Biocompatibility of Resin-based Dental Materials
Abstract
:1. Introduction
2. Local Adverse Reactions and Evaluation Systems
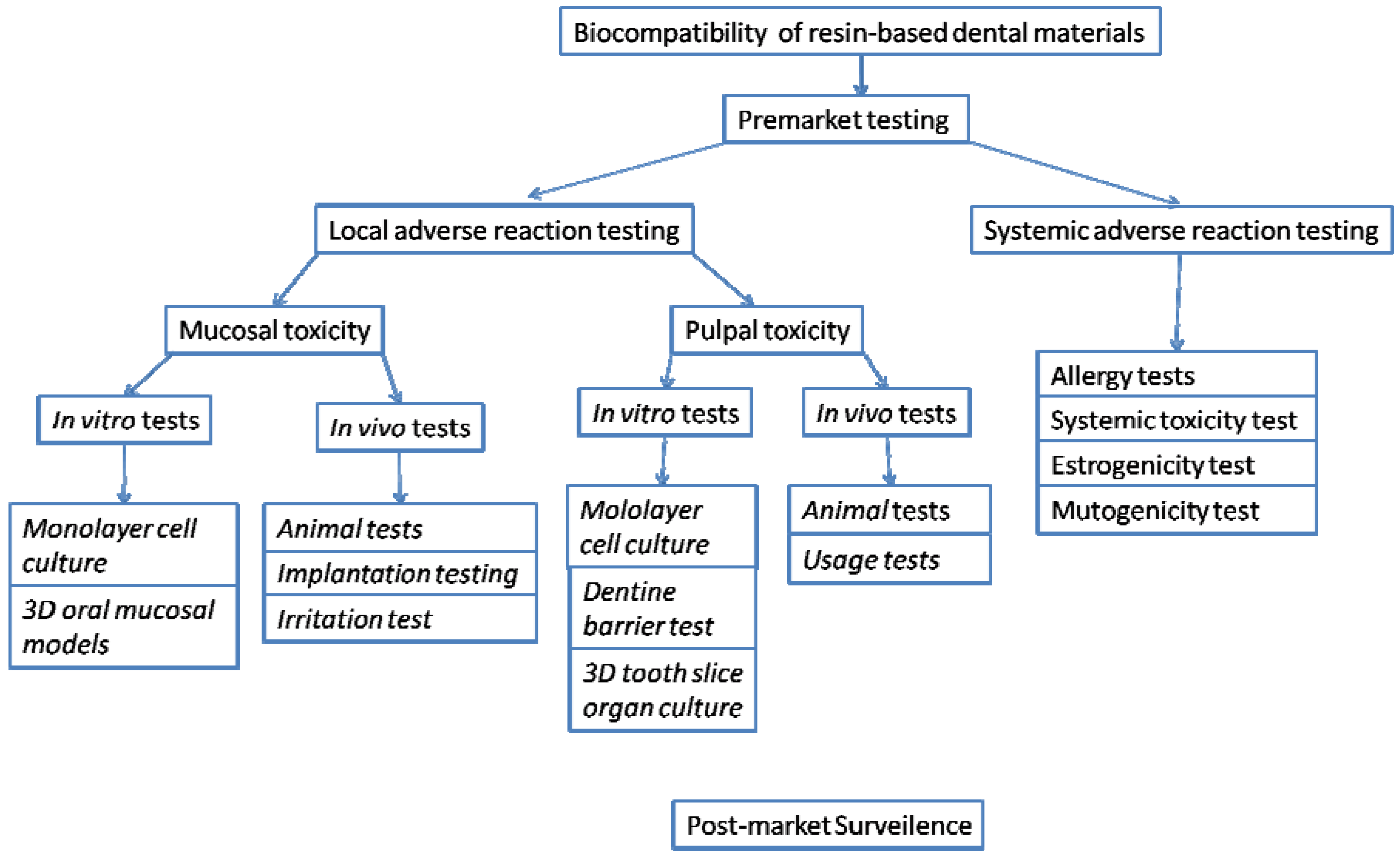
2.1. Mucosal toxicity testing
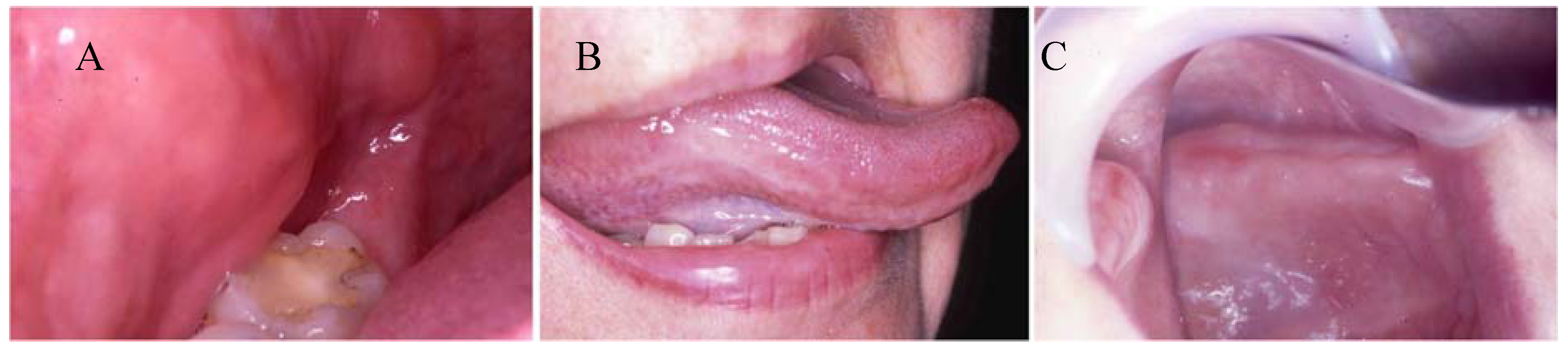
2.1.1. In vitro mucotoxicity tests
Biological system
Cell/material contact
Biological endpoint
MTT assay
Alamar blue assay
Neutral red assay
Propidium iodide assay
LDH assay
Bromodeoxyuridine incorporation assay
3H-thymidine incorporation assay
DNA content measurement
Protein content measurement
Inflammatory mediators measurement
Glutathione determination
Heat-Shock Protein assay
Apoptosis assays
Other assays
| Biological assay | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| MTT | Mitochondrial dehydrogenase activity | Rapid and inexpensive | Toxic to the cells |
| Alamar blue | Chemical reduction of culture medium | Accurate, and non-toxic fluorometric/colorimetric method. | Expensive |
| Neutral red | Membrane damage (stains vital cells) | Non toxic substance | Less accurate than Alamar blue |
| Propidium iodide | Membrane damage (stains dead cells) | It is possible to measure dead cells | Less accurate than Alamar blue |
| LDH | Cell damage | Simple assay, provides additional information when used with other assays. | Poor dynamic range, lack of sensitivity |
| BrdU | Proliferation | Simple, rapid and inexpensive. | Less sensitive |
| 3H-thymidine | Proliferation | Rapid and sensitive | Radioactive assay |
| DNA measurement | Proliferation | Non-radioactive, sensitive and robust. | None |
| Protein content | Proliferation | Easy, rapid and precise | None |
| Inflammatory markers | Inflammation indicators | Clinically relevant | Expensive and time-consuming tests. |
| GSH | Toxicity indicator | Provides additional information about the toxicity of materials. | Expensive and sophisticated. |
| HSP | Stress indicator | Provides additional information about the toxicity of materials. | Expensive and sophisticated. |
| Apoptosis | Cell injury | Sensitive and specific for apoptotic cells | Expensive and requires specific equipment |
2.1.2. In vivo tests
Animal tests
Implantation testing
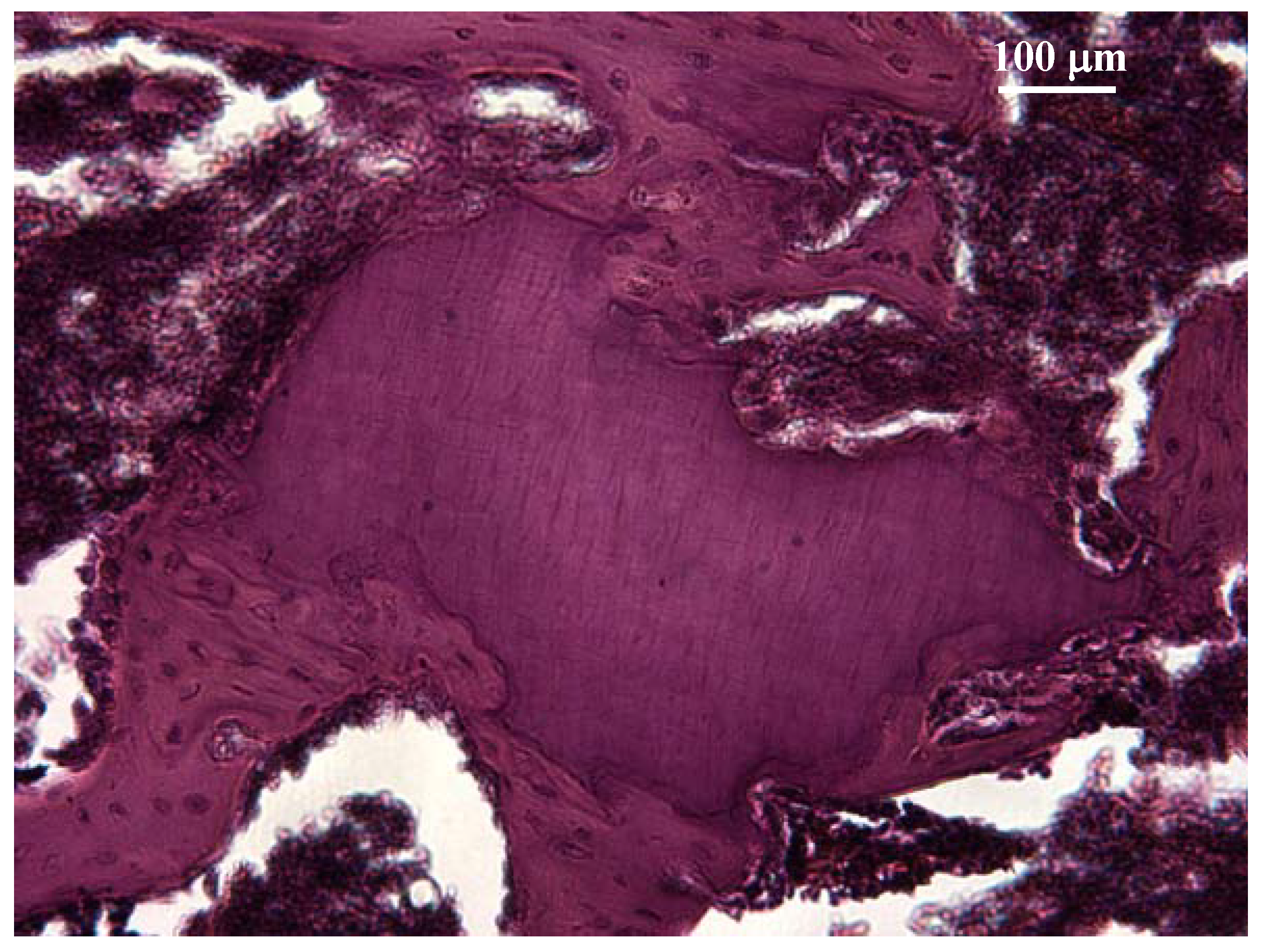
Irritation test
2.2. Pulpal toxicity testing
2.2.1. In vitro pulpal toxicity
Monolayer cell cultures
Dentine barrier systems
3D tooth slice organ culture
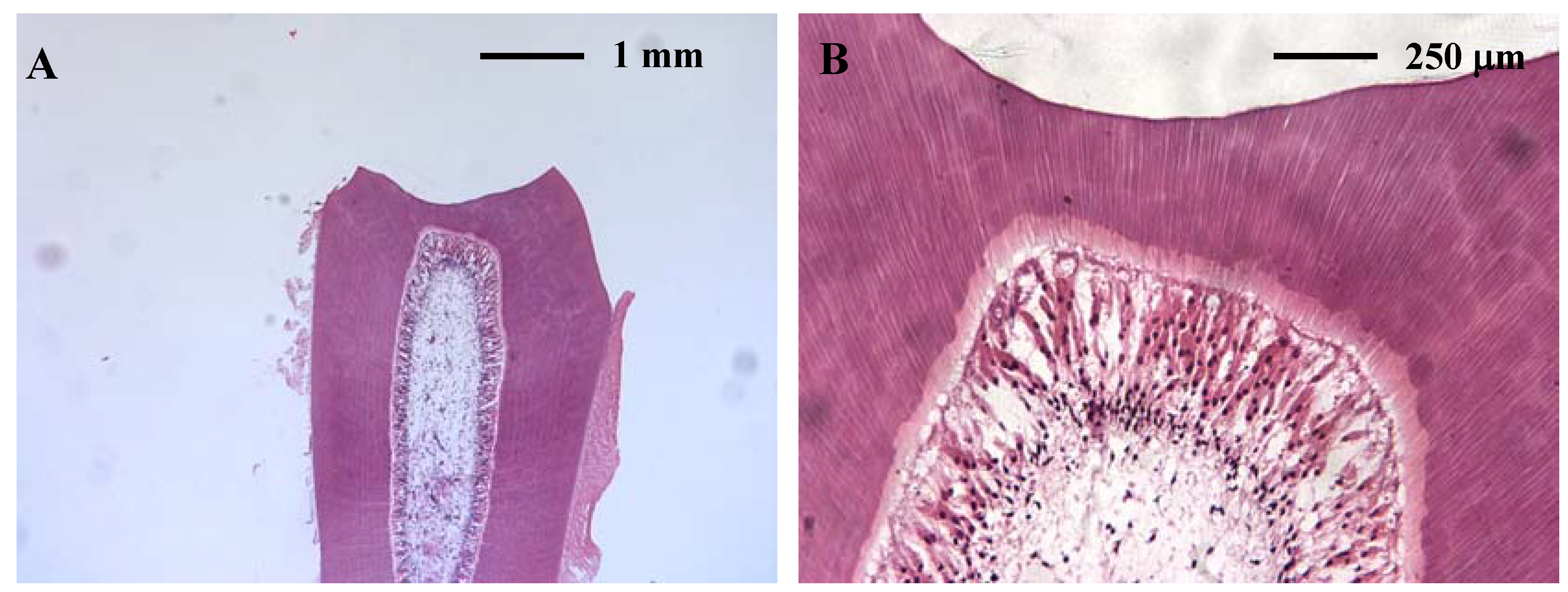
2.2.2. In vivo pulpal toxicity
Animal and usage test
3. Systemic Adverse Reactions
3.1. Allergy tests
3.2. Systemic toxicity
(a) Acute systemic toxicity
(b) Chronic systemic toxicity
3.3. Estrogenicity test
3.4. Genotoxicity (Mutagenicity) test
4. In vitro Biocompatibility Studies of Resin-based Dental Materials
4.1. Initial experiments
4.2. Components of resin-based materials
4.3. Eluates from resin-based materials
4.4. Effect of monomer structure on biocompatibility
4.5. Effects of fillers and additives on biocompatibility
4.6. Long-term Biocompatibility of Resin-based Materials
4.7. In vitro studies of dentine bonding agents
4.8. Studies on the Mechanisms of Monomer Toxicity
5. In Vitro Versus in Vivo Tests
6. Post-Market Surveillance
7. Future Developments
Acknowledgments
References
- Williams, D.F. Definitions in biomaterials; Elsevier: Oxford, UK, 1987. [Google Scholar]
- Wataha, J.C. Principles of biocompatibility for dental practitioners. J. Prosthet. Dent. 2001, 86, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G. Use of cell cultures for toxicity testing of dental materials--advantages and limitations. J. Dent. 1994, 22, S6–S11. [Google Scholar] [CrossRef]
- Scott, A.; Egner, W.; Gawkrodger, D.J.; Hatton, P.V.; Sherriff, M.; van Noort, R.; Yeoman, C.; Grummitt, J. The national survey of adverse reactions to dental materials in the UK: a preliminary study by the UK Adverse Reactions Reporting Project. Br. Dent. J. 2004, 196, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Mjor, I.A. Problems and benefits associated with restorative materials: side-effects and long-term cost. Adv. Dent. Res. 1992, 6, 7–16. [Google Scholar] [PubMed]
- Sadoh, D.R.; Sharief, M.K.; Howard, R.S. Occupational exposure to methyl methacrylate monomer induces generalised neuropathy in a dental technician. Br. Dent. J. 1999, 186, 380–381. [Google Scholar] [PubMed]
- Hensten-Pettersen, A. Skin and mucosal reactions associated with dental materials. Eur. J. Oral Sci. 1998, 106, 707–712. [Google Scholar] [PubMed]
- Michelsen, V.B.; Lygre, H.; Skalevik, R.; Tveit, A.B.; Solheim, E. Identification of organic eluates from four polymer-based dental filling materials. Eur. J. Oral Sci. 2003, 111, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Condon, J.R. Rate of elution of leachable components from composite. Dent. Mater. 1990, 6, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Spahl, W.; Budzikiewicz, H.; Geurtsen, W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J. Dent. 1998, 26, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W. Substances released from dental resin composites and glass ionomer cements. Eur. J. Oral. Sci. 1998, 106, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J. Mater. Sci. Mater. Med. 2007, 18, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Huang, H.M.; Lin, C.Y.; Shih, Y.H. Leached components from dental composites in oral simulating fluids and the resultant composite strengths. J. Oral Rehabil. 1998, 25, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Arenholt-Bindslev, D.; Breinholt, V.; Preiss, A.; Schmalz, G. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin. Oral Invest. 1999, 3, 120–125. [Google Scholar] [CrossRef]
