Research on the Influence of Recycled Fine Powder on Chloride Ion Erosion of Concrete in Different Chloride Salt Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportion and Specimen Preparation
2.2.1. Mix Proportion
2.2.2. Specimen Making
2.3. Test Methods
3. Results and Analysis
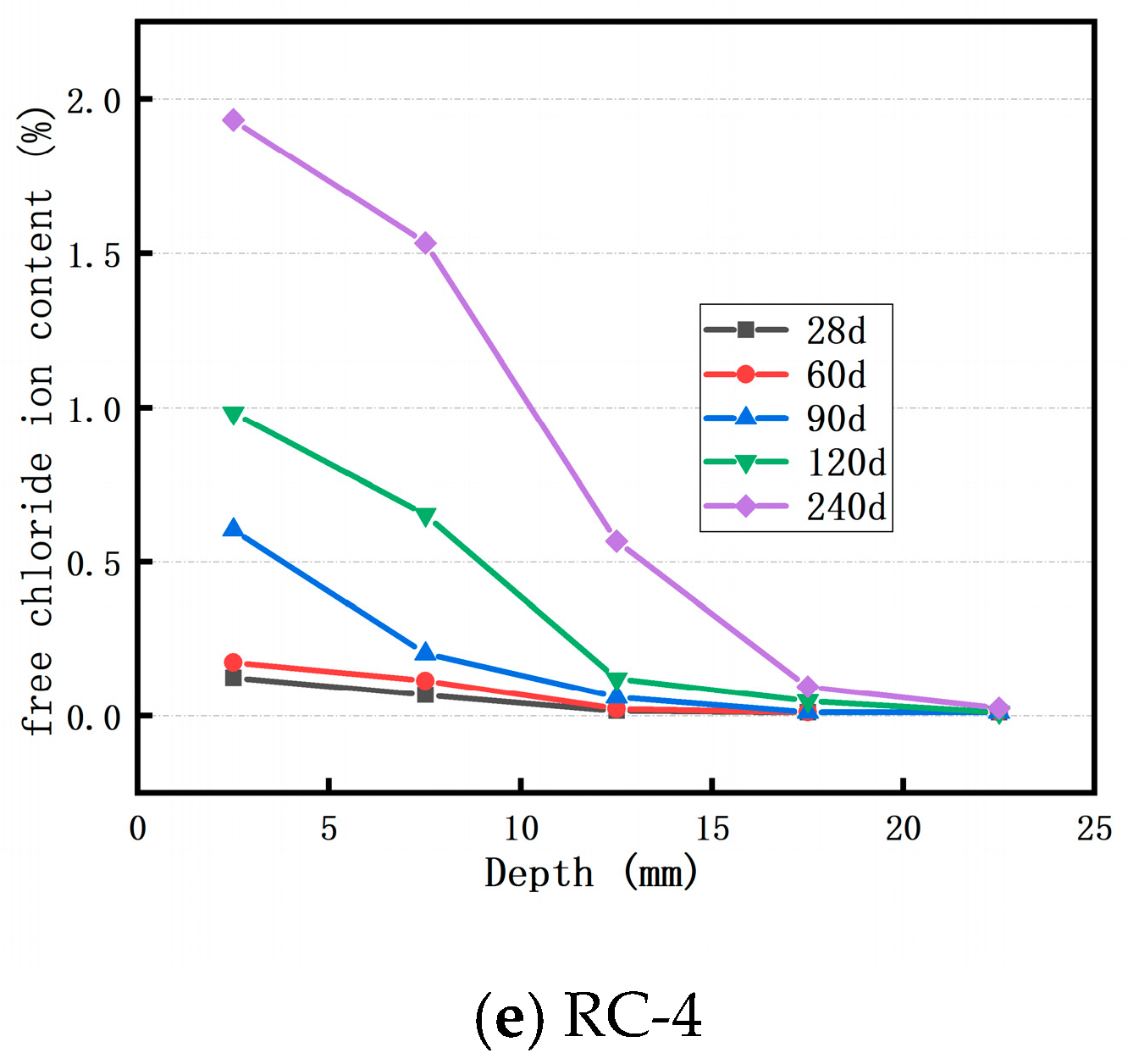
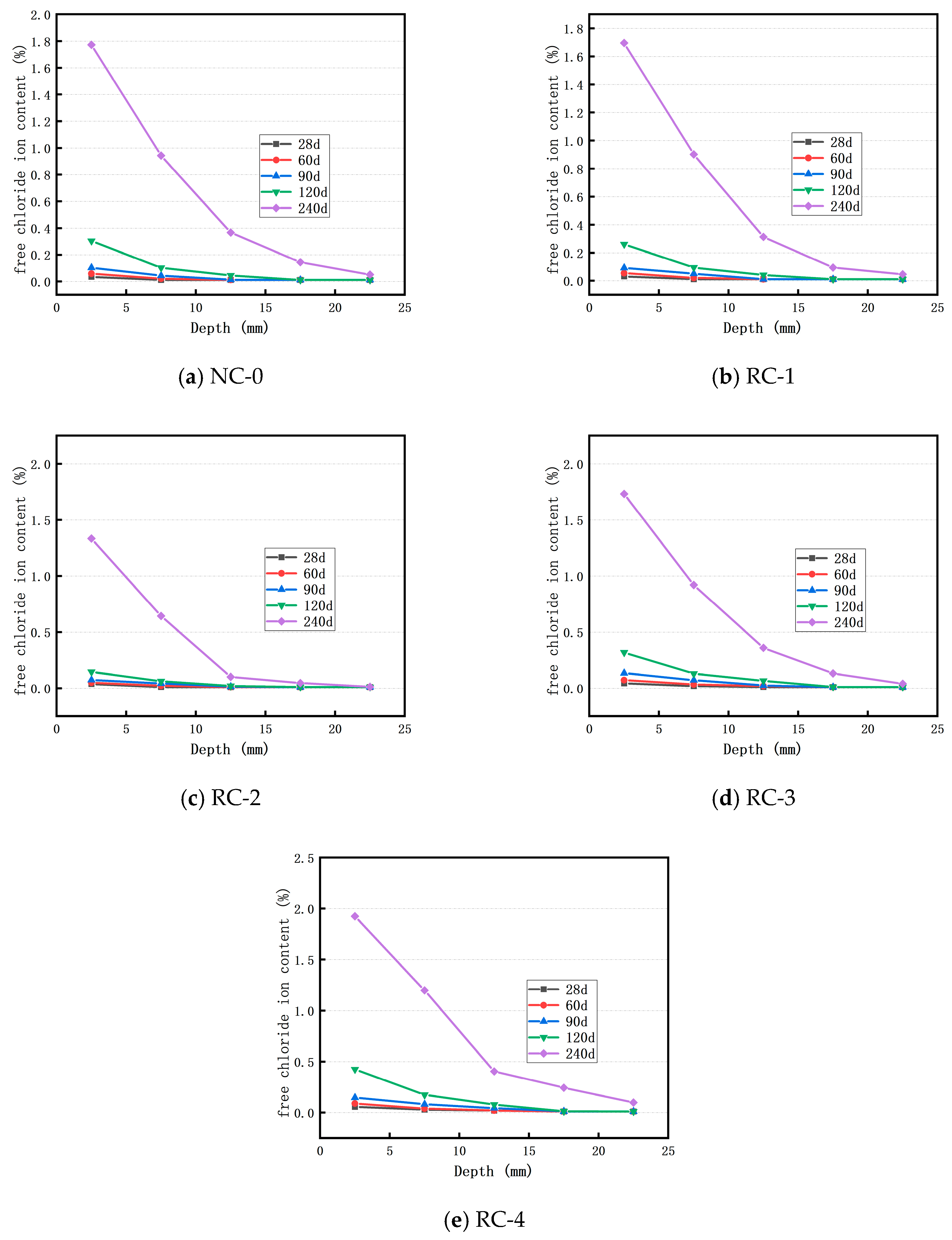
3.1. Distribution of Cl− Ion of Concrete in Sodium Chloride Solution
