The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri
Abstract
:1. Introduction
2. Results
2.1. Morphologies of chitosan scaffolds
| Parameters | Shrimp shells (SHCTS) | Crab shells (CRCTS) | Squid bone plates (SQCTS) | Fungal mycelia (FCTS) | ||
| Size of scaff old (mm) | Before neutralization | Thickness | 4.17 ± 0.16 | 4.84 ± 0.44 | 4.02 ± 0.38 | 4.56 ± 0.40 |
| Diameter | 30.5 ± 0.4 | 30.3 ± 0.4 | 30.4 ± 0.4 | 30.9 ± 0.4 | ||
| After neutralization | Thickness | 4.50 ± 0.40 | 3.25 ± 0.15 | 3.4 ± 0.70 | 4.70 ± 0.40 | |
| Diameter | 24.2 ± 1.0 | 25.9 ± 0.7 | 25.4 ± 0.9 | 28.1 ± 1.7 | ||
| Pore size of chitosan scaffold (μm) | 64 ± 20 | 77 ± 22 | 54 ± 17 | 84 ± 21 | ||
| Amount of absorbed water in scaffold (g/g of scaffold) | 36 ± 4 | 43 ± 3 | 46 ± 0 | 53 ± 2 | ||
| Porosity of water absorbed scaffold (%) | 88 ± 4 | 90 ± 3 | 96 ± 5 | 97 ± 1 | ||
| Tensile strength of scaffold | Force (cN) | 141 ± 37 | 88 ± 09 | 151 ± 31 | 209 ± 20 | |
| Elongation (%) | 15.1 ± 4.4 | 20.3 ± 4.2 | 5.4 ± 1.7 | 7.2 ± 1.5 | ||
| Degradation of scaffold (%) | 2 ± 0.8 | 5 ± 0.7 | 5 ± 0.5 | 11 ± 1.1 | ||
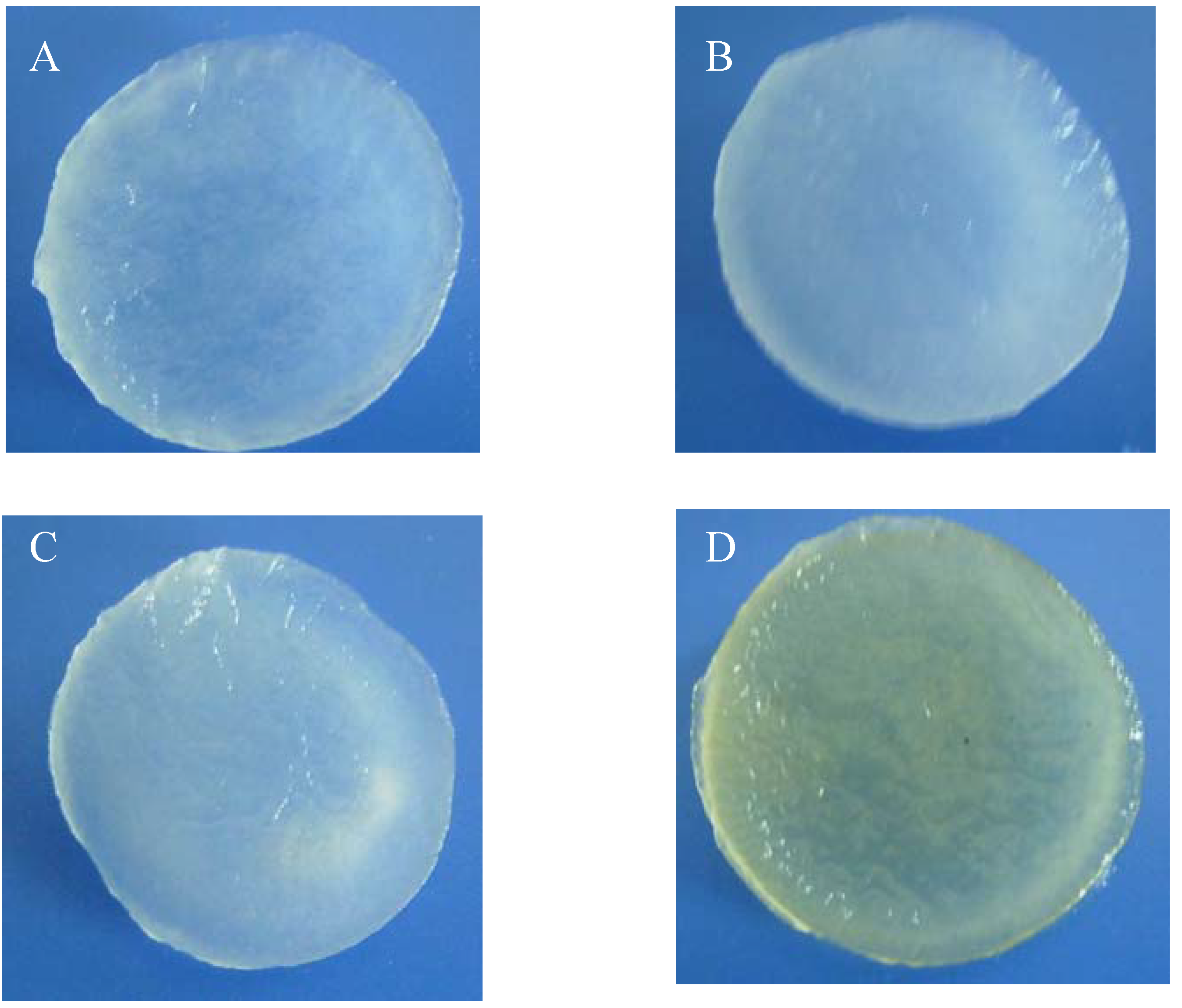