- Michelsen, V.B.; Moe, G.; Strom, M.B.; Jensen, E.; Lygre, H. Quantitative analysis of TEGDMA and HEMA eluted into saliva from two dental composites by use of GC/MS and tailor-made internal standards. Dent. Mater. 2008, 24, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Brooks, S.C.; Walker, D.M. The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: an assay for monomer in saliva. J. Dent. Res. 1988, 67, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.T.; Wataha, J.C.; Sun, Z. In vitro models of biocompatibility: a review. Dent. Mater. 1996, 12, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Willershausen, B.; Schafer, D.; Pistorius, A.; Schulze, R.; Mann, W. Influence of resin-based restoration materials on cytotoxicity in gingival fibroblasts. Eur. J. Med. Res. 1999, 4, 149–155. [Google Scholar] [PubMed]
- Wan, Q.; Rumpf, D.; Schricker, S.R.; Mariotti, A.; Culbertson, B.M. Influence of hyperbranched multi-methacrylates for dental neat resins on proliferation of human gingival fibroblasts. Biomacromolecules 2001, 2, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Szep, S.; Kunkel, A.; Ronge, K.; Heidemann, D. Cytotoxicity of modern dentin adhesives--in vitro testing on gingival fibroblasts. J. Biomed. Mater. Res. 2002, 63, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.; Leyhausen, G.; Leibfritz, D.; Geurtsen, W. Effect of TEGDMA on the intracellular glutathione concentration of human gingival fibroblasts. J. Biomed. Mater. Res. 2002, 63, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Janke, V.; von Neuhoff, N.; Schlegelberger, B.; Leyhausen, G.; Geurtsen, W. TEGDMA causes apoptosis in primary human gingival fibroblasts. J. Dent. Res. 2003, 82, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Issa, Y.; Watts, D.C.; Brunton, P.A.; Waters, C.M.; Duxbury, A.J. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent. Mater. 2004, 20, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Schedle, A.; Franz, A.; Rausch-Fan, X.; Spittler, A.; Lucas, T.; Samorapoompichit, P.; Sperr, W.; Boltz-Nitulescu, G. Cytotoxic effects of dental composites, adhesive substances, compomers and cements. Dent. Mater. 1998, 14, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Kostoryz, E.L.; Tong, P.Y.; Chappelow, C.C.; Eick, J.D.; Glaros, A.G.; Yourtee, D.M. In vitro cytotoxicity of solid epoxy-based dental resins and their components. Dent. Mater. 1999, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kaga, M.; Noda, M.; Ferracane, J.L.; Nakamura, W.; Oguchi, H.; Sano, H. The in vitro cytotoxicity of eluates from dentin bonding resins and their effect on tyrosine phosphorylation of L929 cells. Dent. Mater. 2001, 17, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Konig, F.; Anglmayer, M.; Rausch-Fan, X.; Gille, G.; Rausch, W.D.; Lucas, T.; Sperr, W.; Schedle, A. Cytotoxic effects of packable and nonpackable dental composites. Dent. Mater. 2003, 19, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Kostoryz, E.L.; Eick, J.D.; Glaros, A.G.; Judy, B.M.; Welshons, W.V.; Burmaster, S.; Yourtee, D.M. Biocompatibility of hydroxylated metabolites of BISGMA and BFDGE. J. Dent. Res. 2003, 82, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.T.; Strawn, S.E.; Wataha, J.C.; Craig, R.G. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J. Dent. Res. 1991, 70, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, M.A.; Craig, R.G.; Hanks, C.T.; Filisko, F.E. Cytotoxicity of a BIS-GMA dental composite before and after leaching in organic solvents. J. Biomed. Mater. Res. 1991, 25, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Ratanasathien, S.; Wataha, J.C.; Hanks, C.T.; Dennison, J.B. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J. Dent. Res. 1995, 74, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W.; Spahl, W.; Leyhausen, G. Residual monomer/additive release and variability in cytotoxicity of light-curing glass-ionomer cements and compomers. J. Dent. Res. 1998, 77, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W.; Spahl, W.; Muller, K.; Leyhausen, G. Aqueous extracts from dentin adhesives contain cytotoxic chemicals. J. Biomed. Mater. Res. 1999, 48, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Shaw, L.; Gonzalez, L.; Wataha, J.C.; Krejci, I. Long-term cytotoxicity of resin-based dental restorative materials. J. Oral Rehabil. 2002, 29, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Lockwood, P.E.; Bouillaguet, S.; Noda, M. In vitro biological response to core and flowable dental restorative materials. Dent. Mater. 2003, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Wataha, J.C.; Lockwood, P.E.; Volkmann, K.R.; Kaga, M.; Sano, H. Sublethal, 2-week exposures of dental material components alter TNF-alpha secretion of THP-1 monocytes. Dent. Mater. 2003, 19, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Schweikl, H.; Hiller, K.A. Release of prostaglandin E2, IL-6 and IL-8 from human oral epithelial culture models after exposure to compounds of dental materials. Eur. J. Oral Sci. 2000, 108, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.T.; Anderson, M.; Craig, R.G. Cytotoxic effects of dental cements on two cell culture systems. J. Oral Pathol. 1981, 10, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Feigal, R.J.; Yesilsoy, C.; Messer, H.H.; Nelson, J. Differential sensitivity of normal human pulp and transformed mouse fibroblasts to cytotoxic challenge. Arch. Oral Biol. 1985, 30, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Meryon, S.D. The importance of surface area in the cytotoxicity of zinc phosphate and silicate cements in vitro. Biomaterials 1983, 4, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Hensten-Pettersen, A.; Helgeland, K. Sensitivity of different human cell line in the biologic evaluation of dental resin-based restorative materials. Scand. J. Dent. Res. 1981, 89, 102–107. [Google Scholar] [PubMed]
