Superconformal Electrodeposition of Cobalt into Micron-Scale Trench with Alkynol Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Calculation Details
2.2.2. Material Characterization
3. Results and Discussion
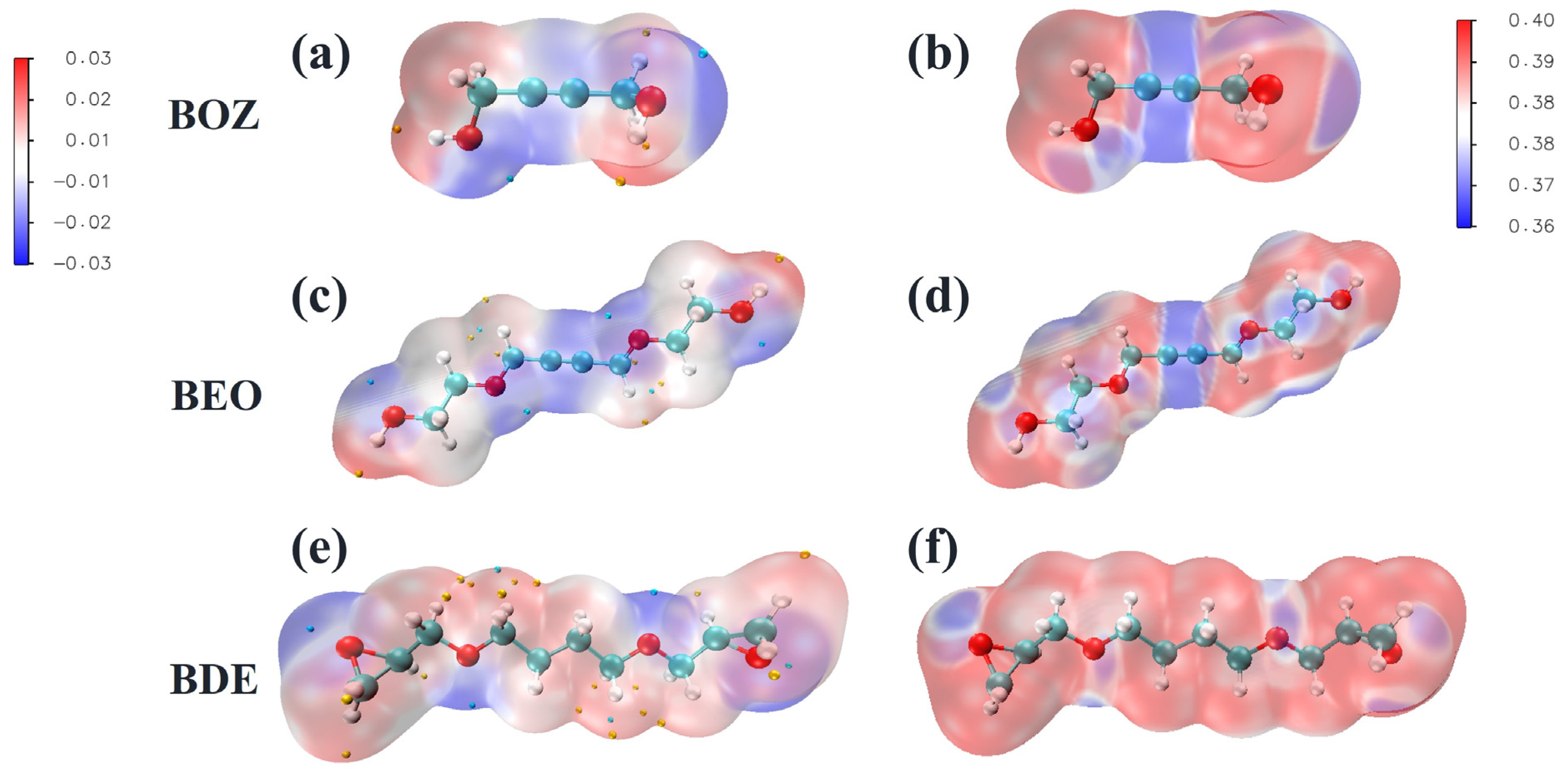
3.1. Molecular Activity Analysis
3.2. Electrochemical Analysis
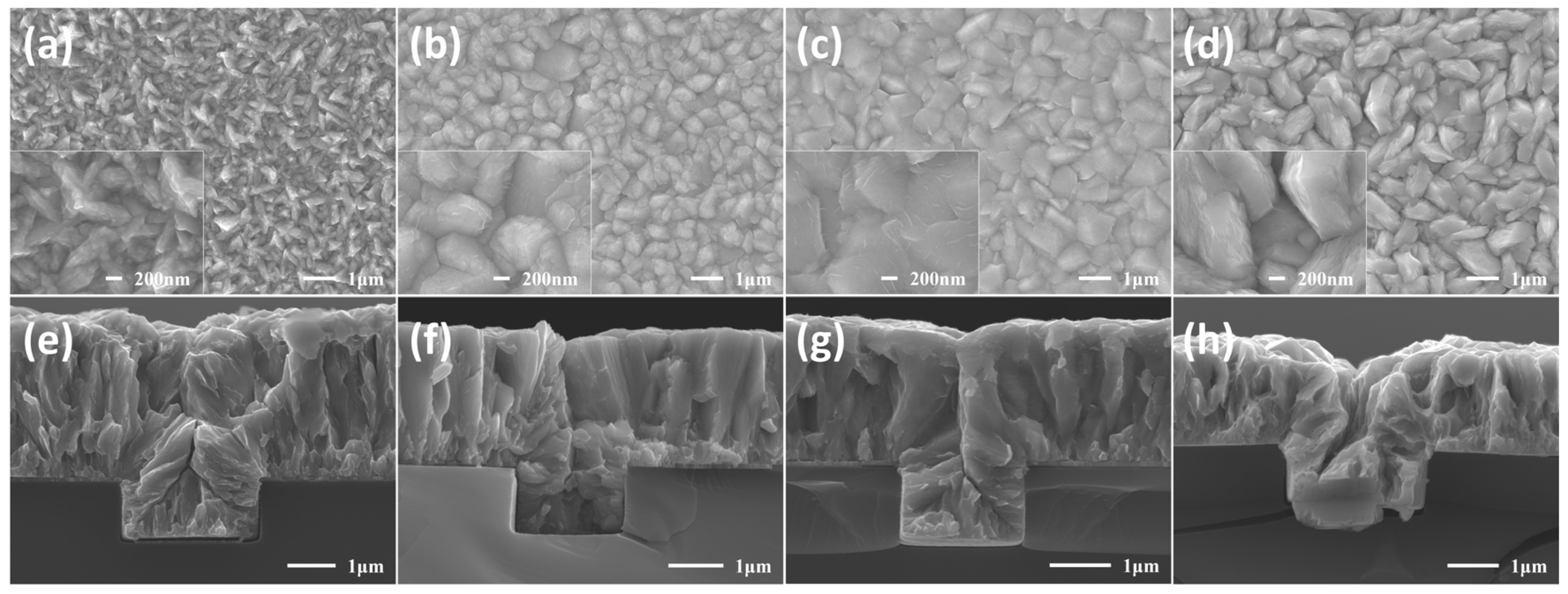
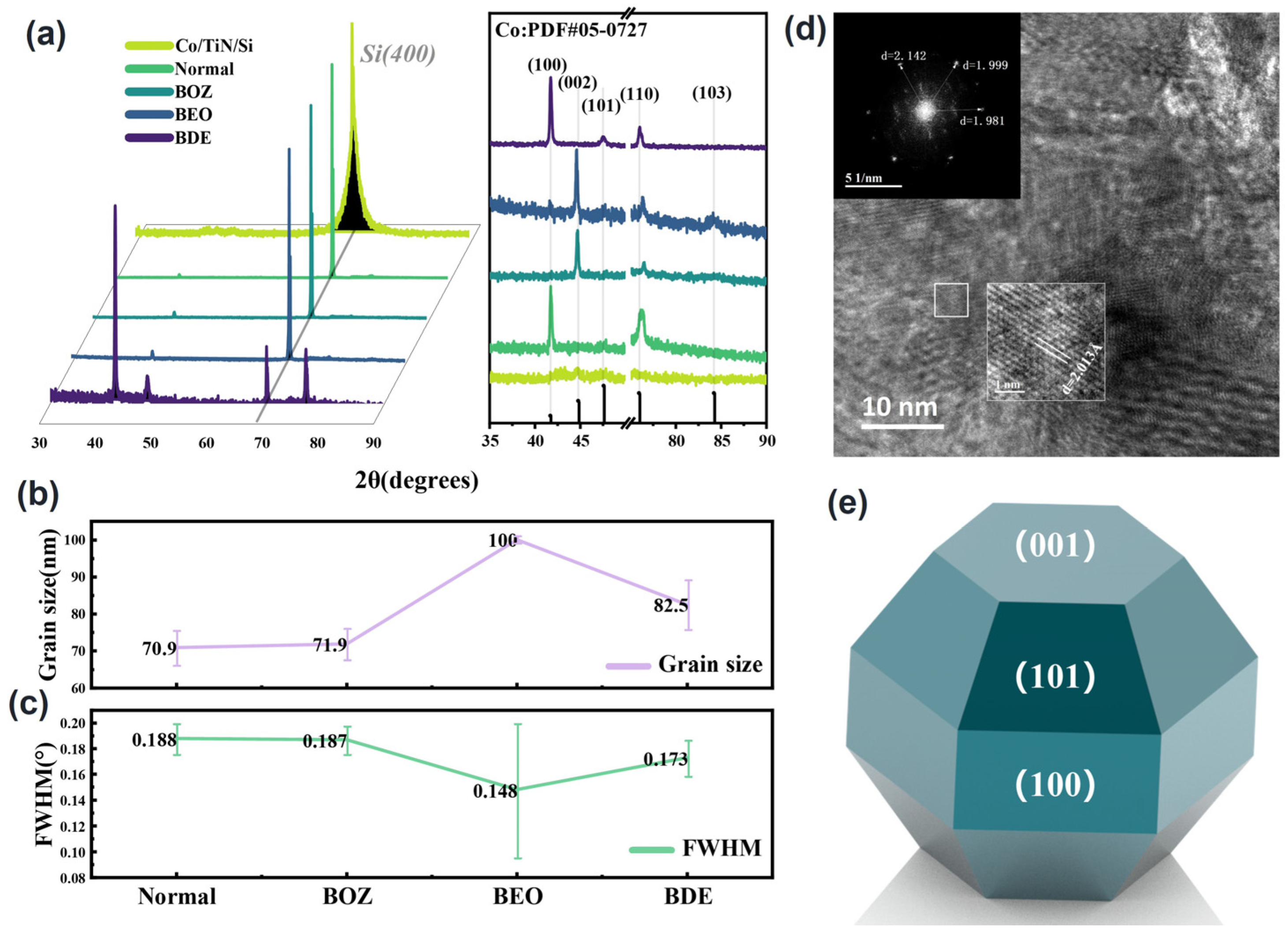
3.3. Morphology and Crystalline Structure Analysis
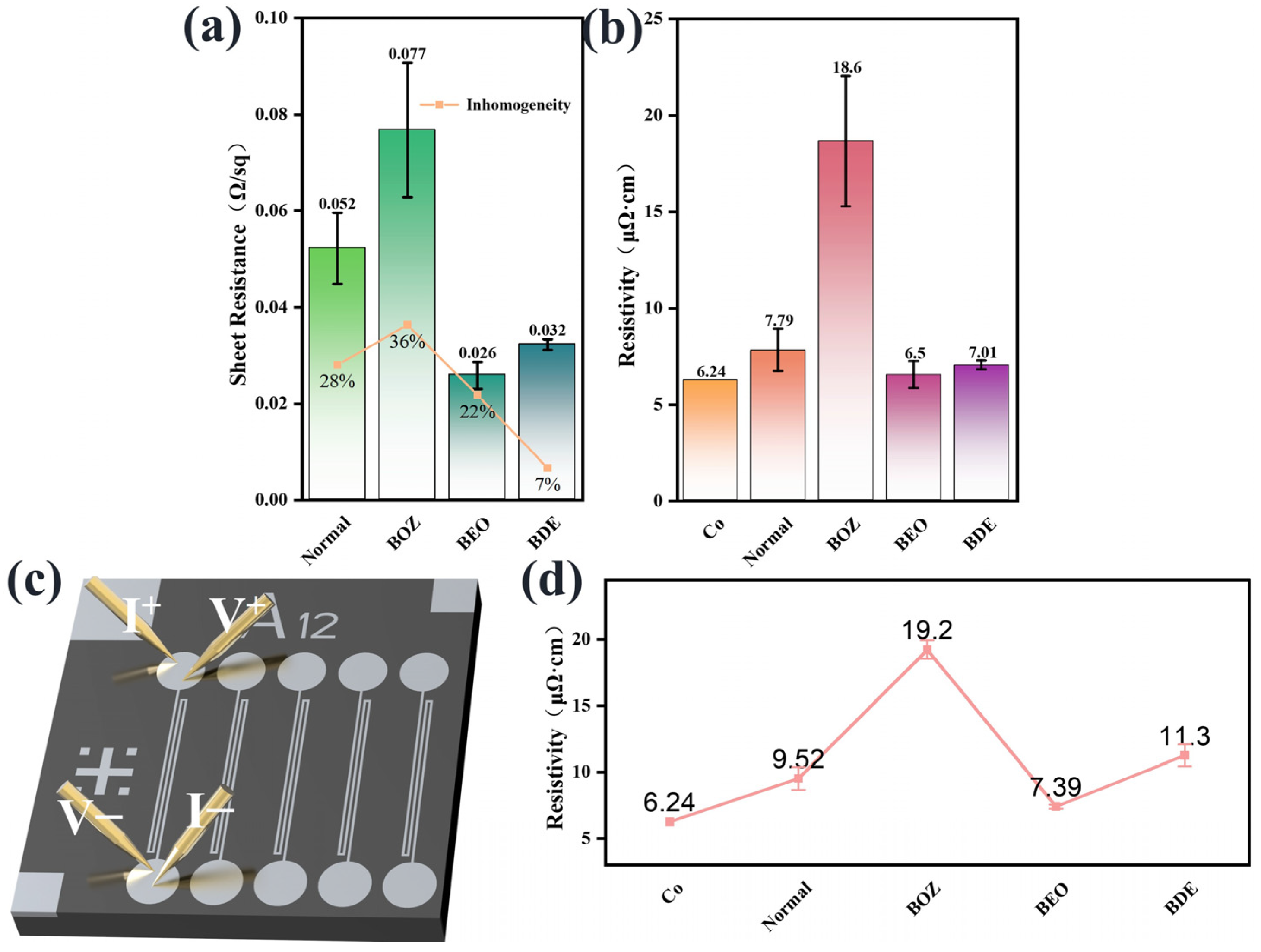
3.4. Electrical Performance Analysis
3.5. Selective Growth of Cobalt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon, A.; van der Straten, O.; Lanzillo, N.A.; Yang, C.-C.; Nogami, T.; Edelstein, D.C. Role of High Aspect-Ratio Thin-Film Metal Deposition in Cu Back-End-of-Line Technology. J. Vac. Sci. Technol. A 2020, 38, 053402. [Google Scholar] [CrossRef]
- Edelstein, D.; Uzoh, C.; Cabral, C.; DeHaven, P.; Buchwalter, P.; Simon, A.; Cooney, E.; Malhotra, S.; Klaus, D.; Rathore, H.; et al. A High Performance Liner for Copper Damascene Interconnects. In Proceedings of the IEEE 2001 International Interconnect Technology Conference (Cat. No.01EX461), San Francisco, CA, USA, 4–6 June 2001; pp. 9–11. [Google Scholar]
- Kuo, C.-Y.; Zhu, J.-H.; Chiu, Y.-P.; Ni, I.-C.; Chen, M.-H.; Wu, Y.-R.; Wu, C.-I. Graphene-All-Around Cobalt Interconnect with a Back-End-of-Line Compatible Process. Nano Lett. 2024, 24, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Xie, S.; Lu, X. The Role of Diethanolamine on Chemical Mechanical Polishing in Alkaline Glycine-Based Slurries for Cobalt Interconnects. ECS J. Solid State Sci. Technol. 2023, 12, 44006. [Google Scholar] [CrossRef]
- Lanzillo, N.A.; Chu, A.; Bhosale, P.; Dechene, D. Power Delivery Design, Signal Routing, and Performance of On-Chip Cobalt Interconnects in Advanced Technology Nodes. IEEE Trans. Very Large Scale Integr. VLSI Syst. 2022, 30, 60–67. [Google Scholar] [CrossRef]