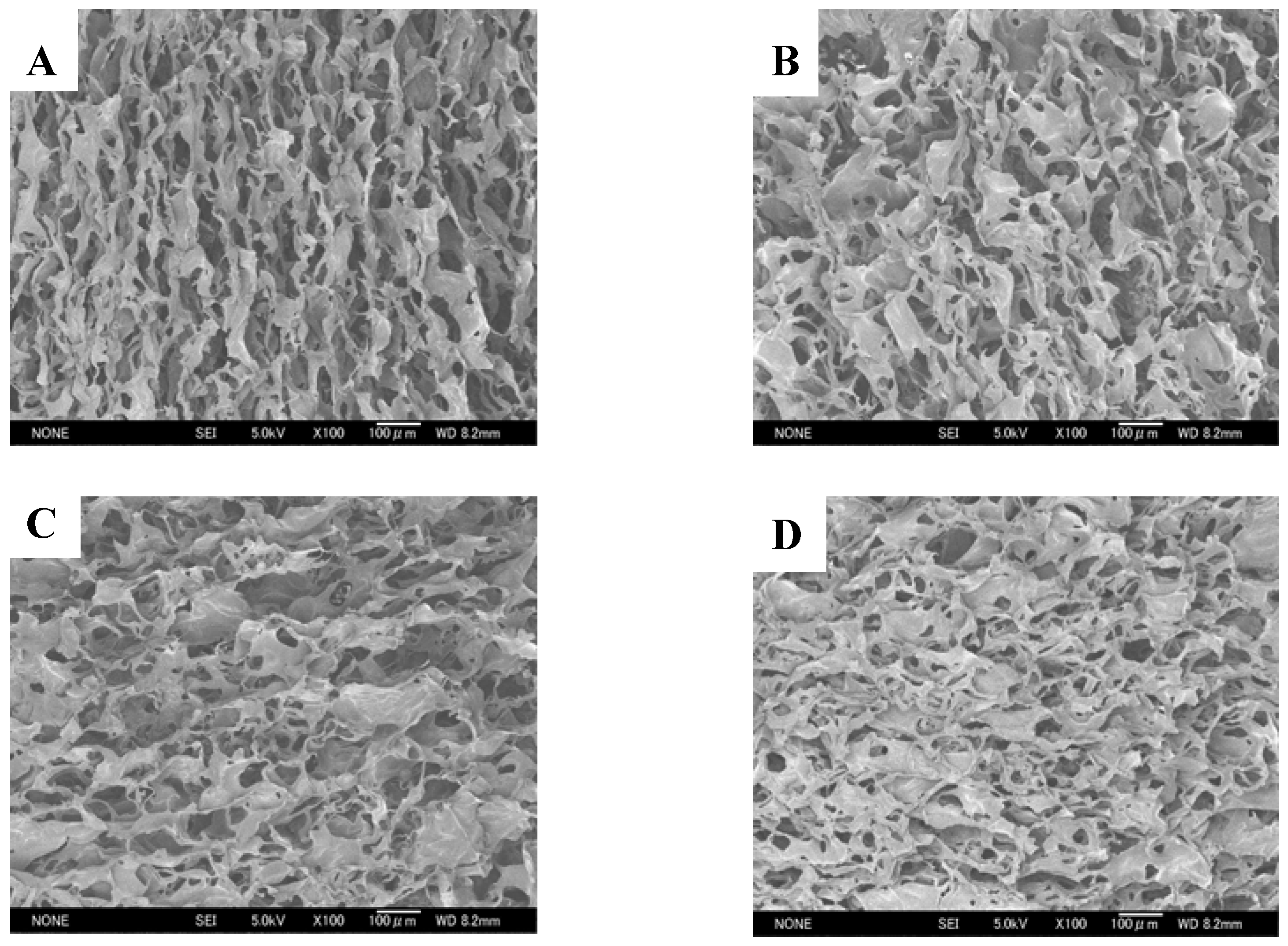
2.2. Water absorption properties of chitosan scaffolds
2.3. Mechanical properties of chitosan scaffolds
2.4. In Vitro degradation of chitosan scaffolds
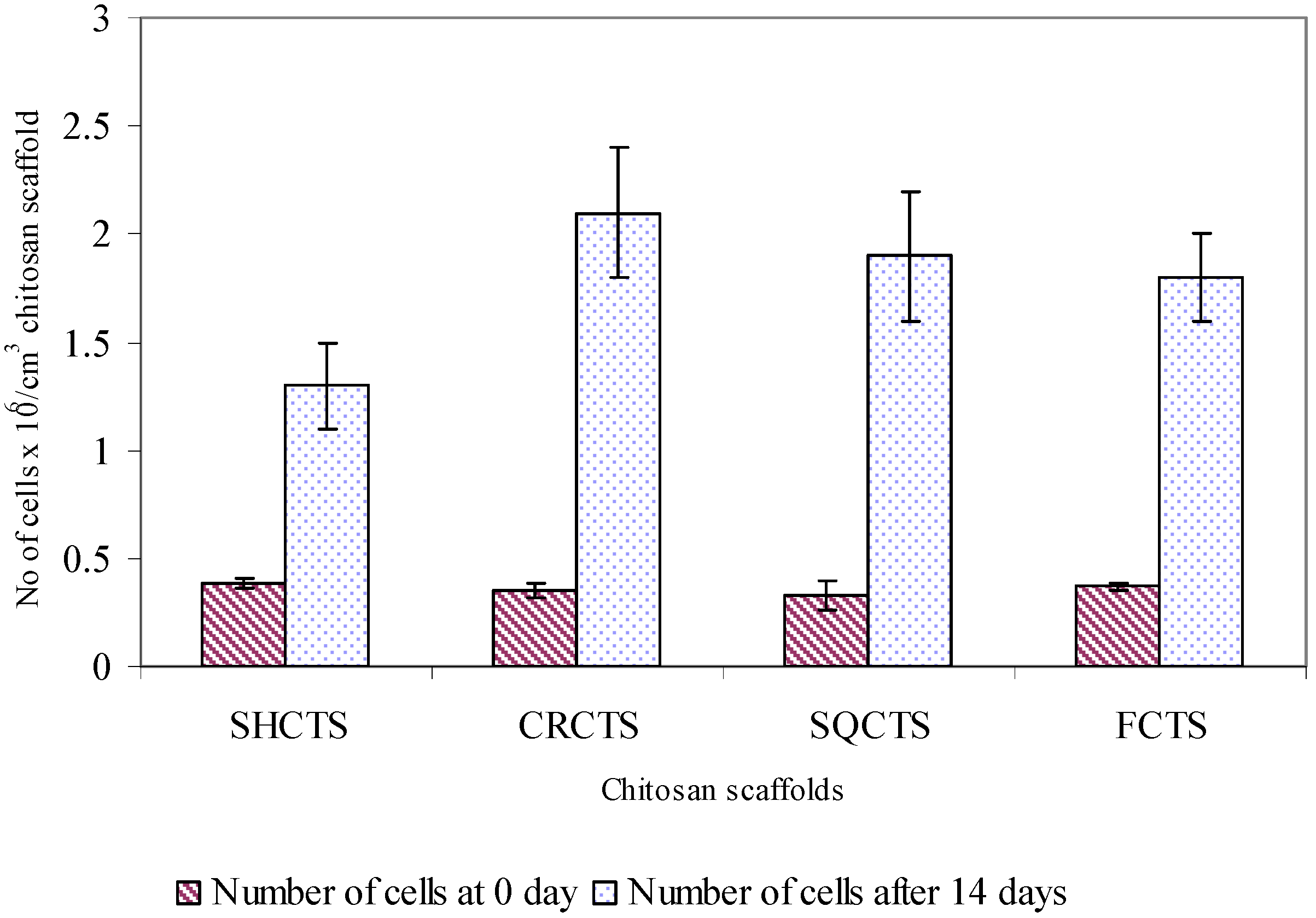
2.5. Assessment of attachment, morphology and proliferation of Swiss mouse embryo fibroblast NIH/3T3 cells on chitosan scaffolds
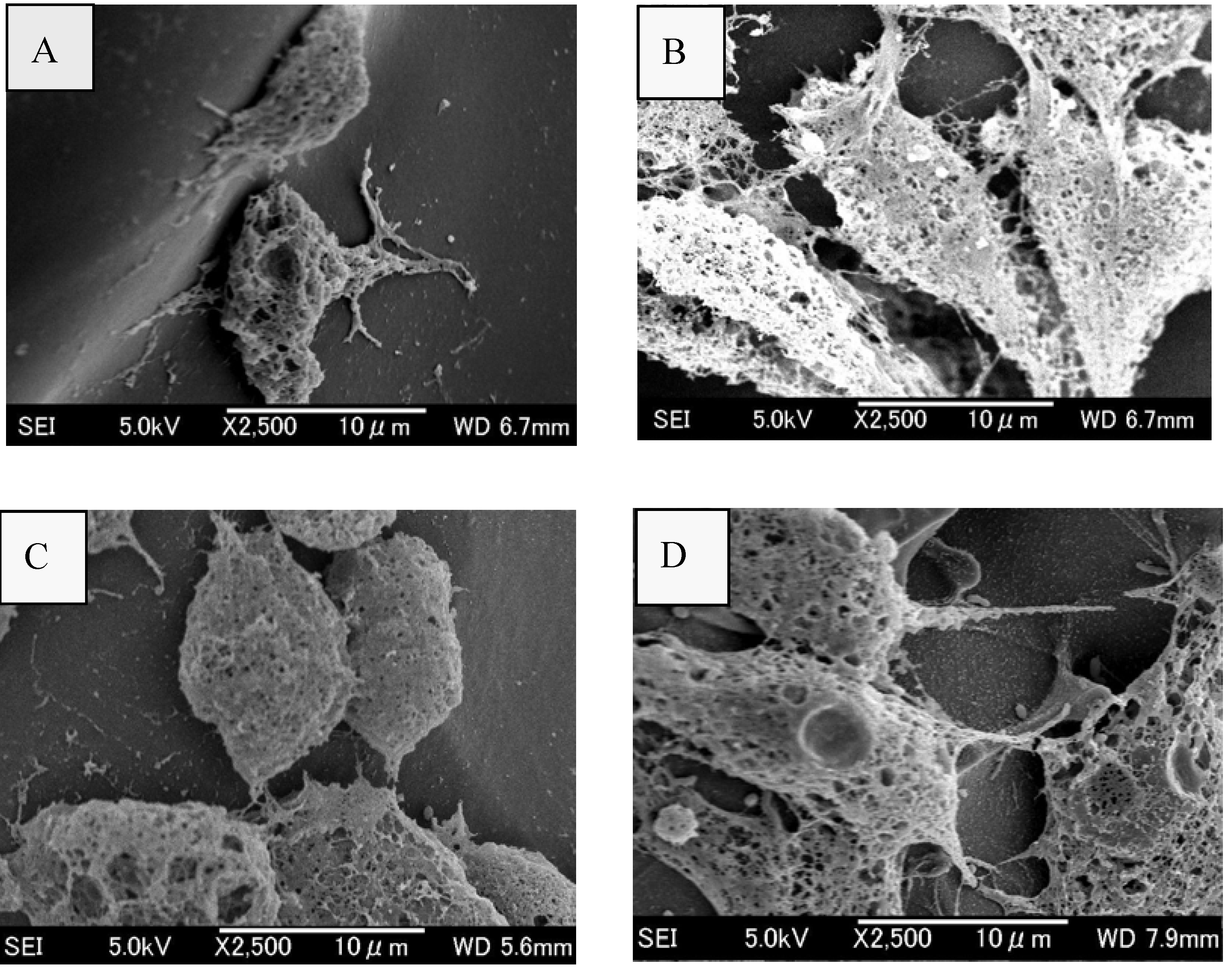
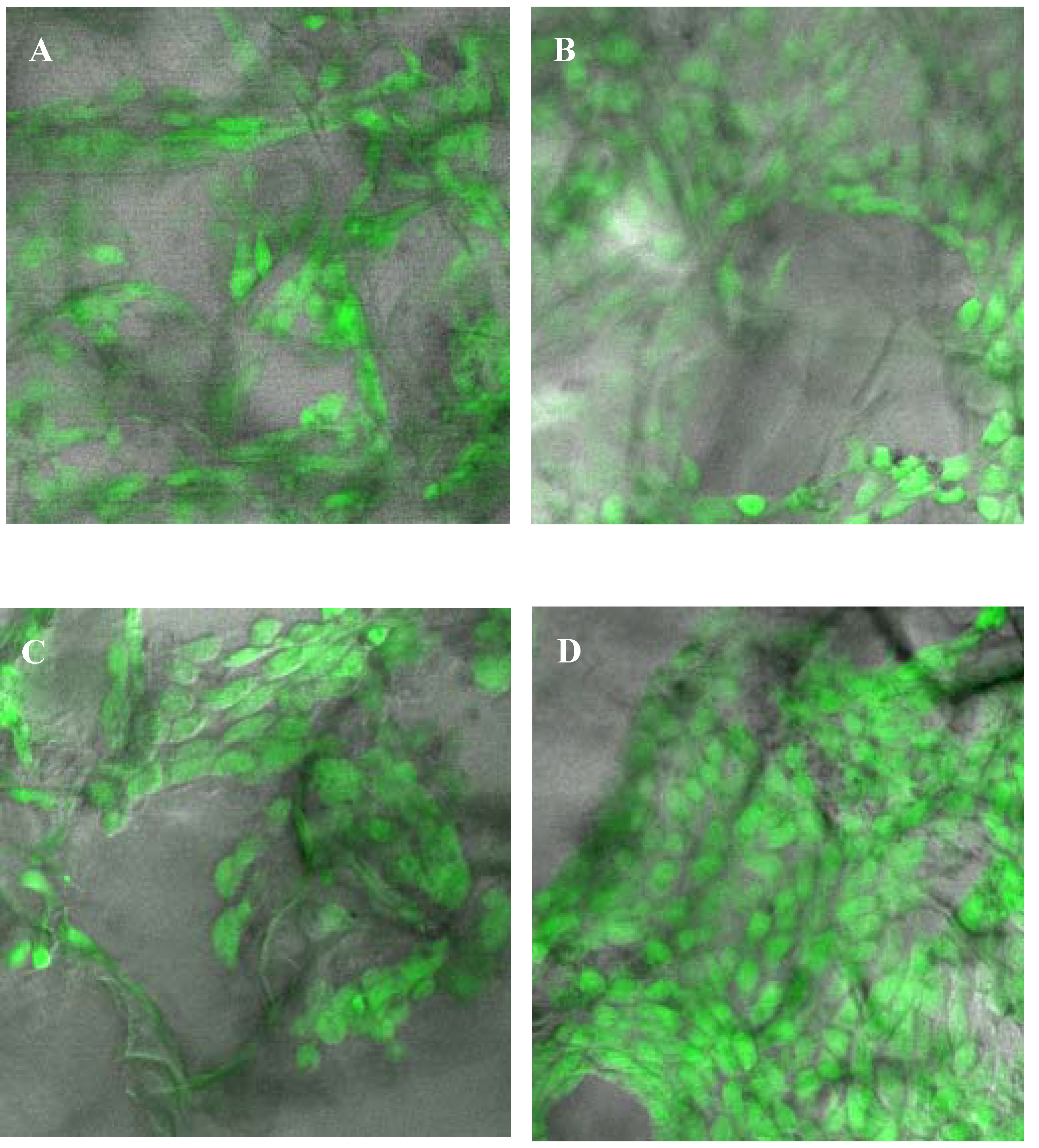
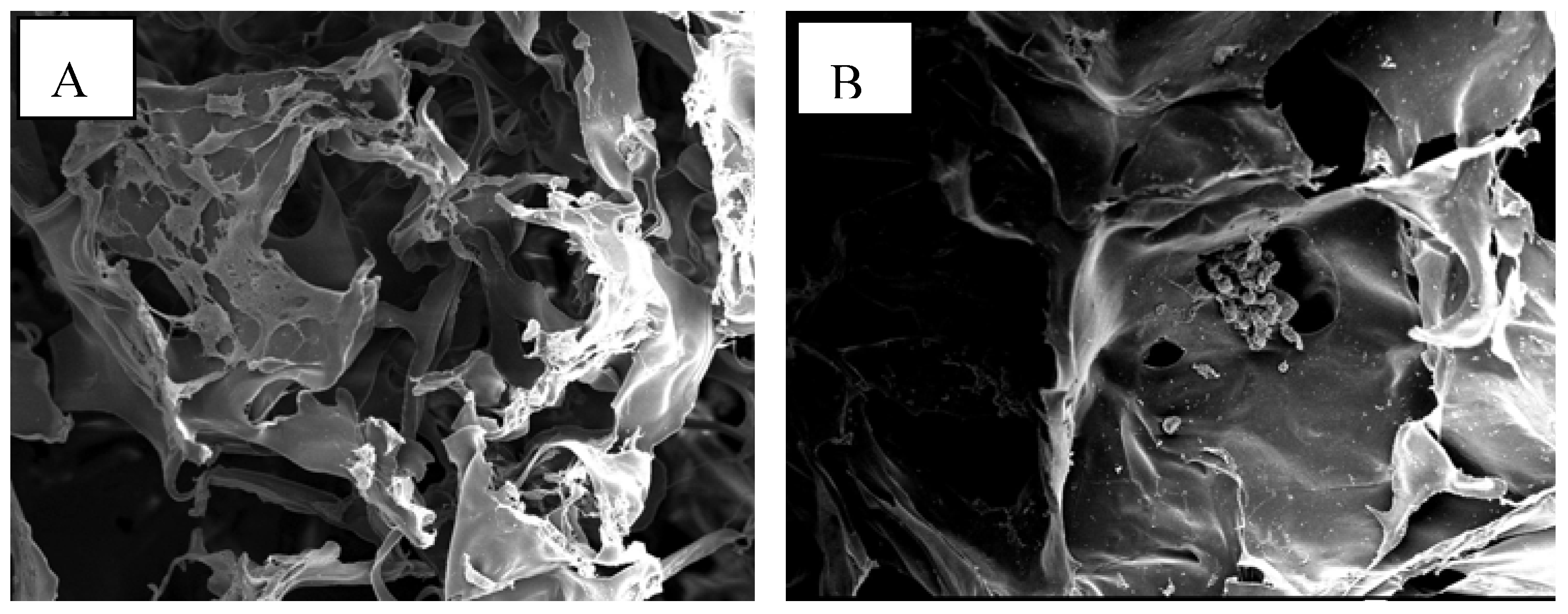
3. Discussion
3.1. Mechanical and biological properties of chitosan scaffolds
3.2. Biocompatibility of chitosan scaffolds
- (1)
- Thickness of scaffold, i.e., the cell growth rate decreased when the thickness of scaffold increased [46],
- (2)
- Longer the lag phase during growth of cells on chitosan scaffold [22],
- (3)
- High or low number of cells inoculated to the scaffold [22], i.e., low inoculum concentration resulted in cellular stress during growth of the cell on the chitosan scaffold and high initial cell concentration resulted in slower growth rate, and
- (4)
- Interaction between the cell and scaffold surface [28].