- Johnson, H.J.; Northup, S.J.; Seagraves, P.A.; Garvin, P.J.; Wallin, R.F. Biocompatibility test procedures for materials evaluation in vitro. I. Comparative test system sensitivity. J. Biomed. Mater. Res. 1983, 17, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.J.; Northup, S.J.; Seagraves, P.A. Biocompatibility test procedures for materials evaluation in vitro. II. Objective methods of toxicity assessment. J. Biomed. Mater. Res. 1985, 19, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A.M.; Thornhill, M.H. Tissue-engineered oral mucosa: a review of the scientific literature. J. Dent. Res. 2007, 86, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A.M.; Smith, K.G.; Thornhill, M.H. Development, optimization and characterization of a full-thickness tissue engineered human oral mucosal model for biological assessment of dental biomaterials. J. Mater. Sci. Mater. Med. 2008, 19, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Scutt, A.M.; Thornhill, M.H.; Van Noort, R. Mucotoxicity of dental composite resins on a tissue-engineered human oral mucosal model. J. Dent. 2008, 36, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Arenholt-Bindslev, D.; Hiller, K.A.; Schweikl, H. Epithelium-fibroblast co-culture for assessing mucosal irritancy of metals used in dentistry. Eur. J. Oral Sci. 1997, 105, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Schuster, U.; Nuetzel, K.; Schweikl, H. An in vitro pulp chamber with three-dimensional cell cultures. J. Endod. 1999, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Schuster, U.; Schmalz, G.; Thonemann, B.; Mendel, N.; Metzl, C. Cytotoxicity testing with three-dimensional cultures of transfected pulp-derived cells. J. Endod. 2001, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, G.L. In vitro evaluation of dental materials. Clin. Mater. 1994, 16, 21–60. [Google Scholar] [CrossRef] [PubMed]
- Sisca, R.F.; Thonard, J.C.; Lower, D.A.; George, W.A. Responses of epithelial-like cells in tissue culture to implant materials. J. Dent. Res. 1967, 46, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Pineda, L.F.; Schneider, P.E.; Rawls, H.R.; Foster, T.A. Biocompatibility testing of an experimental fluoride releasing resin using human gingival epithelial cells in vitro. In Vitro Cell Dev. Biol. 1989, 25, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Leirskar, J.; Helgeland, K. A methodologic study of the effect of dental materials on growth and adhesion of animal cells in vitro. Scand. J. Dent. Res. 1972, 80, 120–133. [Google Scholar] [PubMed]
- Spangberg, L. Kinetic and quantitative evaluation of material cytotoxicity in vitro. Oral Surg. Oral Med. Oral Pathol. 1973, 35, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Felder, S.M.; Gettleman, L.; Alchediak, T. A model culture system with human gingival fibroblasts for evaluating the cytotoxicity of dental materials. In Vitro 1982, 18, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Guess, W.L.; Rosenbluth, S.A.; Schmidt, B.; Autian, J. Agar diffusion method for toxicity screening of plastics on cultured cell monolayers. J. Pharm. Sci. 1965, 54, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Thonemann, B.; Riedel, M.; Elderton, R.J. Biological and clinical investigations of a glass ionomer base material. Dent. Mater. 1994, 10, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.V.; Lameiras, F.S.; Lobato, Z.I. Biological reactivity of zirconia-hydroxyapatite composites. J. Biomed. Mater. Res. 2002, 63, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Choi, S.Y.; Choi, S.H.; Lee, Y.K.; Kim, K.N. The influence of lithium fluoride on in vitro biocompatibility and bioactivity of calcium aluminate-pMMA composite cement. J. Mater. Sci. Mater. Med. 2004, 15, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tyas, M.J. A method for the in vitro toxicity testing of dental restorative materials. J. Dent. Res. 1977, 56, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.; Hasselgren, G.; Tronstad, L. A method for toxicity screening of biomaterials using cells cultured on millipore filters. J. Biomed. Mater. Res. 1979, 13, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Theilig, C.; Tegtmeier, Y.; Leyhausen, G.; Geurtsen, W. Effects of BisGMA and TEGDMA on proliferation, migration, and tenascin expression of human fibroblasts and keratinocytes. J. Biomed. Mater. Res. 2000, 53, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Thonemann, B.; Schmalz, G.; Hiller, K.A.; Schweikl, H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater. 2002, 18, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent. Mater. 2007, 23, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Soheili Majd, E.; Goldberg, M.; Stanislawski, L. In vitro effects of ascorbate and Trolox on the biocompatibility of dental restorative materials. Biomaterials 2003, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chang, Y.C. Cytotoxicity of resin-based restorative materials on human pulp cell cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, L.; Daniau, X.; Lauti, A.; Goldberg, M. Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J. Biomed. Mater. Res. 1999, 48, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kawahara, H.; Kataoka, Y.; Maehara, S.; Izutani, M.; Taguchi, H. Biocompatibility of dental amalgams in vitro during 52 week period. Shika. Rikogaku. Zasshi. 1980, 21, 228–244. [Google Scholar] [PubMed]