- Wang, J.; Ke, D.; Tian, F.; Xiong, L.; Chen, H.; Li, M. Co Contamination of Si: Plating & Aging. Heliyon 2024, 10, e32530. [Google Scholar] [CrossRef]
- Mont, F.W.; Zhang, X.; Wang, W.; Kelly, J.J.; Standaert, T.E.; Quon, R.; Ryan, E.T. Cobalt Interconnect on Same Copper Barrier Process Integration at the 7nm Node. In Proceedings of the 2017 IEEE International Interconnect Technology Conference (IITC), Hsinchu, Taiwan, 16–18 May 2017; IEEE: HsinChu, Taiwan, 2017. [Google Scholar]
- Fang, J.-S.; Su, T.-H.; Cheng, Y.-L.; Huang, C.-W.; Chen, G.-S. Influence of Trace Tungsten Contents on Thin-Film Properties and Electromigration Behaviors of Electroless-Deposited Cobalt Interconnect Lines. Mater. Res. Bull. 2025, 186, 113343. [Google Scholar] [CrossRef]
- Fang, J.-S.; Lee, W.; Cheng, Y.-L.; Lin, C.-I.; Chen, G.-S. The Encaging of Cobalt Interconnect Lines with an Ordered Amino-Based Self-Assembled Monolayer for Electromigration Mitigation Using an All-Wet Electroless Process. Thin Solid Films 2025, 815, 140644. [Google Scholar] [CrossRef]
- Kim, C.; Kang, G.; Jung, Y.; Kim, J.-Y.; Lee, G.-B.; Hong, D.; Lee, Y.; Hwang, S.-G.; Jung, I.-H.; Joo, Y.-C. Robust Co Alloy Design for Co Interconnects Using a Self-Forming Barrier Layer. Sci. Rep. 2022, 12, 12291. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, T.; Younas, R.; Hinkle, C.L. Materials and Device Strategies for Nanoelectronic 3D Heterogeneous Integration. In Proceedings of the 2021 International Conference on Simulation of Semiconductor Processes and Devices (SISPAD), Dallas, TX, USA, 27–29 September 2021; pp. 163–166. [Google Scholar]
- Ichou, H.; Arrousse, N.; Berdimurodov, E.; Aliev, N. Exploring the Advancements in Physical Vapor Deposition Coating: A Review. J. Bio Tribo Corros. 2023, 10, 3. [Google Scholar] [CrossRef]
