2.1. Material Characteristics
The first material used for the test was an aluminium alloy with the designation EN AW-1050A. It is referred to as a pure aluminium alloy due to its very high aluminium content, which is 99.5%. The alloy is characterised by high plasticity, but it has low strength properties and, therefore, is not suitable for machining. Moreover, the alloy is corrosion-resistant and has good weldability and high thermal and heat conductivity, which make it suitable for use in the energy and automotive industries [
22]. The application of aluminium alloy EN AW-1050A in industry is also influenced by its appearance, which is valued for the glossy surface of the material. In
Table 1 the physical properties of the tested aluminium alloy are presented.
The second adherent forming the adhesive joints was non-tempered soda-lime float glass. This type of glass is used in the construction industry and the automotive industry for glazing. Non-tempered glass is a very brittle material, which splits easily and has low impact strength. In order to change the properties of the glass, it is possible to make modifications to increase the strength and flexibility of the glass by applying tempering or bonding of the glass layers with the use of polyvinyl butyral (PVB) foil [
24,
25]. Modifications are necessary in the automotive industry, where a vehicle is subjected to dynamic forces that can easily damage the glass.
The float glass production process allows for an even sheet of glass with a thickness between 0.4 mm and 25 mm to be obtained [
26]. The basic materials included in the composition of the glass selected for the study are as follows: SiO
2—60–75%, Na
2O—12–18%, CaO—5–12% [
27]. In addition to these materials, soda-lime glass also contains other chemical compounds, which often tint the glass to protect the user from UV radiation, as is the case for car windows. The glass, for the samples selected for testing, was not tinted. The properties of the glass are shown in
Table 2.
A two-component epoxy adhesive containing an epoxy resin and curing agent was used to prepare the adhesive joints. The epoxy resin, chosen for the study (trademark: Epidian 5, Ciech Resins, Nowa Sarzyna, Poland) is formed via the reaction of bisphenol A with epichlorodrin [
29]. It occurs in the form of a thick liquid with an amber colour. This resin constitutes the basis of adhesives used to bond glass, wood or metal.
Table 3 shows the characteristics of the Epidian 5 epoxy resin (Ciech Resins, Nowa Sarzyna, Poland).
The curing agent of the adhesive compound was a polyamide curing agent (trademark: PAC, Ciech Resins, Nowa Sarzyna, Poland). This curing agent is available in a liquid form. It belongs to the group of slow-reacting curing agents and has low reactivity. The adhesive compound made up of the above-mentioned components is characterised by low viscosity, which allows for efficient spreading over the surface of the sample and thorough filling of micro-roughness, ensuring proper mechanical adhesion. In addition, it has high chemical resistance to oils and solvents. Once cured, the adhesive composition exhibits high stiffness. The disadvantage of this composition compared to other available epoxy adhesives is the need for the precise dosage of the resin and curing agent [
28,
30,
31]. The adhesive compound was mixed at a ratio of 80 g of curing agent per 100 g of epoxy resin. A mechanical stirrer with a spindle speed of 460 rpm was used to mix the components to obtain a uniform consistency of the adhesive. The process of curing the resulting adhesive was carried out in stages. The first stage was the gelation of the adhesive compound, which began from the moment of mixing the adhesive components. The gelation time was 180 min at 25 °C. The adhesive compound was then pre-cured. A fully cured adhesive joint was obtained after 7 to 14 days [
32].