- Lefebvre, C.A.; Knoernschild, K.L.; Schuster, G.S. Cytotoxicity of eluates from light-polymerized denture base resins. J. Prosthet. Dent. 1994, 72, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Pelka, M.; Danzl, C.; Distler, W.; Petschelt, A. A new screening test for toxicity testing of dental materials. J. Dent. 2000, 28, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Valle, G.F.; Taintor, J.F.; Marsh, C.L. The effect of varying liquid-to-powder ratio to zinc oxide and eugenol of rat pulpal respiration. J. Endod. 1980, 6, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Eick, J.D.; Kostoryz, E.L.; Rozzi, S.M.; Jacobs, D.W.; Oxman, J.D.; Chappelow, C.C.; Glarosa, A.G.; Yourteeb, D.M. In vitro biocompatibility of oxirane/polyol dental composites with promising physical properties. Dent. Mater. 2002, 18, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.S.; Liu, C.C.; Tseng, W.Y.; Jeng, J.H.; Lin, C.P. Cytotoxicity of three dentin bonding agents on human dental pulp cells. J. Dent. 2003, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Schuster, U.; Koch, A.; Schweikl, H. Cytotoxicity of low pH dentin-bonding agents in a dentin barrier test in vitro. J. Endod. 2002, 28, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Nociari, M.M.; Shalev, A.; Benias, P.; Russo, C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Methods 1998, 213, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D.; Lancaster, M.V. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993, 11, 48–50. [Google Scholar] [PubMed]
- Uo, M.; Sjogren, G.; Sundh, A.; Watari, F.; Bergman, M.; Lerner, U. Cytotoxicity and bonding property of dental ceramics. Dent. Mater. 2003, 19, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Kirk, D.; Baumgarten, H.; Ruchel, R. A colorimetric assay for the assessment of cytotoxicity of yeasts. Sabouraudia 1984, 22, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G. Toxicity parameters for cytotoxicity testing of dental materials in two different mammalian cell lines. Eur. J. Oral Sci. 1996, 104, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Ciapetti, G.; Granchi, D.; Stea, S.; Savarino, L.; Verri, E.; Gori, A.; Savioli, F.; Montanaro, L. Cytotoxicity testing of materials with limited in vivo exposure is affected by the duration of cell-material contact. J. Biomed. Mater. Res. 1998, 42, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Sletten, G.B.; Dahl, J.E. Cytotoxic effects of extracts of compomers. Acta. Odontol. Scand. 1999, 57, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hikage, S.; Sato, A.; Suzuki, S.; Cox, C.F.; Sakaguchi, K. Cytotoxicity of dental resin monomers in the presence of S9 mix enzymes. Dent. Mater. J. 1999, 18, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Sinensky, M.C. Indirect cytotoxicity of dental materials: a study with Transwell inserts and the neutral red uptake assay. Altern. Lab. Anim. 2001, 29, 9–13. [Google Scholar] [PubMed]
- Hikage, S.; Nakayama, K.; Saito, T.; Takahashi, Y.; Kamataki, T.; Suzuki, S.; Hongo, T.; Sato, A. Cytotoxicity of bisphenol A glycidyl methacrylate on cytochrome P450-producing cells. J. Oral Rehabil. 2003, 30, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Darzynkievicz, Z. Probing nuclear chromatin by flow cytometry. In Flow Cytometry and Sorting; Melamed, M.R., Lindmo, T., Mendelson, M.L., Eds.; Wiley: New York, NY, USA, 1990; pp. 291–314. [Google Scholar]
- Mantellini, M.G.; Botero, T.M.; Yaman, P.; Dennison, J.B.; Hanks, C.T.; Nor, J.E. Adhesive resin induces apoptosis and cell-cycle arrest of pulp cells. J. Dent. Res. 2003, 82, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Babson, A.L.; Phillips, G.E. A rapid colorimetric assay for serum lactic dehydrogenase. Clin. Chim. Acta. 1965, 12, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Reichl, F.X.; Walther, U.I.; Durner, J.; Kehe, K.; Hickel, R.; Kunzelmann, K.H.; Forth, W. Cytotoxicity of dental composite components and mercury compounds in lung cells. Dent. Mater. 2001, 17, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.A.; Zisterer, D.M.; Tipton, K.F.; O'Sullivan, M.I. In vitro cytotoxicity of a composite resin and compomer. Int. Endod. J. 2002, 35, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Dolbeare, F.; Selden, J.R. Immunochemical quantitation of bromodeoxyuridine: application to cell-cycle kinetics. Methods. Cell. Biol. 1994, 41, 297–316. [Google Scholar] [PubMed]
- Aronsson, G.; Dahlgren, U.I.; Karlsson, S. Human and rat mononuclear cell proliferation show different sensitivity, in vitro, to single constituents of dental composite resins. J. Biomed. Mater. Res. 2000, 53, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M. Flow cytometry of DNA content and other indicators of proliferative activity. Arch. Pathol. Lab. Med. 1989, 113, 591–597. [Google Scholar] [PubMed]