- He, Y.; Mao, S.; Xu, J.; Sun, X.; Gao, J.; Liu, W.; Liu, J.; Chen, X.; Li, J.; Wang, X.; et al. Effect of Carbon on the Formation of Cobalt Silicide and Thermal Stability for DRAM Application: A Comparative Study on PVD and CVD Methods. IEEE Trans. Electron Devices 2025, 72, 653–658. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Pugh, T.; Johnson, A.L.; Kingsley, A.J.; Richards, S.P. Cobalt(I) Olefin Complexes: Precursors for Metal–Organic Chemical Vapor Deposition of High Purity Cobalt Metal Thin Films. Inorg. Chem. 2016, 55, 7141–7151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Wang, S.; Lu, X. The Role of Dipotassium Ethylenediaminetetraacetic Acid and Potassium Oleate on Chemical Mechanical Planarization Relevant to Heterogeneous Materials of Cobalt Interconnects. Mater. Sci. Semicond. Process. 2023, 160, 107410. [Google Scholar] [CrossRef]
- Franz, M.; Jäckel, L.; Hu, X.; Kaßner, L.; Thurm, C.; Rittrich, D.; Helke, C.; Schuster, J.; Daniel, M.; Stahr, F.; et al. Low-Temperature ALD of Metallic Cobalt Using the CoCOhept Precursor: Simulation-Assisted Process Development for Deposition on Temperature Sensitive 3D-Structures. JVST A 2025, 43, 22412. [Google Scholar] [CrossRef]
- Donnecke, S.; Paul, M.; Williams, P.J.H.; Chan, S.; Tse, V.; Sachdeva, J.; Oliver, A.G.; McIndoe, J.S.; Paci, I. Mechanistic Study of the Atomic Layer Deposition of Cobalt: A Combined Mass Spectrometric and Computational Approach. Phys. Chem. Chem. Phys. 2024, 26, 14448–14455. [Google Scholar] [CrossRef]
- Breeden, M.; Wang, V.; Spiegelman, J.; Anurag, A.; Wolf, S.F.; Moser, D.; Kanjolia, R.K.; Moinpour, M.; Woodruff, J.; Nemani, S.; et al. Proximity Effects of the Selective Atomic Layer Deposition of Cobalt on the Nanoscale: Implications for Interconnects. ACS Appl. Nano Mater. 2021, 4, 8447–8454. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, B.; Li, L.; Wang, C. Edge Electrodeposition Effect of Cobalt Under an External Magnetic Field. J. Electroanal. Chem. 2020, 865, 114143. [Google Scholar] [CrossRef]