4. Experimental Section
4.1. Extraction of chitosan from cell wall of fungus Gongronella butleri USDB 0201 grown in solid substrate fermentation
4.2. Preparation of porous chitosan scaffolds
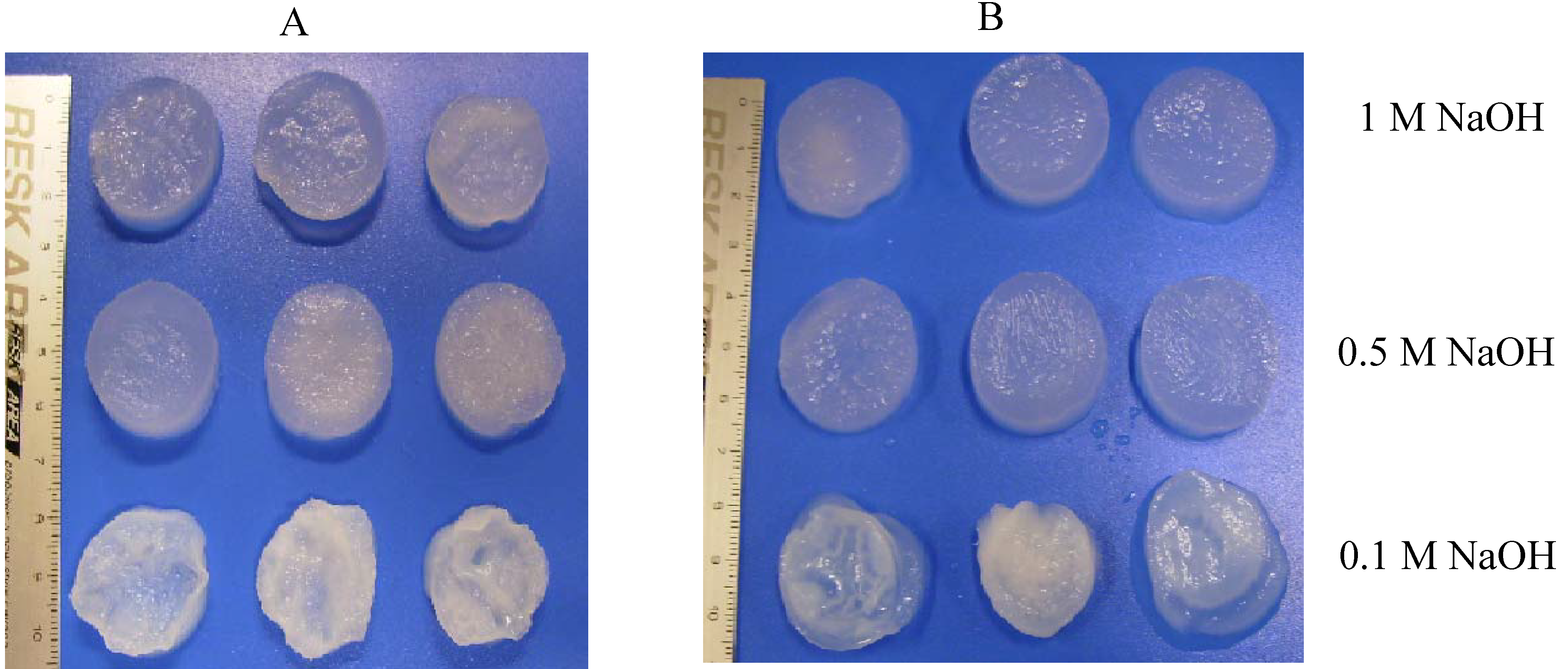
4.3. Observation the morphologies of chitosan scaffolds
4.4. Study the water absorption property of chitosan scaffolds
4.5. Determination of the porosity of water absorbed chitosan scaffolds
4.6. Examination the mechanical properties of chitosan scaffolds
4.7. In vitro degradation of chitosan scaffolds
4.8. Attachment and proliferation of fibroblast NIH/3T3 cells on chitosan scaffolds
4.9. Observation the morphology and viability of fibroblast NIH/3T3 cells on chitosan scaffolds
5. Conclusions
Acknowledgements
References and Notes
- Ng, CH.; Hein, S.; Ogawa, K.; Chandrkrachang, S.; Stevens, W.F. Distribution of D-glucosamine moieties in heterogeneously deacetylated cuttlefish chitin. Carbohydr. Polym. 2007, 69, 382–390. [Google Scholar] [CrossRef]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 2002, 49, 2357. [Google Scholar] [CrossRef]
- Bartniki-Garcia, S. Cell wall chemistry. Annu. Rev. Microbiol. 1968, 22, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Tan, T.K.; Wong, S.M.; Khor, E. The chitosan yield of Zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996, 30, 239–242. [Google Scholar] [CrossRef]
- Crestini, C.; Kovac, B.; Giovannozzi-Sermanni, G. Production and isolation of chitosan by submerged and solid state fermentation from Lentinus edodes. Biotechnol. Bioeng. 1996, 50, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Toan, N.V.; Ng, C.H.; Aye, K.N.; Trang, T.S.; Stevens, W.F. Production of high quality chitin and chitosan from preconditioned shrimp shells. J. Chem. Technol. Biotechnol. 2006, 81, 1113–1118. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeon, Y.; Byun, H.; Park, P. Subacute oral toxicity of chitosan oligosaccharide on Sprague Dawley rats. In Proceedings of the 8th International Chitin and Chitosan Conference and 4th Asia Pacific Chitin and Chitosan Symposium, Tokyo, Kodansha Scientific Ltd, 2001; p. 276.
