The Use of an Edible Mushroom-Derived Renewable Carbon Material as a Highly Stable Electrocatalyst towards Four-Electron Oxygen Reduction
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Physical Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Structural and Surface Characterization
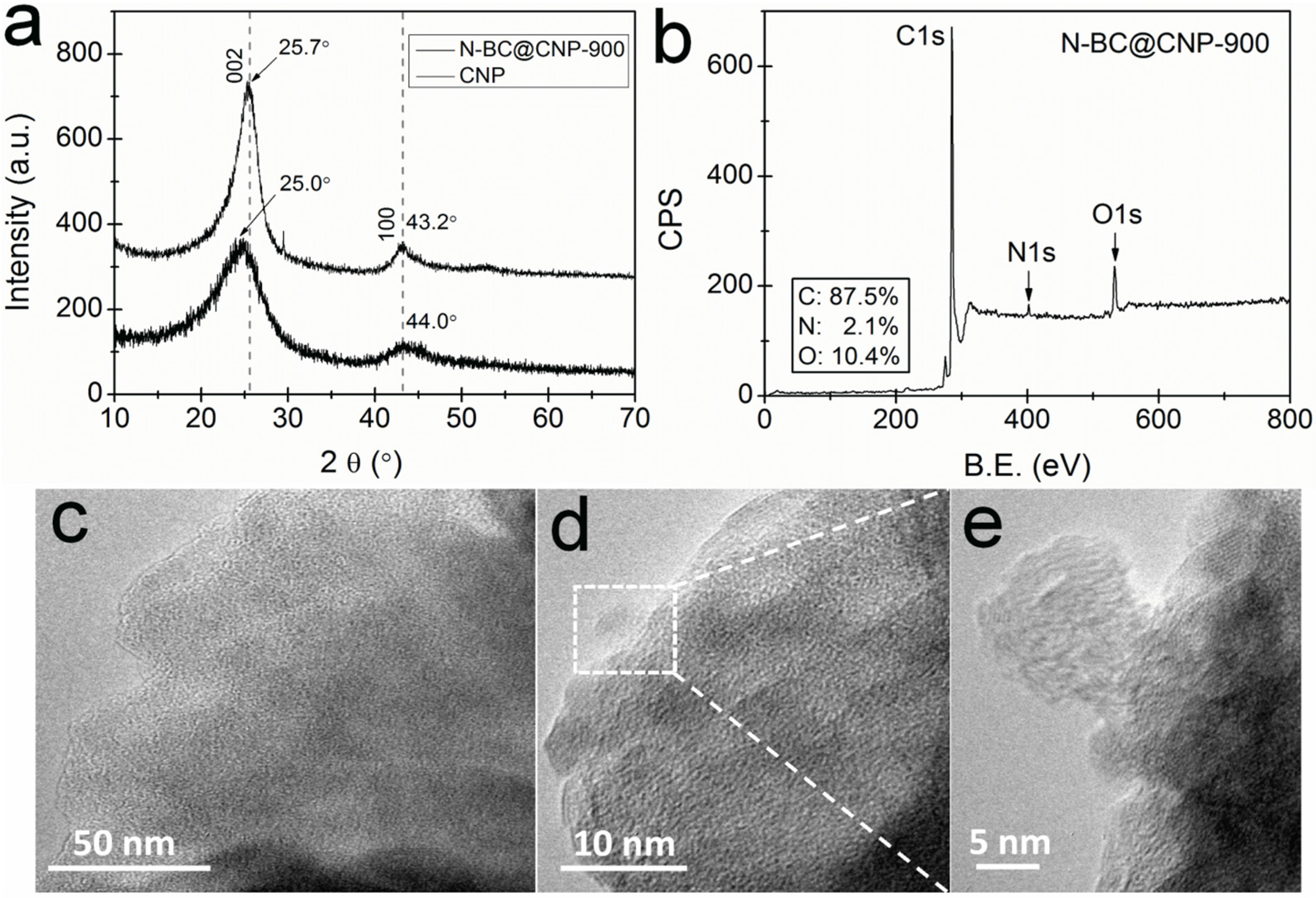
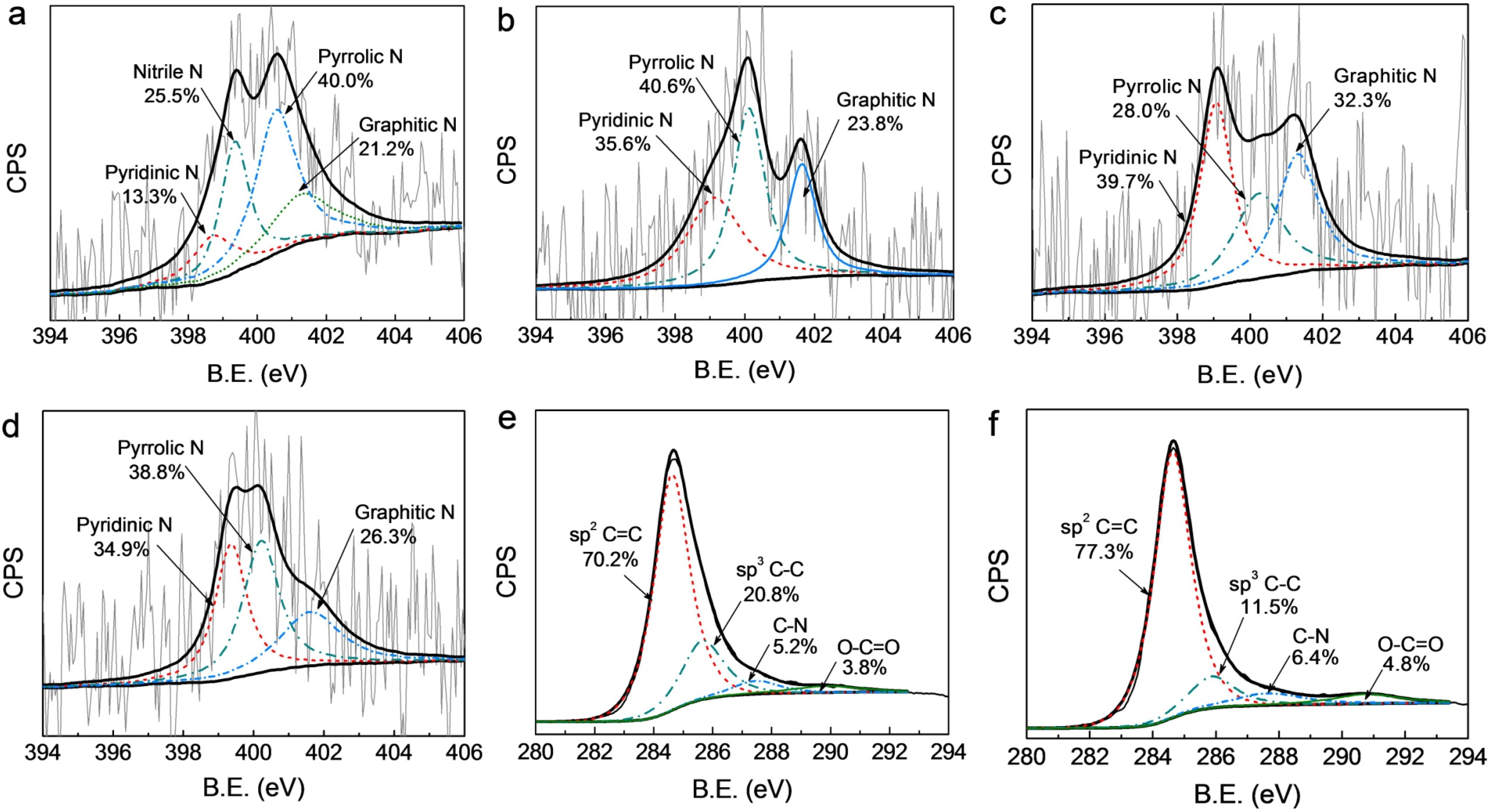
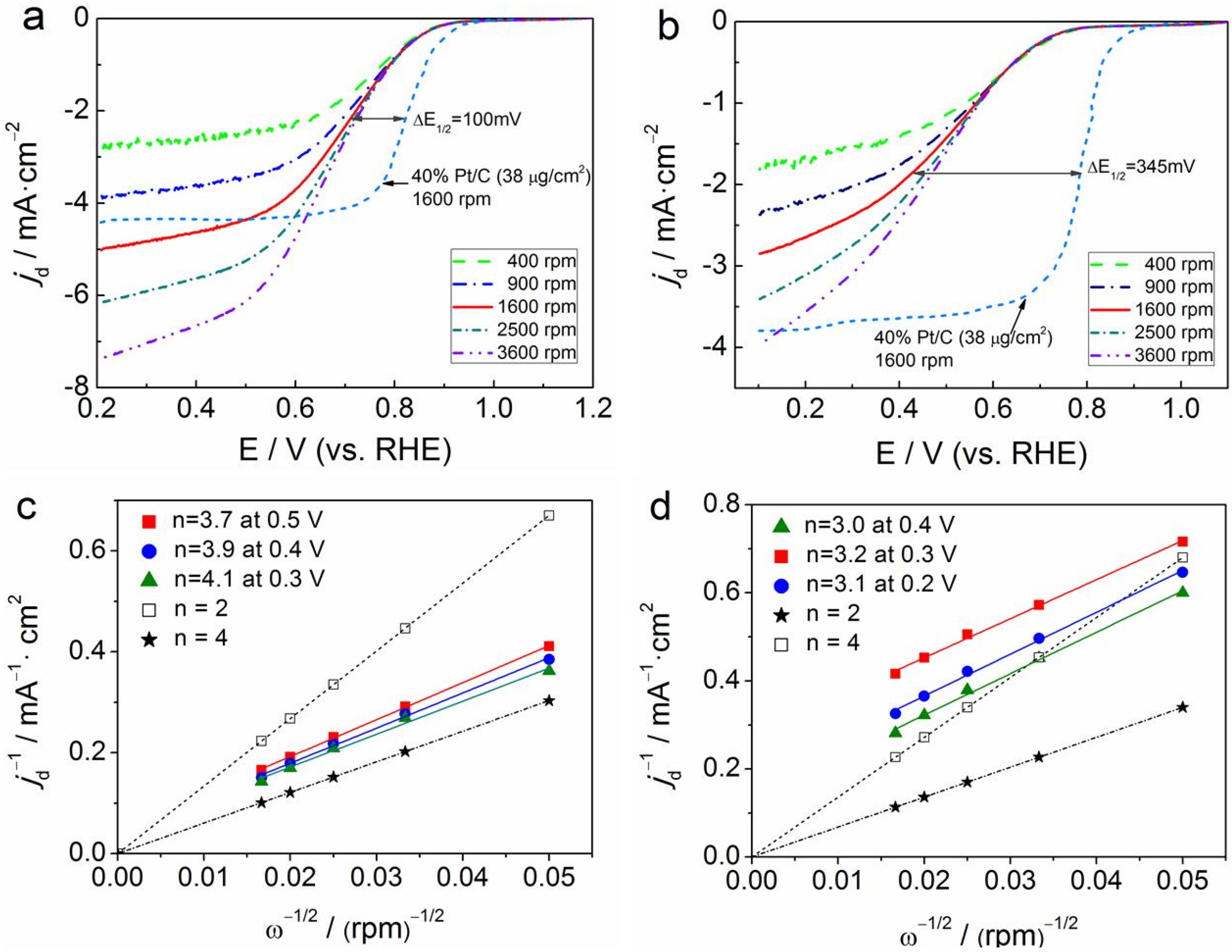
3.2. Electrocatalytic Activity and Stability

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for fuel cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [PubMed]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Z.; Liao, W.L.; Sun, L.T.; Chen, C.G. Synthesis of non-noble nitrogen-containing catalysts for cathodic oxygen reduction reaction: A critical review. Int. J. Electrochem. Sci. 2015, 10, 2467–2477. [Google Scholar]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal-nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.W.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1, e1400129. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, J.; Wang, F.B.; Xia, X.H. Synthesis of nitrogen doped graphene with high electrocatalytic activity toward oxygen reduction reaction. Electrochem. Commun. 2013, 28, 24–26. [Google Scholar] [CrossRef]
- Geng, D.; Liu, H.; Chen, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. Non-noble metal oxygen reduction electrocatalysts based on carbon nanotubes with controlled nitrogen contents. J. Power Sources 2011, 196, 1795–1801. [Google Scholar] [CrossRef]