- Pan, B.; Yao, Y.; Peng, L.; Zhang, Q.; Yang, Y. Ultrasound-Assisted Pulse Electrodeposition of Cobalt Films. Mater. Chem. Phys. 2020, 241, 122395. [Google Scholar] [CrossRef]
- Jin, L.; Lv, Y.-J.; Ran, T.-T.; He, D.; Yang, F.-Z. Molecular Structure with Adsorption Behavior of Butynediol and Its Effects on Cobalt Electrodeposition. Mater. Today Commun. 2025, 45, 112225. [Google Scholar] [CrossRef]
- Brogan, L.J.; Liu, Y.; Huie, M.M.; Reid, J.D.; Kelly, J.; Shobha, H.k.; Huang, H.; Motoyama, K.; Hu, C. Improved Copper Damascene Wires Using Direct Plate on Cobalt Process. ECS Meet. Abstr. 2019, MA2019-01, 1055. [Google Scholar] [CrossRef]
- Proust, M.; Judong, F.; Gilet, J.M.; Liauzu, L.; Madar, R. CVD and PVD Copper Integration for Dual Damascene Metallization in a 0.18 Μm Process. Microelectron. Eng. 2001, 55, 269–275. [Google Scholar] [CrossRef]
- Vorobyova, M.; Biffoli, F.; Giurlani, W.; Martinuzzi, S.M.; Linser, M.; Caneschi, A.; Innocenti, M. PVD for Decorative Applications: A Review. Materials 2023, 16, 4919. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shao, Y.-H.; Ni, Z.-H.; Hu, C.-F.; Qu, X.-P. Low-Temperature Copper–Copper Quasi-Direct Bonding with Cobalt Passivation Layer. AIP Adv. 2022, 12, 115101. [Google Scholar] [CrossRef]
- Jeong, B.H.; Kim, D.W.; Lee, S.Y.; Kim, D.S.; Lee, S.H.; Lee, S.H.; Uematsu, M.; Kokaze, Y.; Taura, Y.; Harada, M.; et al. Enhancing Cu Interconnect Reflow in Back-End-of-Line Metal Wiring with Ultrathin Co Liners. Jpn. J. Appl. Phys. 2025, 64, 11002. [Google Scholar] [CrossRef]
- Zanders, D.; Liu, J.; Obenlüneschloß, J.; Bock, C.; Rogalla, D.; Mai, L.; Nolan, M.; Barry, S.T.; Devi, A. Cobalt Metal ALD: Understanding the Mechanism and Role of Zinc Alkyl Precursors as Reductants for Low-Resistivity Co Thin Films. Chem. Mater. 2021, 33, 5045–5057. [Google Scholar] [CrossRef]