- Shigemasa, Y.; Morimoto, M.; Saimoto, H.; Okamoto, Y.; Minami, S. Applications of chitin and chitosan for biomaterials. In Proceedings of the 3rd Asia Pacific Chitin and Chitosan Symposium, Taiwan, 1998; p. 47.
- Tokura, S.; Nishi, N.; Itoyama, K.; Shirai, A.; Nishimura, S.; Saiki, I.; Azuma, I. Chitin derivatives with biomedical functions. In Chitin World; Karnicki, Z.S., Brzeski, M.M., Bykowski, P. J., Wojtasz-Pajak, A., Eds.; Wirtschaftsverlag NW: Verlag für neue Wissenschaft GmbH: Bremerhaven, Germany, 1994; p. 287. [Google Scholar]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Madihally, S.V.; Mattew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Stevens, W.F. Production of chitin and chitosan and their applications in the medical and biological sector. In Recent Research in Biomedical Aspects of Chitin and Chitosan; Research Signpost: India, 2008; p. 161. [Google Scholar]
- Nwe, N.; Stevens, W.F. Preparation and characteristics of chitosan scaffolds for tissue engineering. In Recent Research in Biomedical Aspects of Chitin and Chitosan; Research Signpost: India, 2008; p. 57. [Google Scholar]
- Gravel, M.; Gross, T.; Vago, R.; Tabrizian, M. Responses of mesenchymal stem cell to chitosan-coralline composites microstructured using coralline as gas forming agent. Biomaterials 2006, 27, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Khor, H.L.; Hutmacher, D.W. In vitro characterization of natural and synthetic dermal matrices cultured with human dermal fibroblasts. Biomaterials 2004, 25, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W.T. Vascular cell responses to polysaccharide materials: in vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7626. [Google Scholar] [CrossRef] [PubMed]
- Thein, H.W.W.; Kitiyanant, Y. Chitosan scaffolds for in vitro buffalo embryonic stem-like cell culture: An approach to tissue engineering. J. Biomed. Mater. Res. 2006, 80B, 92–101. [Google Scholar]
- Ho, M.; Kuo, P.; Hsieh, H.; Hsien, T.; Hou, L.; Lai, J.; Wang, D. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Characterization and evaluation of chitosan matrix for in vitro growth of MCF- 7 breast cancer cell lines. Biomaterials 2004, 25, 5147–5154. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Lee, J.G.; Chen, R.H. Preparation conditions affect the pore size, water vapor permeability and other physical characteristics of resulting chitosan-γ-polyglutamic acid complex scaffolds. In Advance in Chitin Science; The European Chitin Society: Montpellier, France, 2006; Volume 9, p. 241. [Google Scholar]
- Chalonglarp, T.; Sorada, K.; Neeracha, S.; Rath, P.; Tanom, B.; Yasuhiko, T.; Siriporn, D. The influence of molecular weight of chitosan on the physical and biological properties of collagen/chitosan scaffolds. J. Biomater. Sci. Polym. Ed. 2007, 18, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.F.; Sampaio, P.; Barbosa, M.A. Three-dimensional culture of human osteoblastic cells in chitosan sponges: the effect of the degree of acetylation. J. Biomed. Mater. Res. 2006, 76A, 335–346. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Tangsadthakun, C.; Kanokpanont, S.; Sanchavanakit, N.; Pichyangkura, R.; Banaprasert, T.; Tabata, Y.; Damrongsakkul, S. The influence of molecular weight of chitosan on the physical and biological properties of collagen/chitosan scaffolds. J. Biomater. Sci. Polym. Ed. 2007, 18, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Whu, S.W.; Tsai, C.; Wu, Y.; Chen, H.; Hsieh, K. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Aiba, S. Studies on chitosan: 4. Lysozymic hydrolysis of partially N-acetylated chitosans. Int. J. Biol. Macromol. 1992, 14, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Yamaguchi, T.; Sakairi, N.; Nishi, N.; Tokura, S. Antimicrobial activity by fractionated chitosan oligomers. In Advances in Chitin Science; Jacques Andre Publisher: Lyon, France, 1997; Volume 2, p. 156. [Google Scholar]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, S. Bioactivities of chitosan oligosaccharides and their derivatives. In Proceedings of the 3rd Asia Pacific Chitin and Chitosan Symposium, Taiwan, 1998; p. 328.
- Prashanth, K.V.H.; Tharanathan, R.N. Depolymerized products of chitosan as potent inhibitors of tumor-induced angiogenesis. Biochim. Biophys. Acta (BBA), Gen. Subj. 2005, 1722, 22–29. [Google Scholar] [CrossRef]
- Wu, G.; Tsai, G. Immunomodulatory activity of oral administration of the chitosan hydrolytic products in balb/c mice. In Advance in Chitin Science; The European Chitin Society: Montpellier, France, 2006; Volume 9, p. 320. [Google Scholar]
- Okamoto, Y.; Nose, M.; Sashiwa, H.; Morimoto, M.; Saimoto, H.; Shigemasa, Y.; Minami, S. Fundamental study on oral administration of chitin and chitosan in dogs. In Advances in Chitin Science; Jacques Andre Publisher: Lyon, France, 1997; Volume 2, p. 625. [Google Scholar]
- Stevens, W.F. Specific applications require specialized chitosans. In Proceedings of the 7th Asia Pacific Chitin and Chitosan Symposium, 2006; Hanrimwon Printing Co. Ltd: Seoul, S. Korea; p. 494.