- Leyhausen, G.; Abtahi, M.; Karbakhsch, M.; Sapotnick, A.; Geurtsen, W. Biocompatibility of various light-curing and one conventional glass-ionomer cement. Biomaterials 1998, 19, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Ohnishi, S.T.; Barr, J.K. A simplified method of quantitating protein using the biuret and phenol reagents. Anal. Biochem. 1978, 86, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Heil, T.L.; Volkmann, K.R.; Wataha, J.C.; Lockwood, P.E. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J. Oral Rehabil. 2002, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Hedley, D.W.; Chow, S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry 1994, 15, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, L.; Lefeuvre, M.; Bourd, K.; Soheili-Majd, E.; Goldberg, M.; Perianin, A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J. Biomed. Mater. Res. 2003, 66A, 476–482. [Google Scholar] [CrossRef]
- Volk, J.; Engelmann, J.; Leyhausen, G.; Geurtsen, W. Effects of three resin monomers on the cellular glutathione concentration of cultured human gingival fibroblasts. Dent. Mater. 2006, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Walther, U.I.; Siagian, II; Walther, S.C.; Reichl, F.X.; Hickel, R. Antioxidative vitamins decrease cytotoxicity of HEMA and TEGDMA in cultured cell lines. Arch. Oral Biol. 2004, 49, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.; Volk, J.; Leyhausen, G.; Geurtsen, W. ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J. Biomed. Mater. Res. Appl. Biomater. 2005, 75, 272–276. [Google Scholar] [CrossRef]
- Noda, M.; Wataha, J.C.; Lewis, J.B.; Kaga, M.; Lockwood, P.E.; Messer, R.L.; Sano, H. Dental adhesive compounds alter glutathione levels but not glutathione redox balance in human THP-1 monocytic cells. J. Biomed. Mater. Res. Appl. Biomater. 2005, 73, 308–314. [Google Scholar] [CrossRef]
- Lefeuvre, M.; Bourd, K.; Loriot, M.A.; Goldberg, M.; Beaune, P.; Perianin, A.; Stanislawski, L. TEGDMA modulates glutathione transferase P1 activity in gingival fibroblasts. J. Dent. Res. 2004, 83, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, M.; Amjaad, W.; Goldberg, M.; Stanislawski, L. TEGDMA induces mitochondrial damage and oxidative stress in human gingival fibroblasts. Biomaterials 2005, 26, 5130–5137. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Hatayama, T.; Nakamura, M. A possibility for new evaluating method of cytotoxicity by using heat shock protein assay. J. Mater. Sci. Mater. Med. 1997, 8, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Wataha, J.C.; Kaga, M.; Lockwood, P.E.; Volkmann, K.R.; Sano, H. Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J. Dent. Res. 2002, 81, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, A.; Bordador, L.C.; Wang, M.Y.; Hume, W.R.; Jewett, A. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J. Dent. Res. 2005, 84, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Becher, R.; Kopperud, H.M.; Al, R.H.; Samuelsen, J.T.; Morisbak, E.; Dahlman, H.J.; Lilleaas, E.M.; Dahl, J.E. Pattern of cell death after in vitro exposure to GDMA, TEGDMA, HEMA and two compomer extracts. Dent. Mater. 2005.
- Schmalz, G. Concepts in biocompatibility testing of dental restorative materials. Clin. Oral. Invest. 1997, 1, 154–162. [Google Scholar] [CrossRef]
- Zmener, O. Tissue response to a new methacrylate-based root canal sealer: preliminary observations in the subcutaneous connective tissue of rats. J. Endod. 2004, 30, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Steinbrunner, R.L.; Setcos, J.C.; Kafrawy, A.H. Connective tissue reactions to glass ionomer cements and resin composites. Am. J. Dent. 1991, 4, 281–284. [Google Scholar] [PubMed]
- Schmalz, G.; Schmalz, C. Toxicity tests on dental filling materials. Int. Dent. J. 1981, 31, 185–192. [Google Scholar] [PubMed]
- Tassery, H.; Remusat, M.; Koubi, G.; Pertot, W.J. Comparison of the intraosseous biocompatibility of Vitremer and super EBA by implantation into the mandible of rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ellender, G.; Feik, S.A.; Gaviria, C. The biocompatibility testing of some dental amalgams in vivo. Aust. Dent. J. 1990, 35, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.M.; Do Nascimento, A.B.; Hebling, J.; De Souza Costa, C.A. In vivo evaluation of the biocompatibility of three current bonding agents. J. Oral. Rehabil. 2006, 33, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E. Potential of dental adhesives to induce mucosal irritation evaluated by the HET-CAM method. Acta. Odontol. Scand. 2007, 65, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Al, R.H.; Dahl, J.E.; Morisbak, E.; Polyzois, G.L. Irritation and cytotoxic potential of denture adhesives. Gerodontology 2005, 22, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E.; Polyzois, G.L. Irritation test of tissue adhesives for facial prostheses. J. Prosthet. Dent. 2000, 84, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E.; Frangou-Polyzois, M.J.; Polyzois, G.L. In vitro biocompatibility of denture relining materials. Gerodontology 2006, 23, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Freeman, C.; Blackwood, K. Processed bovine dentine as a bone substitute. Br. J. Oral Maxillofac. Surg. 2008, 46, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.A.; Vaerten, M.A.; Edwards, C.A.; Hanks, C.T. Cytotoxic effects of current dental adhesive systems on immortalized odontoblast cell line MDPC-23. Dent. Mater. 1999, 15, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Aranha, A.M.; Giro, E.M.; Souza, P.P.; Hebling, J.; de Souza Costa, C.A. Effect of curing regime on the cytotoxicity of resin-modified glass-ionomer lining cements applied to an odontoblast-cell line. Dent. Mater. 2006, 22, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Lanza, C.R.; de Souza Costa, C.A.; Furlan, M.; Alecio, A.; Hebling, J. Transdentinal diffusion and cytotoxicity of self-etching adhesive systems. Cell. Biol. Toxicol. 2008, in press. [Google Scholar]