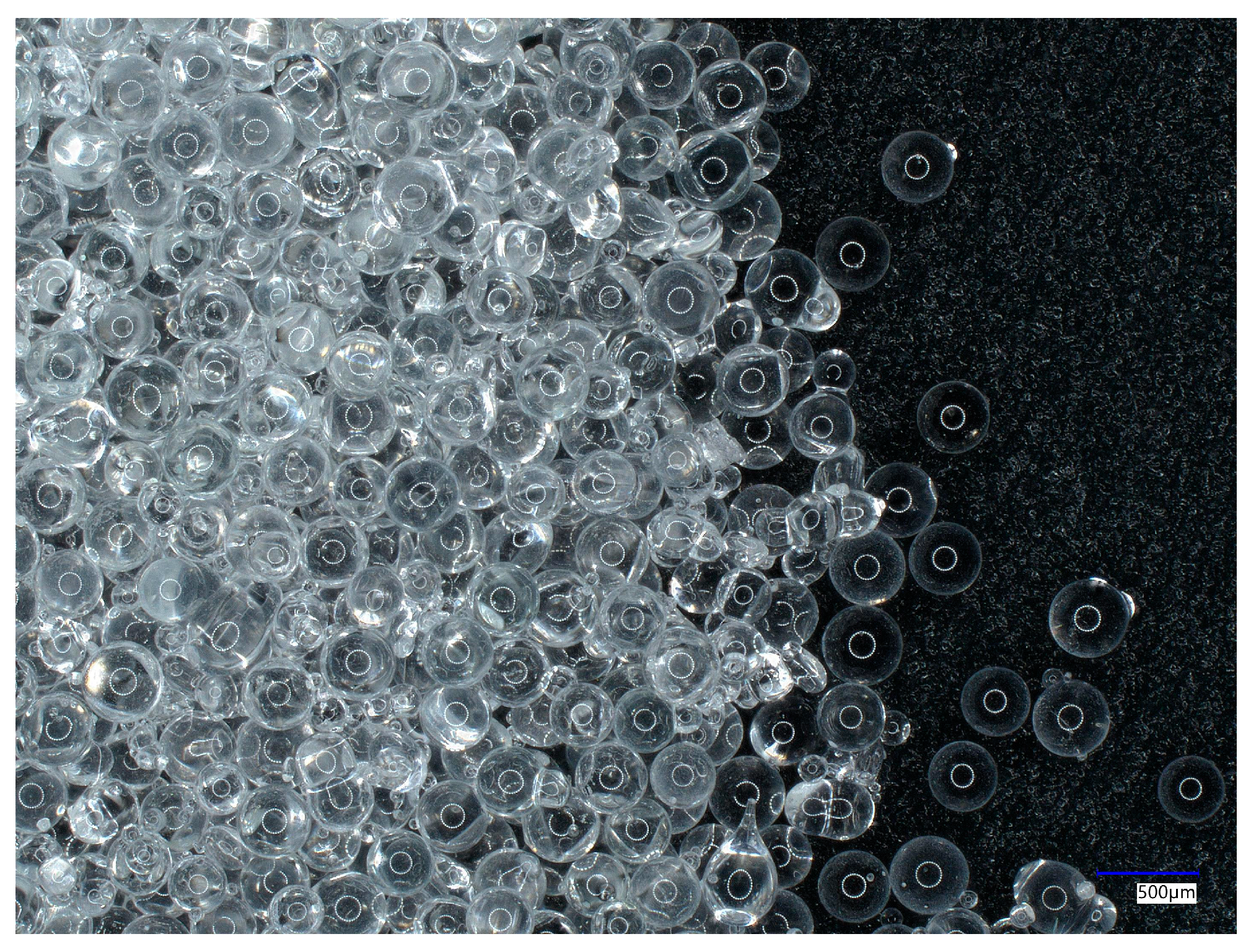
Two sanding agents, copper slag and glass beads, were used in the study. Copper slag is a by-product of copper ore smelting. The photos were taken at close range using a microscope (model: VHX 500, Keyence, Osaka, Japan). The microscopic view of copper slag is presented in
Figure 1.
It is formed by depositing slag on the surface of the molten metal, and then, it is collected for air hardening. The hardening treatment produces granules with sharp, angular edges and a black-red colour [
33,
34]. Copper slag is widely used as a sandblasting agent due to the easy availability of this material and its low price. Moreover, it is a non-toxic, environmentally friendly material. The hardness of this material is 7 on the Mohs scale. The chemical composition of the material used in the study consisted of the following: SiO
2 32.0–46.0%, CaO 18.5–29.0%, FeO 4.5–6.0%, Al2O3 10.0–14.5%, MgO 8.0–12.5% [
35]. This material, due to its properties, is widely used as an abrasive; its hardness allows it to be used in copper slag sandblasting to clean mainly metal or concrete surfaces. The grain size of the copper slag used in the study ranged from 0.2 to 0.8 mm.
The second sandblasting agent was glass beads. The microscopic view of glass beads is presented in
Figure 2.
This material allows for the very thorough cleaning of metal surfaces and makes them smooth and satin. This agent is suitable to process thin and brittle materials susceptible to surface damage. The glass beads do not cause aggressive surface abrasion of the workpiece, and as in the case of the copper slag, they can be used multiple times. The hardness of glass beads is 6 on the Mohs scale. The chemical composition of the glass beads used in the study was as follows: SiO
2 70–73%,Na
2O + K
2O 13–15%, CaO 7–11%, MgO 3–5%, Al
2O
3 0.5–2.0% [
36]. The size of the glass beads selected for the study ranged from 0.42 to 0.80 mm.