- Hasegawa, M.; Isogai, A.; Onabe, F. Preparation of low-molecular weight chitosan using phosphoric acid. Carbohydr. Polym. 1993, 20, 279–283. [Google Scholar] [CrossRef]
- Chang, C.; Liao, Y.; Li, S. Preparation of low molecrular weight chitosan and chitooligosaccharides by the enzymatic hydrolysis of chitosan. In Proceedings of the 3rd Asia Pacific Chitin and Chitosan Symposium, Taiwan, 1998; p. 233.
- Xing, R.; Liu, S.; Yu, H.; Guo, Z.; Wang, P.; Li, C.; Li, Z.; Li, P. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation. Carbohydr. Res. 2005, 340, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kang, H.; Lee, Y.; Kim, K.; Kim, H.; Seo, Y.; Park, B.; Choi, M.; Son, T. Preparation and cytocompatibility of chitosan oligomer by nitrous acid depolymerisation for growth factor immobilization. J. Chitin Chitosan 2006, 11, 42–46. [Google Scholar]
- Li, J.; Du, Y.; Yang, J.; Feng, T.; Li, A.; Chen, P. Preparation and characterisation of low molecular weight chitosan and chito-oligomers by a commercial enzyme. Polym. Degrad. Stab. 2005, 87, 441–448. [Google Scholar] [CrossRef]
- Thomas, P.; Philip, B. Production of partially degraded chitosan with desired molecular weight. In Advance in Chitin Science; Universitat Potsdam: Potsdam, Germany, 2000; Volume 4, p. 63. [Google Scholar]
- Nwe, N.; Stevens, W.F. Production of fungal chitosan by solid substrate fermentation followed by enzymatic extraction. Biotechnol. Lett 2002, 24, 131–134. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W.F.; Tokura, S.; Tamura, H. Characterization of chitosan and chitosan-glucan complex extracted from cell wall of fungus Gongronella butleri USDB 0201 by enzymatic method. Enzyme Microb. Technol. 2008, 42, 242–251. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W.F.; Montet, D.; Tokura, S.; Tamura, H. Decomposition of myceliar matrix and extraction of chitosan from Gongronella butleri USDB 0201 and Absidia coerulea ATCC 14076. Int. J. Biol. Macromol. 2008, 43, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.S.; Liu, H.F.; Yin, Y.J.; Yao, K.D. The Properties of chitosan-gelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials 2003, 24, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chu, X.; Huang, N.; Wang, T.; Wang, Y.; Shi, X.; Ding, Y.; Gu, Z. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009, 30, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Lertwattanaseri, T.; Ichikawa, N.; Mizoguchi, T.; Tanaka, Y.; Chirachanchai, S. Microwave technique for efficient deacetylation of chitin nanowhiskers to a chitosan nanoscaffold. Carbohydr. Res. 2009, 344, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, N.; Ravichandran, P.; Reddy, P.N.; Ramamurty, N.; Pal, S.; Rao, K.P. Collagen-chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials 2001, 22, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yamaguchi, I.; Shinomiya, K.; Tanaka, J. Development of the chitosan tube prepared from crab tendon for nerve regeneration. Sci. Technol. Adv. Mater. 2003, 4, 261–268. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; He, B.; Chen, J. A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human neofetal dermal fibroblasts. Biomaterials 2001, 22, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Tsai, S.; Ho, M.; Wang, D.; Liu, C.; Hsieh, C.; Tseng, H.; Hsieh, H. Analysis of freeze-gelation and cross-linking processes for preparing porous chitosan scaffolds. Carbohydr. Polym. 2007, 67, 124–132. [Google Scholar] [CrossRef]
- Wang, A.; Ao, Q.; Cao, W.; Yu, M.; He, Q.; Kong, L.; Zhang, L.; Gong, Y.; Zhang, X. Porous chitosan tubular scaffolds with knitted outer wall and controllable inner structure for nerve tissue engineering. J. Biomed. Mater. Res. 2006, 79A, 36–46. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, A.; Wu, H.; Wang, Z.; Wen, D. Porous-conductive chitosan scaffolds for tissue engineering II. in vitro and in vivo degradation. J. Mater. Sci. - Mater. Med. 2005, 16, 1017–1028. [Google Scholar] [CrossRef]
- Rudall, K.M. The chitin/protein complexes of insect cuticles. Adv. Ins. Physiol. 1963, 1, 257–314. [Google Scholar]
- Mao, J.S.; Zhao, L.G.; Yin, Y.J.; Yao, K.D. Structure and properties of bilayer chitosan-gelatin scaffolds. Biomaterials 2003, 24, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, P.; Pan, L.F.; Mei, N.; Wu, C.G.; Chen, X.; Shao, Z.Z. Growth of human fetal lung fibroblasts on the natural biomaterial-chitosan scaffold. Acta Chim. Sinica 2004, 62, 992–997. [Google Scholar]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Mueller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Wake, M.C.; Patrick, C.W., Jr.; Mikos, A.G. Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 1994, 3, 339–343. [Google Scholar] [PubMed]
- Salem, A.K.; Stevens, R.; Peason, R.G.; Davies, M.C.; Tendler, S.J.; Roberts, C.J.; Willams, P.M.; Shakesheff, K.M. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J. Biomed. Mater. Res. 2002, 61, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Young, G.; Shortkroff, S.; Louie, L.K.; Sledge, C.B.; Yannas, I.V.; Spector, M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997, 18, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Kuberka, M.; Von, H.D.; Schoof, H.; Heschel, I.; Rau, G. Magnification of the pore size in biodegradable collagen sponges. Int. J. Artif. Organs 2002, 1, 67–73. [Google Scholar]
- Claase, M.B.; Grijpma, D.W.; Mendes, S.C.; De, Bruijn, J.D.; Feijen, J. Porous PEOT/PBT scaffolds for bone tissue engineering: preparation, characterization, and in vitro bone marrow cell culturing. J. Biomed. Mater. Res. 2003, 64A, 291–300. [Google Scholar] [CrossRef]
- Wan, W.; Wu, H.; Wen, D. Porous-conductive chitosan scaffolds for tissue engineering, 1 Preparation and characterization. Macromol. Biosci. 2004, 4, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Takamori, Y.; Sashiwa, H.; Saimoto, H.; Okamoto, Y.; Minami, S.; Matsuhashi, A.; Shigemasa, Y. Degradation of chitin in vivo. In Proceedings of the 2nd Asia Pacific Chitin and Chitosan Symposium, Bangkok, Thailand, 1996; p. 249.