- Browne, R.M.; Tyas, M.J. Biological testing of dental restorative materials in vitro--a review. J. Oral Rehabil. 1979, 6, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Hetem, S.; Jowett, A.K.; Ferguson, M.W. Biocompatibility testing of a posterior composite and dental cements using a new organ culture model. J. Dent. 1989, 17, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hikage, S.; Atsuta, M.; Sato, A. Biological evaluation of biomaterials using cultured chick embryo femurs (3). Shika. Zairyo. Kikai. 1989, 8, 642–647. [Google Scholar] [PubMed]
- Beele, H.; Thierens, H.; Deveux, R.; Goethals, E.; de Ridder, L. Skin organ culture model to test the toxicity of polyoxyethylene networks. Biomaterials 1992, 13, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Lumley, P.J.; Ross, H.F.; Smith, A.J. Tooth slice organ culture for cytotoxicity assessment of dental materials. Biomaterials 2000, 21, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Saw, T.Y.; Cao, T.; Yap, A.U.; Lee Ng, M.M. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol. In Vitro 2005, 19, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.; Gangler, P.; Krehan, F.; Wutzler, P. [Scotchbond dentin-bonding material in the biological test chain--is an adhesive composite-filling technic pulp-compatible?]. Zahn. Mund. Kieferheilkd. Zentralbl. 1989, 77, 243–251. [Google Scholar] [PubMed]
- Heitmann, T.; Unterbrink, G. Direct pulp capping with a dentinal adhesive resin system: a pilot study. Quintessence Int. 1995, 26, 765–770. [Google Scholar] [PubMed]
- White, K.C.; Cox, C.F.; Kanka, J., 3rd; Dixon, D.L.; Farmer, J.B.; Snuggs, H.M. Pulpal response to adhesive resin systems applied to acid-etched vital dentin: damp versus dry primer application. Quintessence Int. 1994, 25, 259–268. [Google Scholar] [PubMed]
- Harnirattisai, C.; Hosoda, H. Pulpal responses to various dentin bonding systems in dentin cavities. Dent. Mater. J. 1991, 10, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Fuks, A.B.; Funnell, B.; Cleaton-Jones, P. Pulp response to a composite resin inserted in deep cavities with and without a surface seal. J. Prosthet. Dent. 1990, 63, 129–134. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, J.W.; Sjostrom, S. The effect of glass ionomer cement and composite resin fillings on marginal gingiva. J. Clin. Periodontol. 1991, 18, 200–203. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, J.W.; Sjostrom, S.; Wing, K. , The effect of different types of composite resin fillings on marginal gingiva. J. Clin. Periodontol. 1987, 14, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; Hebling, J.; Smith, A.J.; Giro, E.M.; Oliveira, M.F.; de Souza Costa, C.A. Reactionary dentinogenesis after applying restorative materials and bioactive dentin matrix molecules as liners in deep cavities prepared in nonhuman primate teeth. J. Oral Rehabil. 2006, 33, 452–461. [Google Scholar] [CrossRef] [PubMed]
- de Souza Costa, C.A.; Teixeira, H.M.; Lopes do Nascimento, A.B.; Hebling, J. Biocompatibility of resin-based dental materials applied as liners in deep cavities prepared in human teeth. J. Biomed. Mater. Res. Appl. Biomater. 2007, 81, 175–184. [Google Scholar] [CrossRef]
- de Souza Costa, C.A.; Hebling, J.; Randall, R.C. Human pulp response to resin cements used to bond inlay restorations. Dent. Mater. 2006, 22, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Ollier, S. Allergy: The Facts; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Phielepeit, T.; Legrum, W. The toxicity of palladium. Dtsch. Zahnarztl. Z. 1986, 41, 1257–1260. [Google Scholar] [PubMed]
- Al-Waheidi, E.M. Allergic reaction to nickel orthodontic wires: a case report. Quintessence Int. 1995, 26, 385–387. [Google Scholar] [PubMed]
- Hubler, W.R., Jr.; Hubler, W.R., Sr. Dermatitis from a chromium dental plate. Contact Dermatitis 1983, 9, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.A. Contact dermatitis to metals. Dermatol. Ther. 2004, 17, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, A.I.; Oquist, G. Location of the allergenic monomer in warm-polymerized acrylic dentures. Part I: Causes of denture sore mouth, incidence of allergy, different allergens and test methods on suspicion of allergy to denture material - a survey of the literature. Case report, allergenic analysis of denture and test casting. Swed. Dent. J. 1980, 4, 241–252. [Google Scholar] [PubMed]
- Crissey, J.T. Stomatitis, dermatitis, and denture materials. Arch. Dermatol. 1965, 92, 45–48. [Google Scholar] [CrossRef] [PubMed]
- van Joost, T.; van Ulsen, J.; van Loon, L.A. Contact allergy to denture materials in the burning mouth syndrome. Contact Dermatitis 1988, 18, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, A.I.; Oquist, G. Location of the allergenic monomer in warm-polymerized acrylic dentures. Part II: Experiments aimed at establishing guidelines for production of acrylic dentures suited for patients allergic to acrylic monomer and complementary investigations. Swed. Dent. J. 1980, 4, 253–260. [Google Scholar] [PubMed]