The first stage of the sample preparation was to cut the materials used to a specified size. For the preparation of the EN AW-1050A aluminium alloy samples, a sheet of a 3 mm thickness and dimensions of 2 × 1 m was used. The aluminium alloy sheet was cut into 100 × 25 mm samples using Waterjet Eckert Combo portal cutting machine.
As for the glass samples, samples with dimensions of 125 × 25 mm and a thickness of 4 mm were purchased. The brittleness of the glass, which has not been subjected to any additional treatment that would strengthen the glass structure, does not allow for the strength testing of adhesive lap joints consisting of only 2 elements, in this case a glass sample and an aluminium alloy sample. In overlap joints, the non-tempered glass, under the influence of the forces acting in the strength testing machine, cracks in front of the adhesive layer of the adhesive joint [
9]. It is also possible to damage the material because of the stress during the fixing of the adhesive joint in the holder of the strength testing machine.
The glass samples were cut to the length of the overlap. This allowed the glass sample to be placed between the two aluminium alloy samples. The glass samples were cut using a special knife. In order to determine the length of the glass sample, the formula for limiting the overlap length lgr was used. The values of the coefficient of longitudinal elasticity and the thickness of the material were adopted only for glass [
37].
The values are as follows:
E = 7.2 ∙ 104 MPa—Young’s modulus of glass
g = 4 mm—thickness of glass
gk = 0.10 mm—adhesive joint layer thickness
Gk = 2200 MPa–shear modulus of the adhesive
The value of the shear modulus of the adhesive in calculating the limiting length of the single-lap was selected for the adhesive formed from the Epidian 5 epoxy resin mixed with the polyamide curing agent in a ratio of 10:6. The calculated limiting length of the overlap was 16.36 mm. The length of the glass sample and, thus, the overlap of the adhesive joint, adopted for the test, was 22 mm. This was due to the difficulty of cutting the sample below 22 mm in length while maintaining the required dimension.
After obtaining the samples of the required dimensions, the focus was made on the surface treatment of adherends. Three groups of samples were selected at this stage.
The first group consisted of samples for which surfaces were untreated. In the second group, the samples were sandblasted with copper slag, and in the third group, they weresandblasted with glass beads.
Sandblasting was carried out manually using a sandblasting gun (Yato Yt-2375, Shanghai, China) and a compressor (Stanley 24l, NU AIR POLSKA Sp. z o.o., Warsaw, Poland). The jet of the sandblasting agent was directed perpendicularly to the surface of the sample. The distance between the nozzle and the treated material was 10 ± 1 cm. The treated sample was inclined at an angle of 45 ± 1° during sandblasting to the work surface. The pressure of the compressor during the sandblasting operation was 0.6 MPa. As for the surface of the aluminium alloy samples, only the overlap part of the specimen was treated. However, the glass samples were sandblasted on both sides. The sandblasting operation of each adherent lasted until a homogeneous structure was obtained on the surface of the samples. During the sandblasting operation, the temperature and humidity were respectively 23 ± 1 °C and 20 ± 1%.
Adhesive joints consisting of two of the same aluminium alloy samples and one glass sample were prepared in the study. The design of this joint was determined by the brittleness of the glass, which does not allow a standard overlapping adhesive joint to be created. The purpose of the adopted design was to enable the performance of strength tests without damaging the glass at a location other than the adhesive layer [
9].
Figure 3 shows the design of the adhesive joint and the dimensions.
The adhesive joints created in the study differed in the way of surface treatment of the samples.