- Wei, C.-C.; Chou, E.; Shih, S.; Lin, S.-M. Bottom-up Filling of Damascene Trenches with Cobalt By Electroplating Process. Meet. Abstr. 2015, MA2015-02, 949. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, J.; Wang, L.; Han, H.; Ma, Y.; Xin, B.; Wang, Z. Acceleration Mechanism of Triethanolamine in Electroless Bath for Pure Cobalt Deposition. J. Electrochem. Soc. 2023, 170, 112503. [Google Scholar] [CrossRef]
- Guo, L.; Li, S.; He, Z.; Fu, Y.; Qiu, F.; Liu, R.; Yang, G. Electroplated Copper Additives for Advanced Packaging: A Review. ACS Omega 2024, 9, 20637–20647. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, P.; Chen, P.; Hang, T.; Li, M.; Wu, Y. Super-Flat and Uni-Oriented Cobalt Film Electrodeposited by Modulating the Crystal Nucleation and Growth Behavior. Appl. Surf. Sci. 2024, 645, 158795. [Google Scholar] [CrossRef]
- Rigsby, M.A.; Spurlin, T.A.; Reid, J.D. Alternative Metals for Advanced Interconnects: Cobalt and Beyond. Meet. Abstr. 2021, MA2021-01, 936. [Google Scholar] [CrossRef]
- Josell, D.; Wheeler, D.; Moffat, T.P. Modeling Extreme Bottom-Up Filling of Through Silicon Vias. J. Electrochem. Soc. 2012, 159, D570. [Google Scholar] [CrossRef]
- Yang, L.; Radisic, A.; Deconinck, J.; Vereecken, P.M. Modeling the Bottom-Up Filling of Through-Silicon Vias through Suppressor Adsorption/Desorption Mechanism. J. Electrochem. Soc. 2013, 160, D3051. [Google Scholar] [CrossRef]
- Josell, D.; Moffat, T.P. Superconformal Copper Deposition in Through Silicon Vias by Suppression-Breakdown. J. Electrochem. Soc. 2018, 165, D23. [Google Scholar] [CrossRef]
- Josell, D.; Moffat, T.P. Extreme Bottom-Up Filling of through Silicon Vias and Damascene Trenches with Gold in a Sulfite Electrolyte. J. Electrochem. Soc. 2013, 160, D3035. [Google Scholar] [CrossRef]