- Yu, Y.M.; Zhang, J.H.; Xiao, C.H.; Zhong, J.D.; Zhang, X.H.; Chen, J.H. High active hollow nitrogen-doped carbon microspheres for oxygen reduction in alkaline media. Fuel Cells 2012, 12, 506–510. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Gao, S.; Wei, X.; Fan, H.; Li, L.; Geng, K.; Wang, J. Nitrogen-doped carbon shell structure derived from natural leaves as a potential catalyst for oxygen reduction reaction. Nano Energy 2015, 13, 518–526. [Google Scholar] [CrossRef]
- Maruyama, J.; Abe, I. Carbonized hemoglobin functioning as a cathode catalyst for polymer electrolyte fuel cells. Chem. Mater. 2006, 18, 1303–1311. [Google Scholar] [CrossRef]
- Liu, F.; Peng, H.; You, C.; Fu, Z.; Huang, P.; Song, H.; Liao, S. High-performance doped carbon catalyst derived from nori biomass with melamine promoter. Electrochim. Acta 2014, 138, 353–359. [Google Scholar] [CrossRef]
- Guo, C.Z.; Liao, W.; Chen, C.G. Design of a non-precious metal electrocatalyst for alkaline electrolyte oxygen reduction by using soybean biomass as the nitrogen source of electrocatalytically active center structures. J. Power Sources 2014, 269, 841–847. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Bai, L.; Han, L.; Dong, S. Nitrogen, cobalt-codoped carbon electrocatalyst for oxygen reduction reaction using soy milk and cobalt salts as precursors. Electrochem. Commun. 2013, 34, 68–72. [Google Scholar] [CrossRef]
- Iwazaki, T.; Obinata, R.; Sugimoto, W.; Takasu, Y. High oxygen-reduction activity of silk-derived activated carbon. Electrochem. Commun. 2009, 11, 376–378. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Wang, H.; Ji, S.; Key, J.; Wang, R. Fe (III)-induced N enrichment in the surface of carbon materials derived from silk fibroins and its effect on electrocatalytic oxygen reduction. J. Electrochem. Soc. 2014, 161, F795–F802. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Ji, S.; Feng, H.; Linkov, V.; Wan, R. Biomass-derived activated carbon as high-performance non-precious electrocatalyst for oxygen reduction. RSC Adv. 2013, 3, 12039–12042. [Google Scholar] [CrossRef]
- Zhao, H.; Hui, K.S.; Hui, K.N. Synthesis of nitrogen-doped multilayer graphene from milk powder with melamine and their application to fuel cells. Carbon 2014, 76, 1–9. [Google Scholar] [CrossRef]
- Guo, C.Z.; Chen, C.G.; Luo, Z.L. A novel nitrogen-containing electrocatalyst for oxygen reduction reaction from blood protein pyrolysis. J. Power Sources 2014, 245, 841–845. [Google Scholar] [CrossRef]
- Guo, C.Z.; Liao, W.L.; Li, Z.B.; Chen, C.G. Exploration of the catalytically active site structures of animal biomass-modified on cheap carbon nanospheres for oxygen reduction reaction with high activity, stability and methanol-tolerant performance in alkaline medium. Carbon 2015, 85, 279–288. [Google Scholar] [CrossRef]
- Yuan, Z. Flammulina velutipes health function & processing use. Acad. Period. Farm Prod. Proc. 2015, 6, 125–128. (In Chinese) [Google Scholar]
- Guo, C.Z.; Liao, W.L.; Chen, C.G. Fe/N/C catalysts derived from blood protein and their electrocatalytic activity towards the oxygen reduction reaction in acidic solution. Chin. Sci. Bull. 2014, 59, 3424–3429. [Google Scholar] [CrossRef]
