [CrossRef]
- Dimitrova, T.D.; Leal-Calderon, F.; Gurkov, T.D.; Campbell, B. Disjoining pressure vs. thickness isotherms of thin emulsion films stabilized by proteins. Langmuir 2001, 17, 8069–8077. [Google Scholar] [CrossRef]
- Cao, T.; Szilagyi, I.; Oncsik, T.; Borkovec, M.; Trefalt, G. Aggregation of colloidal particles in the presence of multivalent co-ions: The inverse Schulze–Hardy rule. Langmuir 2015, 31, 6610–6614. [Google Scholar] [CrossRef] [PubMed]
- Danov, K.D.; Kralchevsky, P.A. The standard free energy of surfactant adsorption at air/water and oil/water interfaces: Theoretical vs. empirical approaches. Colloid J. 2012, 74, 172–185. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Bhat, J.I.; Manjunatha, M.N. Effect of dielectric constant on the solvation of sodium citrate. Int. J. Chem. Res. 2012, 3, 6–14. [Google Scholar]
- Perera, J.; Weerasekera, M.; Kottegoda, N. Slow release anti-fungal skin formulations based on citric acid intercalated layered double hydroxides nanohybrids. Chem. Cent. J. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Kralchevsky, P.A.; Danov, K.D.; Denkov, N.D. Chemical physics of colloid systems and interfaces. In Handbook of Surface and Colloid Chemistry, 3rd ed.; Birdi, K.S., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 197–377. [Google Scholar]
- Angarska, J.K.; Dimitrova, B.St.; Kralchevsky, P.A.; Danov, K.D. Adsorption of sodium dodecyl sulfate in the presence of magnesium sulfate. An estimation of the adsorption of magnesium ions in Stern layer based on the surface tension data. In Collection of Scientific Studies in Natural Sciences; University Publisher Ep. K. Preslavsky: Shumen, Bulgaria, 2002; pp. 192–209. [Google Scholar]
- Hill, T.L. An Introduction to Statistical Thermodynamics; Dover Publications: New York, NY, USA, 1987. [Google Scholar]


| System | κ∞ (nm−1) Debye Parameter | κexp (nm−1) Experimental Slope | κa (nm−1) Equation (5.12) |
|---|---|---|---|
| 30 mM NaCl | 0.580 | 0.57 ± 0.01 | 0.580 |
| 10 mM Na2SO4 | 0.580 | 0.59 ± 0.01 | 0.603 |
| 5 mM Na3Citrate | 0.580 | 0.68 ± 0.01 | 0.712 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danov, K.D.; Basheva, E.S.; Kralchevsky, P.A. Effect of Ionic Correlations on the Surface Forces in Thin Liquid Films: Influence of Multivalent Coions and Extended Theory. Materials 2016, 9, 145. https://doi.org/10.3390/ma9030145
Danov KD, Basheva ES, Kralchevsky PA. Effect of Ionic Correlations on the Surface Forces in Thin Liquid Films: Influence of Multivalent Coions and Extended Theory. Materials. 2016; 9(3):145. https://doi.org/10.3390/ma9030145
Chicago/Turabian StyleDanov, Krassimir D., Elka S. Basheva, and Peter A. Kralchevsky. 2016. "Effect of Ionic Correlations on the Surface Forces in Thin Liquid Films: Influence of Multivalent Coions and Extended Theory" Materials 9, no. 3: 145. https://doi.org/10.3390/ma9030145
APA StyleDanov, K. D., Basheva, E. S., & Kralchevsky, P. A. (2016). Effect of Ionic Correlations on the Surface Forces in Thin Liquid Films: Influence of Multivalent Coions and Extended Theory. Materials, 9(3), 145. https://doi.org/10.3390/ma9030145