3.2. Distribution of Cl− Ion of Concrete in Brine Solution of Salt Lake
3.3. Effect of Soaking Time on Diffusion Coefficient Df of Free Cl− Ion
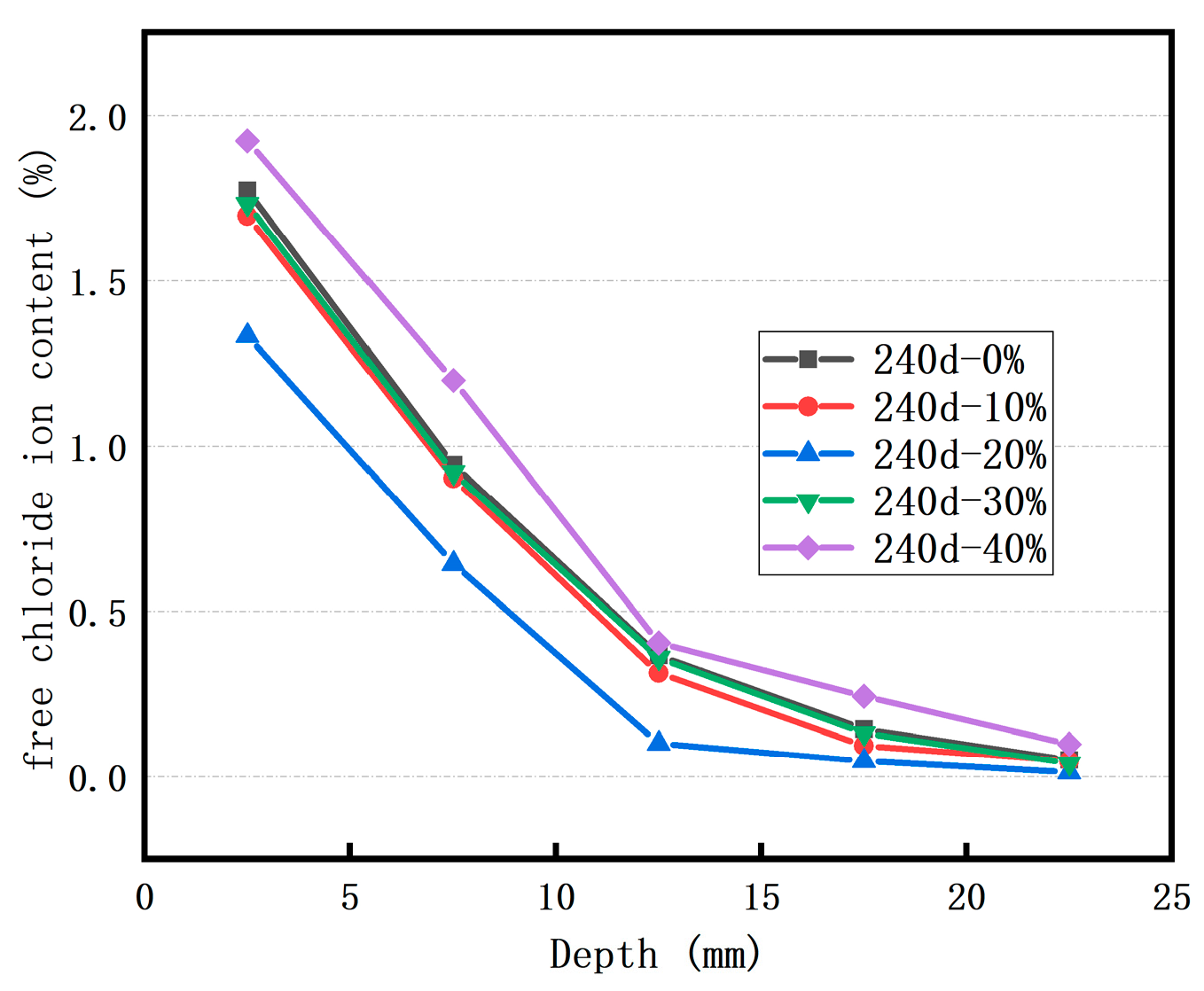
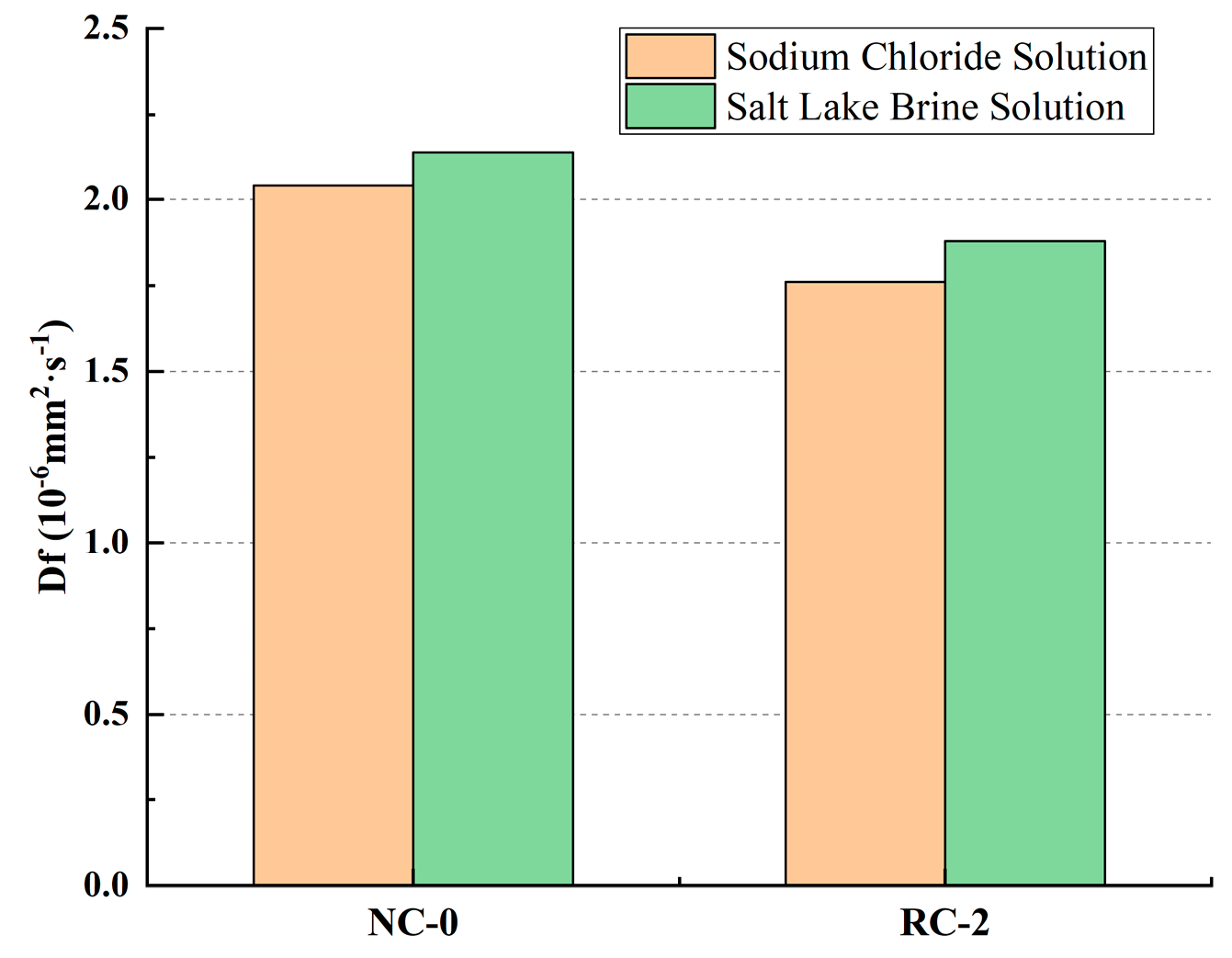
3.4. Comparative Analysis of Cl− Ion Diffusion Properties After Soaking in Two Kinds of Salt Solution
3.4.1. Cl− Ion Erosion Concentration