- Favaro, M.; Perini, L.; Agnoli, S.; Durante, C.; Granozzi, G.; Gennaro, A. Electrochemical behavior of N and Ar implanted highly oriented pyrolytic graphite substrates and activity toward oxygen reduction reaction. Electrochim. Acta 2013, 88, 477–487. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Maruyama, J.; Okamura, J.; Miyazaki, K. Two-step carbonization as a method of enhancing catalytic properties of hemoglobin at the fuel cell cathode. J. Phys. Chem. C 2007, 111, 6597–6600. [Google Scholar] [CrossRef]
- Favaro, M.; Carraro, F.; Cattelan, M.; Colazzo, L.; Durante, C.; Sambi, M.; Gennaro, A.; Agnoli, S.; Granozzi, G. Multiple doping of graphene oxide foams and quantum dots: New switchable systems for oxygen reduction and water remediation. J. Mater. Chem. A 2015, 3, 14334–14347. [Google Scholar] [CrossRef]
- Ding, W.; Wei, Z.D.; Chen, S.G.; Qi, X.Q.; Yang, T.; Hu, J.S.; Wang, D.; Wan, L.-J.; Alvi, S.F.; Li, L. Space-confinement-induced synthesis of pyridinic- and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew. Chem. Int. Edit. 2013, 52, 11755–11759. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Vijh, A.K. Non-noble metal-carbonized aerogel composites as electrocatalysts for the oxygen reduction reaction. Electrochem. Commun. 2003, 5, 272–275. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L. Electrochemical Methods, 2nd ed.; Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Favaro, M.; Ferrighi, L.; Fazio, G.; Colazzo, L.; Di Vaentin, C.; Durante, C.; Sedona, F.; Gennaro, A.; Agnoli, S.; Granozzi, G. Single and multiple doping in graphene quantum dots: Unraveling the origin of selectivity in the oxygen reduction reaction. ACS Catal. 2015, 5, 129–144. [Google Scholar] [CrossRef]