- Josell, D.; Moffat, T.P. Superconformal Bottom-Up Nickel Deposition in High Aspect Ratio through Silicon Vias. J. Electrochem. Soc. 2016, 163, D322. [Google Scholar]
- Josell, D.; Silva, M.; Moffat, T.P. Superconformal Bottom-Up Cobalt Deposition in High Aspect Ratio Through Silicon Vias. ECS Trans. 2016, 75, 25–30. [Google Scholar] [CrossRef]
- Josell, D.; Moffat, T.P. Bottom-Up Electrodeposition of Zinc in through Silicon Vias. J. Electrochem. Soc. 2015, 162, D129. [Google Scholar] [CrossRef]
- Rigsby, M.; Brogan, L.; Doubina, N.; Spurlin, T.; Zhou, J.; Reid, J. Superconformal Cobalt Fill Through the Use of Sacrificial Oxidants. Meet. Abstr. 2017, MA2017-02, 931. [Google Scholar] [CrossRef]
- Rigsby, M.A.; Brogan, L.J.; Doubina, N.V.; Liu, Y.; Opocensky, E.C.; Spurlin, T.A.; Zhou, J.; Reid, J.D. The Critical Role of pH Gradient Formation in Driving Superconformal Cobalt Deposition. J. Electrochem. Soc. 2019, 166, D3167–D3174. [Google Scholar] [CrossRef]
- Wu, J.; Wafula, F.; Branagan, S.; Suzuki, H.; Van Eisden, J. Mechanism of Cobalt Bottom-Up Filling for Advanced Node Interconnect Metallization. J. Electrochem. Soc. 2019, 166, D3136–D3141. [Google Scholar] [CrossRef]
- Kiruba, M. Butynediol’s Role Beyond Brightening Additive During Electrodeposition of Cobalt. J. Electrochem. Soc. 2022, 169, 032507. [Google Scholar] [CrossRef]
- Kiruba, M.; Jeyabharathi, C. Discerning the Oscillatory Electrochemical Response During Electrodeposition of Cobalt in the Presence of But-2-Yne-1,4-Diol. J. Solid State Electrochem. 2020, 24, 2997–3002. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, S.; Li, F.; Zhang, M.; Ren, X.; Li, M. Tailoring Crystalline Orientation of Electrodeposited Cobalt by Alkynol Additives. Electrochim. Acta 2024, 497, 144593. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, Q.; Liu, Z.; Yang, Y. Influence of Butynediol and Tetrabutylammonium Bromide on the Morphology and Structure of Electrodeposited Cobalt in the Presence of Saccharin. Mater. Chem. Phys. 2019, 228, 37–44. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Borji, F.; Pour, A.N.; Karimi, J.; Izadyar, M.; Keyvanloo, Z.; Hashemian, M. The Molecular Adsorption of Carbon Monoxide on Cobalt Surfaces: A Dft Study. Prog. React. Kinet. Mech. 2017, 42, 89–98. [Google Scholar] [CrossRef]
- Bai, X.; Duan, Z.; Nan, B.; Wang, L.; Tang, T.; Guan, J. Unveiling the Active Sites of Ultrathin Co-Fe Layered Double Hydroxides for the Oxygen Evolution Reaction. Chin. J. Catal. 2022, 43, 2240–2248. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Q.; Wei, S.; Guo, X.; Chimtali, P.J.; Xu, W.; Chen, S.; Cao, Y.; Zhang, P.; Zhu, K.; et al. Anion Additive Integrated Electric Double Layer and Solvation Shell for Aqueous Zinc Ion Battery. Small Methods 2024, 8, 2301115. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Garcia, E.M. Electrochemical Recycling of Cobalt from Cathodes of Spent Lithium-Ion Batteries. J. Power Sources 2007, 171, 953–959. [Google Scholar] [CrossRef]
- Hamulić, D.; Milošev, I.; Lützenkirchen-Hecht, D. The Effect of the Deposition Conditions on the Structure, Composition and Morphology of Electrodeposited Cobalt Materials. Thin Solid Films 2018, 667, 11–20. [Google Scholar] [CrossRef]
- Kang, J.; Sung, M.; Byun, J.; Kwon, O.J.; Kim, J.J. Proton Sensitive Additive for Cobalt Electrodeposition. J. Electrochem. Soc. 2020, 167, 122510. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Shi, F.-N.; Xu, G.; Qi, Y.; Li, D.; Zhang, Z.; Zhang, Y.; You, H. Synthesis of Hierarchical Cobalt Dendrites Based on Nanoflake Self-Assembly and Their Microwave Absorption Properties. RSC Adv. 2016, 6, 40844–40853. [Google Scholar] [CrossRef]