3.4.2. Free Cl− Ion Diffusion Coefficient Df
3.5. SEM Analysis
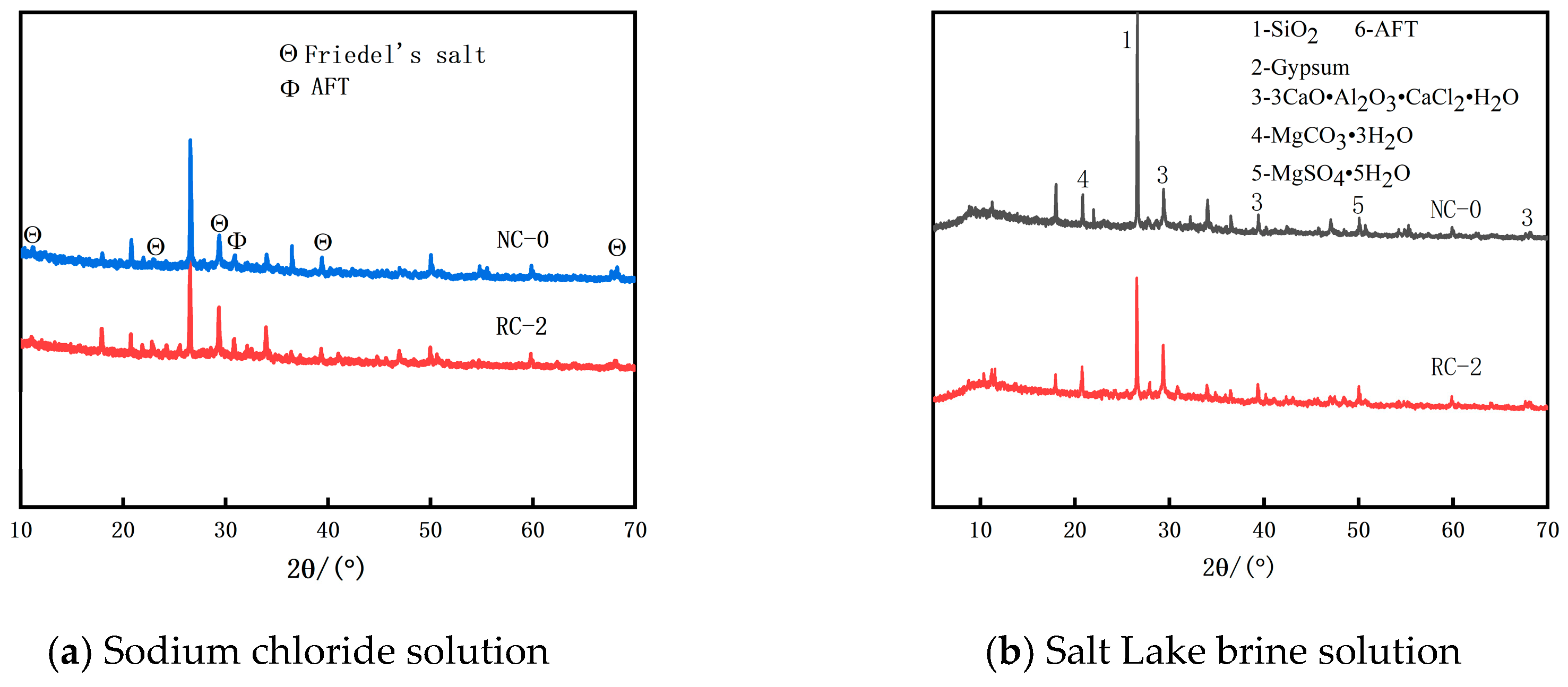
3.6. XRD Analysis
4. Discussion
5. Conclusions
- The Cl− ion concentration in concrete at the same depth increases as the soaking time lengthens. Incorporating an appropriate amount of RFP into concrete can enhance the concrete’s resistance to Cl− ion penetration. When the RFP replacement rate is 20%, the concrete demonstrates good resistance to Cl− ion erosion in both the sodium chloride solution and the Salt Lake brine solution.
- In both the sodium chloride solution and the Salt Lake brine solution, as the soaking time increases, the free chloride ion diffusion coefficient (Df) of the RFP concrete shows a downward trend, indicating that after the RFP concrete is fully cured, the recycled fine powder undergoes secondary hydration, which improves the compactness of the concrete and enhances its durability. The hydration products of the RFP concrete are rich in mineral components such as tricalcium aluminate hydrate, which can react with the free Cl− in the concrete to form Friedel’s salt, solidifying part of the Cl−, thus effectively delaying the diffusion rate of Cl− in the concrete.