- Rao, C.V.; Cabrera, C.R.; Ishikawa, Y. In search of the active site in nitrogen-doped carbon nanotube electrodes for the oxygen reduction reaction. J. Phys. Chem. Lett. 2010, 1, 2622–2627. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, C.Z.; Chen, C.; Fan, M.; Gong, J.; Zhang, Y.; Zhao, T.; Sun, Y.; Xu, X.; Li, M.; et al. High content of pyridinic- and pyrrolic-nitrogen-modified carbon nanotubes derived from blood biomass for the electrocatalysis of oxygen reduction reaction in alkaline medium. Electrochim. Acta 2015, 168, 386–393. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Sun, L.; Liao, W.; Li, Z. The Use of an Edible Mushroom-Derived Renewable Carbon Material as a Highly Stable Electrocatalyst towards Four-Electron Oxygen Reduction. Materials 2016, 9, 1. https://doi.org/10.3390/ma9010001
Guo C, Sun L, Liao W, Li Z. The Use of an Edible Mushroom-Derived Renewable Carbon Material as a Highly Stable Electrocatalyst towards Four-Electron Oxygen Reduction. Materials. 2016; 9(1):1. https://doi.org/10.3390/ma9010001
Chicago/Turabian StyleGuo, Chaozhong, Lingtao Sun, Wenli Liao, and Zhongbin Li. 2016. "The Use of an Edible Mushroom-Derived Renewable Carbon Material as a Highly Stable Electrocatalyst towards Four-Electron Oxygen Reduction" Materials 9, no. 1: 1. https://doi.org/10.3390/ma9010001
APA StyleGuo, C., Sun, L., Liao, W., & Li, Z. (2016). The Use of an Edible Mushroom-Derived Renewable Carbon Material as a Highly Stable Electrocatalyst towards Four-Electron Oxygen Reduction. Materials, 9(1), 1. https://doi.org/10.3390/ma9010001