- Ikeda, T.; Yanagiguchi, K.; Matsunaga, T.; Yamada, S.; Ohara, N. In vivo studies on the biodegradation process of chitin and chitosan. In Advance in Chitin Science; The European Chitin Society: Montpellier, France, 2006; Volume 9, p. 411. [Google Scholar]
- Ren, D.; Yi, H.; Zhang, H.; Xie, W.; Wang, W.; Ma, X. A preliminary study on fabrication of nanoscale fibrous chitosan membranes in situ by biospecific degradation. J. Membr. Sci. 2006, 280, 99–107. [Google Scholar] [CrossRef]
- Nakamura, H.; Hashimoto, S. Up-to-date review about clinical trials of glucosamine supplement. Chitin Chitosan Res. 2006, 12, 94–95. [Google Scholar]
- Ganno, T.; Yamada, S.; Ohara, N.; Matsunaga, T.; Yanagiguchi, K.; Ikeda, T.; Ishizaki, H.; Hayashi, Y. Early gene expression analyzed by cDNA microarray and real-time PCR in osteoblasts cultured with chitosan monomer. J. Biomed. Mater. Res. 2007, 82A, 188–194. [Google Scholar] [CrossRef]
- Sayo, T.; Sakai, S.; Sugiyama, Y.; Matahira, Y.; Sakai, K.; Inoue, S. Effect of N-acetylglucosamine on hyaluronan production in the skin cells. In Advance in Chitin Science; The European Chitin Society: Montpellier, France, 2006; Volume 9, p. 33. [Google Scholar]
- Oh-nishi, A.; Okamura, Y.; Tsuka, T.; Okamoto, Y.; Matahira, Y.; Minami, S. Effect on experimental damaged cartilage repair by N-acetyl-D-glucosamine and fish collagen peptide. In Advance in Chitin Science; The European Chitin Society: Montpellier, France, 2006; Volume 9, p. 293. [Google Scholar]
- Matahira, Y.; Sakai, K.; Misawa, Y. Characterization, physiological function and application of N-acetylglucosamine. Chitin Chitosan Res. 2006, 12, 92–93. [Google Scholar]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrϕd, O. Degradation of partially N-acetylated chitosans with hen egg white and human lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblasts-like cells. Clin. Oral Implants Res. 2004, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Aiba, S. Preparation of N-acetylchitooligosaccharides from lysozymic hydrolysates of partially N-acetylated chitosans. Carbohydr. Res. 1994, 261, 297–306. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, L.; Guo, X.; Yu, Y. Culture of primary rat hepatocytes within porous chitosan scaffolds. J. Biomed. Mater. Res. 2003, 67A, 938–943. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.J.; Jones, J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int. J. Oral Maxillofac Surg. 2007, 36, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R.; Lamar, P.C.; Smith, C.S. Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol Vitro 2006, 20, 942–953. [Google Scholar] [CrossRef]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Stevens, W.F. Optimization of solid substrate fermentation for the production of fungal chitosan. J. Chitin Chitosan 2006, 11, 11–15. [Google Scholar]
- Nwe, N.; Stevens, W.F. Effect of urea on fungal chitosan production in solid substrate fermentation. Proc. Biochem. 2004, 39, 1639–1642. [Google Scholar] [CrossRef]
- Nwe, N.; Stevens, W.F. Chitosan isolation from the chitosan-glucan complex of fungal cell wall using amylolytic enzymes. Biotechnol. Lett. 2002, 24, 1461–1464. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Saikhun, J.; Stevens, W.F.; Kitiyanant, Y. Chitosan scaffolds and membranes for tissue engineering. In Proceedings of the 7th Asia Pacific Chitin and Chitosan Symposium, Hanrimwon Printing Co. Ltd: Seoul, S. Korea, 2006; Kim, S.K., No, H.K., Park, R.D., Eds.; p. 341.
- Oliveira, J.M.; Rodrigues, M.T.; Silva, S.S.; Malafaya, P.B.; Gomes, M.E.; Viegas, C.A.; Dias, I.R.; Azevedo, J.T.; Mano, J.F.; Reis, R.L. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 2006, 27, 6123–6137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nwe, N.; Furuike, T.; Tamura, H. The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri. Materials 2009, 2, 374-398. https://doi.org/10.3390/ma2020374
Nwe N, Furuike T, Tamura H. The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri. Materials. 2009; 2(2):374-398. https://doi.org/10.3390/ma2020374
Chicago/Turabian StyleNwe, Nitar, Tetsuya Furuike, and Hiroshi Tamura. 2009. "The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri" Materials 2, no. 2: 374-398. https://doi.org/10.3390/ma2020374
APA StyleNwe, N., Furuike, T., & Tamura, H. (2009). The Mechanical and Biological Properties of Chitosan Scaffolds for Tissue Regeneration Templates Are Significantly Enhanced by Chitosan from Gongronella butleri. Materials, 2(2), 374-398. https://doi.org/10.3390/ma2020374