- Compared with erosion in the sodium chloride solution, after soaking in Salt Lake brine for 240 days, the Cl− ion diffusion coefficients Df of NC-0 and RC-2 are 2.14 × 10−6 mm2·s−1 and 1.88 × 10−6 mm2·s−1, respectively, increasing by 0.1 × 10−6 mm2·s−1 and 0.12 × 10−6 mm2·s−1 compared with the erosion in the sodium chloride solution. The results show that the damage degree in the Salt Lake solution is stronger than in the sodium chloride solution at the latter age. This indicates that as the soaking time increases, the inhibitory effect of sulfate ions in the Salt Lake brine on the penetration of chloride ions gradually disappears. Sulfate erosion leads to the generation of a large amount of volume-expanding substances, increasing the number of pores in the concrete, which makes the penetration of chloride ions easier.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RFP | recycled fine powders |
| Df | the free chloride ion diffusion coefficient |
| Friedel’s salt | 3CaO·Al2O3·CaCl2·10H2O |
| C3A | tricalcium aluminate hydrate |
| Cf | the mass percentage of Cl− content relative to the mass of concrete |
References
- Xiao, J.-Z. Recycled Concrete; China Architecture & Building Press: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Xiao, J.-Z.; Zhang, H.-H.; Tang, Y.-X.; Lv, Z.-Y.; Ye, T.-H.; Duan, Z.-H.; Sui, T.-B.; Xiao, X.-W. Principles for waste concrete recycling and basic problems of recycled concrete. Chin. Sci. Bull. 2023, 68, 510–523. (In Chinese) [Google Scholar] [CrossRef]
- Arora, M.; Raspall, F.; Fearnley, L.; Sliva, A. Urban mining in buildings for a circular economy: Planning, process and feasibility prospects. Resour. Conserv. Recycl. 2021, 174, 105754. [Google Scholar] [CrossRef]
- Xiao, J.-Z.; Li, W.-G.; Fan, Y.-H.; Xiao, H. An overview of study on recycled aggregate concrete in China (1996–2011). Constr. Build. Mater. 2012, 31, 364–383. [Google Scholar] [CrossRef]
- Behera, M.; Bhattacharyya, S.-K.; Minocha, A.-K.; Deoliya, R.; Maiti, S. Recycled aggregate from C&D waste & its use in concrete—A breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar]
- Xiao, J.-Z.; Ye, T.-H.; Sui, T.-B.; Pan, Z.-S. Fundamental Problems and Applications of Recycled Fine Powder Derived from Waste Concrete. Mater. Rep. 2023, 37, 22120116. [Google Scholar]
- Singh, A.; Miao, X.-Z.; Zhou, X.; Deng, Q.; Li, J.-N.; Duan, Z.-H. Use of recycled fine aggregates and recycled powders in sustainable recycled concrete. J. Build. Eng. 2023, 77, 107370. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhu, Z.-Y.; Ma, L.-Y. Evaluation of waste concrete recycled powder (WCRP) on the preparation of low-exothermic cement. J. Build. Eng. 2022, 53, 104511. [Google Scholar] [CrossRef]
- Duan, Z.-H.; Hou, S.-D.; Xiao, J.-Z.; Li, B. Study on the essential properties of recycled powders from construction and demolition waste. J. Clean. Prod. 2023, 253, 119865. [Google Scholar] [CrossRef]
- Kaya, Y.-B.; Aytekin, B.; Kaya, T.; Mardani, A. Investigation of pozzolanic activity of recycled concrete powder: Effect of cement fineness, grain size distribution and water/cement ratio. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Cheng, Z.-Q.; Wang, K.-C.; Zhou, J.-J.; Wu, H.-L. Mechanical properties evaluation of sustainable engineered cementitious composites containing recycled fine powders. J. Build. Eng. 2023, 71, 106438. [Google Scholar] [CrossRef]
- Xiao, J.-Z.; Xiao, Y.; Liu, Y.; Ding, T. Carbon emission analyses of concretes made with recycled materials considering CO2 uptake through carbonation absorption. Struct. Concr. 2020, 22, E58–E73. [Google Scholar] [CrossRef]