- Doubina, N.V.; Spurlin, T.A.; Opocensky, E.C.; Reid, J.D. The Effect of Thermal Annealing on Cobalt Film Properties and Grain Structure. MRS Adv. 2020, 5, 1919–1927. [Google Scholar] [CrossRef]
- Bron, M.; Holze, R. Spectroelectrochemical Investigation of the Adsorption and Oxidation of Unsaturated C4-Alcohols. Surf. Sci. 2000, 457, 178–184. [Google Scholar] [CrossRef]


| Additive | HOMO (eV) | LUMO (eV) | ΔE (eV) |
|---|---|---|---|
| BOZ | −7.341 | 0.066 | 7.407 |
| BEO | −6.874 | 0.825 | 7.698 |
| BDE | −7.319 | 0.872 | 8.192 |
| I | I/I0 | RTC(100) | I | I/I0 | RTC(002) | I | I/I0 | RTC(101) | |
|---|---|---|---|---|---|---|---|---|---|
| PDF 05-0727 | 20 | 1 | 33% | 60 | 1 | 33% | 100 | 1 | 33% |
| Normal | 843 | 42.1 | 95% | 69 | 1.2 | 3% | 118 | 1.2 | 3% |
| BOZ | 61 | 3.1 | 17% | 867 | 14.5 | 79% | 80 | 0.8 | 4% |
| BEO | 141 | 7.1 | 35% | 719 | 12.0 | 59% | 129 | 1.3 | 6% |
| BDE | 2211 | 110.6 | 97% | 23 | 0.4 | 0% | 274 | 2.7 | 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, Y.; Wu, T.; Duan, Y.; Zhu, L.; Liu, Q.; Wang, Y.; Yu, W. Superconformal Electrodeposition of Cobalt into Micron-Scale Trench with Alkynol Derivatives. Materials 2025, 18, 1747. https://doi.org/10.3390/ma18081747
Xu W, Li Y, Wu T, Duan Y, Zhu L, Liu Q, Wang Y, Yu W. Superconformal Electrodeposition of Cobalt into Micron-Scale Trench with Alkynol Derivatives. Materials. 2025; 18(8):1747. https://doi.org/10.3390/ma18081747
Chicago/Turabian StyleXu, Wei, Yedi Li, Tingjun Wu, Yu Duan, Lei Zhu, Qiang Liu, Yiying Wang, and Wenjie Yu. 2025. "Superconformal Electrodeposition of Cobalt into Micron-Scale Trench with Alkynol Derivatives" Materials 18, no. 8: 1747. https://doi.org/10.3390/ma18081747
APA StyleXu, W., Li, Y., Wu, T., Duan, Y., Zhu, L., Liu, Q., Wang, Y., & Yu, W. (2025). Superconformal Electrodeposition of Cobalt into Micron-Scale Trench with Alkynol Derivatives. Materials, 18(8), 1747. https://doi.org/10.3390/ma18081747