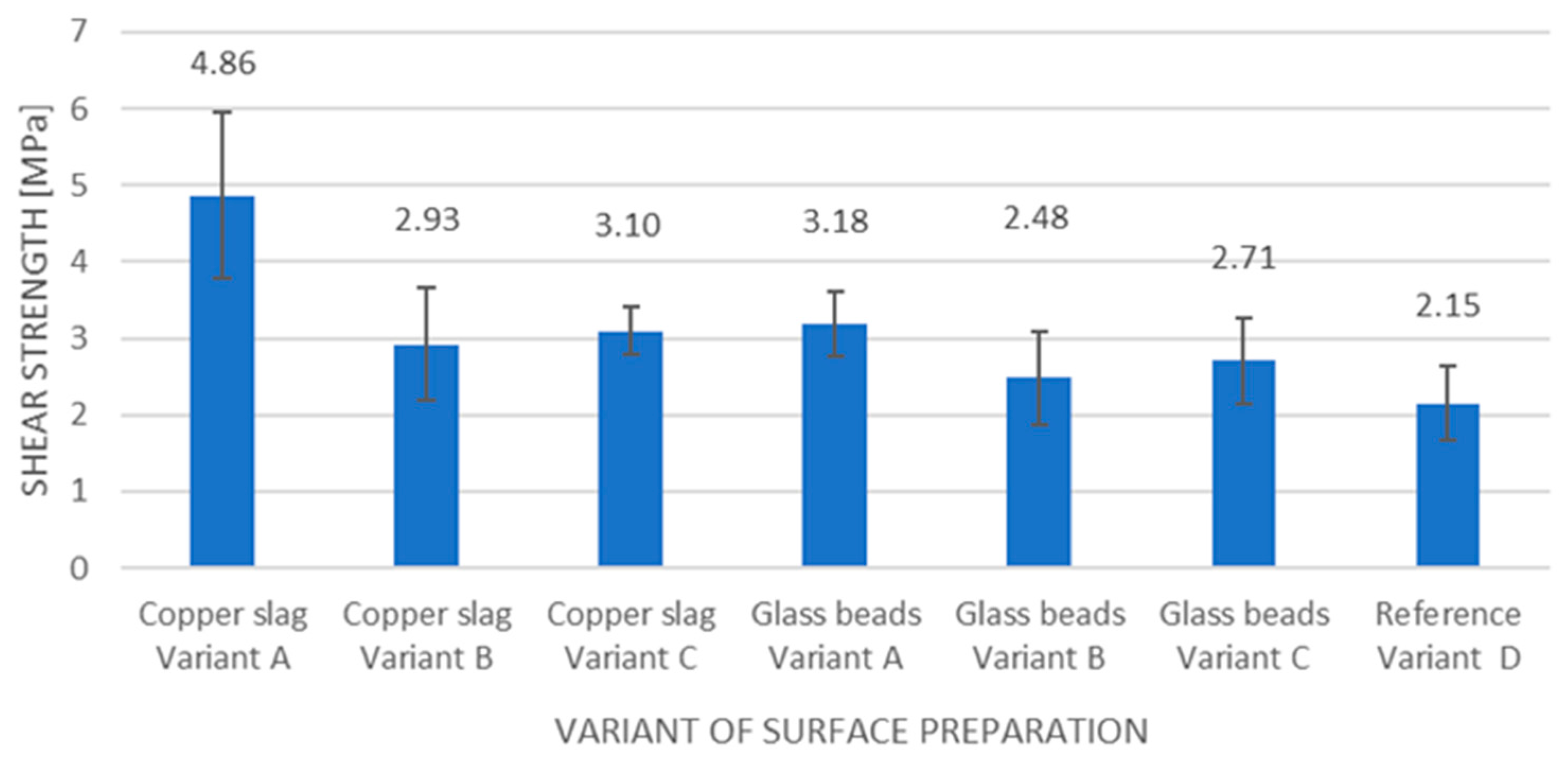
Table 4 shows the configuration diagram of the sample surface treatment with the designation that will be used further on in the article. Configurations A, B and C were divided into joints with the surface of the samples treated by using copper slag and those with the surface of the samples treated by using glass beads. The adhesive joint samples in configuration D were not sandblasted and were therefore the reference variant for both sandblasting agents. Five adhesive joints were prepared for each configuration variant. In total, there were 35 adhesive joints.
The preparation of the adhesive joints was in two stages. The staged way in which the adhesive joints were created resulted from the need to maintain the accuracy of the arrangement of the samples in relation to each other. In the first stage, the focus was on bonding one aluminium alloy sample to the glass. Previously prepared samples were degreased with a Loctite 7063 degreasing agent. The purpose of this treatment was to remove contaminants created during the processing of the materials. The degreasing process was carried out by applying the degreasing agent to the surface of the sample and then wiping it in a single motion using a dust-free swab. This action was repeated twice. In the third degreasing of the sample surface, the cleaning agent was left to evaporate.
The prepared adhesive was applied to the surface of the aluminium alloy, and then, the aluminium alloy sample was combined with the glass sample. The resulting joint was left to pre-cure for a period of 48 h. During the curing process, the joint was pressed with a 0.04 MPa.
In the second stage of the adhesive joint preparation, the second sample of the aluminium alloy was bonded to the glass. This stage started after the pre-curing time had elapsed. The process of cleaning the samples and joining the components followed the same scheme as described before. The adhesive joints consisting of two aluminium samples and a glass sample were left for a period of seven days to allow for a full cure of the adhesive. The pressure of 0.04 MPa was also used at this stage. During the preparation of the adhesive joints and their cure, the temperature and humidity were, respectively, 23 ± 1 °C and 20 ± 1%.