- Mao, X.-Q.; Qu, W.-J.; Zhu, P.; Xiao, J.-Z. Influence of recycled powder on chloride penetration resistance of green reactive powder concrete. Constr. Build. Mater. 2020, 251, 119049. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Wu, H.-X.; Cao, C.-W. Chloride permeability of concrete mixed with activity recycled powder obtained from C&D waste. Constr. Build. Mater. 2019, 199, 652–663. [Google Scholar]
- Bogas, J.-A.; Carrica, A.; Real, S. Durability of concrete produced with recycled cement from waste concrete. Mater. Today Proc. 2022, 58, 1149–1154. [Google Scholar] [CrossRef]
- Sun, C.; Chen, L.-L.; Xiao, J.-Z.; Singh, A.; Zeng, J.-H. Compound utilization of construction and industrial waste as cementitious recycled powder in mortar. Resour. Conserv. Recycl. 2021, 170, 105561. [Google Scholar] [CrossRef]
- Bian, Y.-D.; Qiu, X.; Zhao, J.-H.; Zhong, L.; Ouyang, J.-N. Influence of Recycled Concrete Fine Powder on Durability of Cement Mortar. Fluid Dyn. Mater. Process. 2024, 20, 45–58. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, L.; Liu, R.-G.; Zhu, P.-H.; Liu, H.; Wang, X.-J.; Yu, J.; Chen, Y.-C. Chloride penetration of concrete exposed to dry-wet cycle with various dry-wet ratios and temperature. Constr. Build. Mater. 2023, 400, 132883. [Google Scholar] [CrossRef]
- Shi, X.-M.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Chu, H.-Q.; Wang, T.-T.; Guo, M.-Z.; Zhu, Z.-Y.; Jiang, L.-H.; Pan, C.-L.; Liu, T. Effect of stray current on stability of bound chlorides in chloride and sulfate coexistence environment. Constr. Build. Mater. 2019, 194, 247–256. [Google Scholar] [CrossRef]
- Chen, L.-L.; Chen, X.-D.; Wang, L.; Ning, Y.-J.; Ji, T. Compressive strength, pore structure, and hydration products of slag foam concrete under sulfate and chloride environment. Constr. Build. Mater. 2023, 394, 132141. [Google Scholar] [CrossRef]
- Wang, R.-Q.; Huo, Y.-P.; Wang, T.; Hou, P.; Gong, Z.; Li, G.-D.; Li, C.-Y. Machine Learning Method to Explore the Correlation between Fly Ash Content and Chloride Resistance. Materials 2024, 17, 1192. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Jang, S.-Y.; Cho, J.-Y.; Kim, J.-Y. A novel short-term immersion test to determine the chloride ion diffusion coefficient of cementitious materials. Constr. Build. Mater. 2014, 57, 169–178. [Google Scholar] [CrossRef]
- ASTMC1202; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 1994.
- Tang, L.-P.; Nilsson, L.O. Rapid determination of the chloride diffusivity in concrete by applying an electric field. Mater. J. 1993, 89, 49–53. [Google Scholar]
- Yuan, M.-S. Experimental Study on Anti-Chloride Permeability Performance of Ceramic Powder Concrete. Master’s Thesis, East China Jiaotong University, Shanghai, China, 2018. (In Chinese). [Google Scholar]
- Dong, Y.; Ma, Y.-S.; Peng, N.-B.; Qiu, J.-C. Study on SO42−/Cl− Erosion Resistance and Mechanism of Recycled Concrete Containing Municipal Solid Waste Incineration (MSWI) Powder. Materials 2022, 15, 5352. [Google Scholar] [CrossRef]
- Bayraktar, O.-Y.; Tunçtan, M.; Benli, A.; Türkel, İ.; Kızılay, G.; Kaplan, G. A study on sustainable foam concrete with waste polyester and ceramic powder: Properties and durability. J. Build. Eng. 2024, 95, 110253. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, Z.R.; Niu, D.T. Understanding the acceleration impact of load and flowing water on the chloride ion transport properties of fly ash-based geopolymer concrete. Cem. Concr. Compos. 2023, 114, 105146. [Google Scholar] [CrossRef]