- Goncalves, T.S.; Morganti, M.A.; Campos, L.C.; Rizzatto, S.M.; Menezes, L.M. Allergy to auto-polymerized acrylic resin in an orthodontic patient. Am. J. Orthod. Dentofacial. Orthop. 2006, 129, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Rosenbauer, E.U. Residual methyl methacrylate monomer, water sorption, and water solubility of hypoallergenic denture base materials. J. Prosthet. Dent. 2004, 92, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Culliton, C.R.; Meenaghan, M.A.; Sorensen, S.E.; Greene, G.W.; Eick, J.D. A critical evaluation of the acute systemic toxicity test for dental alloys using histopathologic criteria. J. Biomed. Mater. Res. 1981, 15, 565–575. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.A. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ. Health. Perspect. 1993, 101, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.M.; Beckman, B.S.; Hill, S.M.; McLachlan, J.A.; Walters, M.R.; Arnold, S.F. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ. Health. Perspect. 1996, 104, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, M.; Olea, N.; Brotons, J.A.; Olea-Serrano, M.F.; Ruiz de Almodovar, J.M.; Pedraza, V. The E-screen assay: a comparison of different MCF7 cell stocks. Environ. Health. Perspect. 1995, 103, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C.; Chung, K.L.; Fernandez, M.F.; Olea, N.; Serrano, F.O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ. Health. Perspect. 1995, 103, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Perez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health. Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Schafer, T.E.; Lapp, C.A.; Hanes, C.M.; Lewis, J.B.; Wataha, J.C.; Schuster, G.S. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J. Biomed. Mater. Res. 1999, 45, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, J.; Horiuchi, R.; Nakamura, T.; Iino, Y.; Ishida, T.; Takigawa, H.; Izuo, M. Effects of estrogen and endocrine therapeutic agents on the estrogen receptor, progesterone receptor and DNA synthesis in MCF-7 human breast cancer cells using the whole cell uptake method. Nippon. Naibunpi. Gakkai. Zasshi. 1987, 63, 1351–1363. [Google Scholar] [PubMed][Green Version]
- Gaido, K.W.; Leonard, L.S.; Lovell, S.; Gould, J.C.; Babai, D.; Portier, C.J.; McDonnell, D.P. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol. Appl. Pharmacol. 1997, 143, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Nakamura, M. Estrogenic activity of dental materials and bisphenol-A related chemicals in vitro. Dent. Mater. J. 2000, 19, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, H.; Imazato, S.; Narimatsu, M.; Matsuo, M.; Ebisu, S. Estrogenicity of fissure sealants and adhesive resins determined by reporter gene assay. J. Dent. Res. 2000, 79, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Ishibashi, H.; Miyahara, M.; Shinohara, R.; Shiraishi, F.; Arizono, K. Effects of dental resin metabolites on estrogenic activity in vitro. J. Mater. Sci. Mater. Med. 2003, 14, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Tarumi, H.; Imazato, S.; Narimatsu, M.; Ebisu, S. In vitro estrogenicity of resin composites. J. Dent. Res. 2004, 83, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; McCann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G. Glutaraldehyde-containing dentin bonding agents are mutagens in mammalian cells in vitro. J. Biomed. Mater. Res. 1997, 36, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.P.; Eisentrager, A.; Jahnen-Dechent, W.; Dott, W.; Hollender, J. Effect of sample preparation on the in vitro genotoxicity of a light curable glass ionomer cement. Biomaterials 2003, 24, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.W.; Huang, F.M.; Huang, M.S.; Chang, Y.C. Assessment of the genotoxicity of resin and zinc-oxide eugenol-based root canal sealers using an in vitro mammalian test system. J. Biomed. Mater. Res. 2002, 59, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chou, M.Y.; Chang, Y.C. Dentin bonding agents induce c-fos and c-jun protooncogenes expression in human gingival fibroblasts. Biomaterials 2003, 24, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kostoryz, E.L.; Wetmore, L.A.; Brockmann, W.G.; Yourtee, D.M.; Eick, J.D. Genotoxicity assessment of oxirane-based dental monomers in mammalian cells. J. Biomed. Mater. Res. 2004, 68A, 660–667. [Google Scholar] [CrossRef]
- Kleinsasser, N.H.; Wallner, B.C.; Harreus, U.A.; Kleinjung, T.; Folwaczny, M.; Hickel, R.; Kehe, K.; Reichl, F.X. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J. Dent. 2004, 32, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kleinsasser, N.H.; Schmid, K.; Sassen, A.W.; Harreus, U.A.; Staudenmaier, R.; Folwaczny, M.; Glas, J.; Reichl, F.X. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials 2006, 27, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G. Triethylene glycol dimethacrylate induces large deletions in the hprt gene of V79 cells. Mutat. Res. 1999, 438, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G.; Rackebrandt, K. The mutagenic activity of unpolymerized resin monomers in Salmonella typhimurium and V79 cells. Mutat. Res. 1998, 415, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Altmannberger, I.; Hanser, N.; Hiller, K.A.; Bolay, C.; Brockhoff, G.; Spagnuolo., G.; Galler, K.; Schmalz, G. The effect of triethylene glycol dimethacrylate on the cell cycle of mammalian cells. Biomaterials 2005, 26, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G.; Bey, B. Mutagenicity of dentin bonding agents. J. Biomed. Mater. Res. 1994, 28, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G.; Gottke, C. Mutagenic activity of various dentine bonding agents. Biomaterials 1996, 17, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Schmalz, G.; Weinmann, W. Mutagenic activity of structurally related oxiranes and siloranes in Salmonella typhimurium. Mutat. Res. 2002, 521, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kostoryz, E.L.; Tong, P.Y.; Chappelow, C.C.; Glaros, A.G.; Eick, J.D.; Yourtee, D.M. In vitro toxicity of spiroorthocarbonate monomers designed for non-shrinking dental restoratives. J. Biomater. Sci. Polym. Ed. 2000, 11, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tronstad, L.; Spangberg, L. Biologic tests of a methyl methacrylate composite material. Scand J. Dent. Res. 1974, 82, 93–98. [Google Scholar] [PubMed]