- Xu, F.; Yang, Z.-Q.; Liu, W.-Q. Experimental investigation on the effect of sulfate attack on chloride diffusivity of cracked concrete subjected to composite solution. Constr. Build. Mater. 2020, 237, 117643. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Z.-G.; Su, X.; Feng, Y.-X.; Xu, T.-N.; Zheng, Z.-D.; Liu, Y.; Li, P.-P. Improving sulfate and chloride resistance in eco-friendly marine concrete: Alkali-activated slag system with mineral admixtures. Constr. Build. Mater. 2024, 411, 134333. [Google Scholar] [CrossRef]
- Yang, F.; Ma, Y.; Li, L.-C.; Liu, S.; Hai, R.; Zhu, Z.-Y. Early-Age Behaviour of Portland Cement Incorporating Ultrafine Recycled Powder: Insights into Hydration, Setting, and Chemical Shrinkage. Materials 2024, 17, 5551. [Google Scholar] [CrossRef]
- Brown, P.-W.; Badger, S. The distributions of bound sulfates and chlorides in concrete subjected to mixed NaCl, MgSO4, Na2SO4 attack. Cem. Concr. Res. 2000, 30, 1535–1542. [Google Scholar] [CrossRef]
- Xu, J.-X.; Zhang, C.-K.; Jiang, L.-H.; Tang, L.; Gao, G.-F.; Xu, Y.-P. Releases of bound chlorides from chloride-admixed plain and blended cement pastes subjected to sulfate attacks. Constr. Build. Mater. 2013, 45, 53–59. [Google Scholar] [CrossRef]







| Material | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | CO2 | SO3 |
|---|---|---|---|---|---|---|---|
| Cement | 67.7 | 12.0 | 3.02 | 6.77 | 0.09 | 6.12 | 2.37 |
| RFP | 34.5 | 25.8 | 4.44 | 6.04 | 0.15 | 25.80 | 0.44 |
| Material | Fineness (45 μm)/% | Bulk Density/(kg·m−3) | Apparent Density/(kg·m−3) | Water Demand Ratio/(%) | Specific Surface Area/(m2/kg) | Activity Index/(%) |
|---|---|---|---|---|---|---|
| RFP | 30.78 | 2483 | 945 | 116 | 467 | 0.73 |
| Materials | Bulk Density /kg·m−3 | Apparent Density /kg·m−3 | Clay Content/% | Water Content/% |
|---|---|---|---|---|
| Coarse aggregate | 1510 | 2673 | 0.33 | 0.38 |
| Fine aggregate | 1501 | 2673 | 1.14 | 1.15 |
| Cl− | Mg2+ | SO42− | Ca+ | K+ | Na+ | Li+ | |
|---|---|---|---|---|---|---|---|
| Salt Lake brine | 201 | 153 | 104 | 0.35 | 79 | 12 | 0.24 |
| NO. | Replacement Rate/% | W/C | Amount of Materials/(kg/m3) | |||
|---|---|---|---|---|---|---|
| Cement | RFP | Gravel | Sand | |||
| NC-0 | 0 | 0.45 | 455 | 0 | 1032 | 595 |
| RC-1 | 10 | 0.45 | 410 | 46 | 1032 | 595 |
| RC-2 | 20 | 0.45 | 364 | 91 | 1032 | 595 |
| RC-3 | 30 | 0.45 | 319 | 137 | 1032 | 595 |
| RC-4 | 40 | 0.45 | 273 | 182 | 1032 | 595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhao, G.; Li, Y. Research on the Influence of Recycled Fine Powder on Chloride Ion Erosion of Concrete in Different Chloride Salt Environments. Materials 2025, 18, 2018. https://doi.org/10.3390/ma18092018
Chen L, Zhao G, Li Y. Research on the Influence of Recycled Fine Powder on Chloride Ion Erosion of Concrete in Different Chloride Salt Environments. Materials. 2025; 18(9):2018. https://doi.org/10.3390/ma18092018
Chicago/Turabian StyleChen, Lijun, Gang Zhao, and Ying Li. 2025. "Research on the Influence of Recycled Fine Powder on Chloride Ion Erosion of Concrete in Different Chloride Salt Environments" Materials 18, no. 9: 2018. https://doi.org/10.3390/ma18092018
APA StyleChen, L., Zhao, G., & Li, Y. (2025). Research on the Influence of Recycled Fine Powder on Chloride Ion Erosion of Concrete in Different Chloride Salt Environments. Materials, 18(9), 2018. https://doi.org/10.3390/ma18092018