- Lefebvre, C.A.; Schuster, G.S.; Rueggeberg, F.A.; Tamareselvy, K.; Knoernschild, K.L. Responses of oral epithelial cells to dental resin components. J. Biomater. Sci. Polym. Ed. 1996, 7, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pelka, M.; Distler, W.; Petschelt, A. Elution parameters and HPLC-detection of single components from resin composite. Clin. Oral Invest. 1999, 3, 194–200. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Achilias, D.S. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J. Biomed. Mater. Res. Appl. Biomater. 2005, 74, 617–626. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiyasat, A.S.; Darmani, H.; Milhem, M.M. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin. Oral. Invest. 2005, 9, 21–25. [Google Scholar] [CrossRef]
- Michelsen, V.B.; Moe, G.; Skalevik, R.; Jensen, E.; Lygre, H. Quantification of organic eluates from polymerized resin-based dental restorative materials by use of GC/MS. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2007, 850, 83–91. [Google Scholar] [CrossRef]
- Wataha, J.C.; Rueggeberg, F.A.; Lapp, C.A.; Lewis, J.B.; Lockwood, P.E.; Ergle, J.W.; Mettenburg, D.J. In vitro cytotoxicity of resin-containing restorative materials after aging in artificial saliva. Clin. Oral Invest. 1999, 3, 144–149. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials – fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Nalcaci, A.; Oztan, M.D.; Yilmaz, S. Cytotoxicity of composite resins polymerized with different curing methods. Int. Endod. J. 2004, 37, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Galler, K.; Schmalz, G.; Cosentino, C.; Rengo, S.; Schweikl, H. Inhibition of phosphatidylinositol 3-kinase amplifies TEGDMA-induced apoptosis in primary human pulp cells. J. Dent. Res. 2004, 83, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Ahn, S.J.; Yang, H.C. Involvement of oxidative stress in mutagenicity and apoptosis caused by dental resin monomers in cell cultures. Dent. Mater. 2006, 22, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Jontell, M.; Hanks, C.T.; Bratel, J.; Bergenholtz, G. Effects of unpolymerized resin components on the function of accessory cells derived from the rat incisor pulp. J. Dent. Res. 1995, 74, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Schwengberg, S.; Bohlen, H.; Kleinsasser, N.; Kehe, K.; Seiss, M.; Walther, U.I.; Hickel, R.; Reichl, F.X. In vitro embryotoxicity assessment with dental restorative materials. J. Dent. 2005, 33, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.; Leyhausen, G.; Mai, U.E.; Geurtsen, W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J. Dent. Res. 1998, 77, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Imazato, S.; Russell, R.R.; Noiri, Y.; Ebisu, S. Influence of resin monomers on growth of oral streptococci. J. Dent. Res. 2004, 83, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Salomon, J.P.; Pertot, W.J.; Dejou, J. The coherence between 3 evaluation methods of biocompatibility. J. Biol. Buccale. 1992, 20, 211–217. [Google Scholar] [PubMed]
- Stanley, H.R. Biological evaluation of dental materials. Int. Dent. J. 1992, 42, 37–46. [Google Scholar] [PubMed]
- Lygre, G.B.; Gjerdet, N.R.; Gronningsaeter, A.G.; Bjorkman, L. Reporting on adverse reactions to dental materials--intraoral observations at a clinical follow-up. Community Dent. Oral Epidemiol. 2003, 31, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Vamnes, J.S.; Lygre, G.B.; Gronningsaeter, A.G.; Gjerdet, N.R. Four years of clinical experience with an adverse reaction unit for dental biomaterials. Community Dent. Oral Epidemiol. 2004, 32, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, R.; Gjerdet, N.R.; Schedle, A.; Bjorkman, L.; Berglund, A. An overview of the current status of national reporting systems for adverse reactions to dental materials. J. Dent. 2004, 32, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.M.; Latta, M.A. Self-healing dental composites and related methods. US Pat. 2008/147366, 2008. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moharamzadeh, K.; Brook, I.M.; Van Noort, R. Biocompatibility of Resin-based Dental Materials. Materials 2009, 2, 514-548. https://doi.org/10.3390/ma2020514
Moharamzadeh K, Brook IM, Van Noort R. Biocompatibility of Resin-based Dental Materials. Materials. 2009; 2(2):514-548. https://doi.org/10.3390/ma2020514
Chicago/Turabian StyleMoharamzadeh, Keyvan, Ian M. Brook, and Richard Van Noort. 2009. "Biocompatibility of Resin-based Dental Materials" Materials 2, no. 2: 514-548. https://doi.org/10.3390/ma2020514
APA StyleMoharamzadeh, K., Brook, I. M., & Van Noort, R. (2009). Biocompatibility of Resin-based Dental Materials. Materials, 2(2), 514-548. https://doi.org/10.3390/ma2020514





