The Biodegradation of Acrylic-Coated Woven Fabrics by Gordonia alkanivorans S7: A Novel Approach for Sustainable Textile Waste Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Textile Material
2.1.2. Biological Material
2.2. Methods
2.2.1. Biodegradation Experiments
2.2.2. Mass Reduction
2.2.3. Construction Characteristics of Coated Woven Fabric Before and After the Biodegradation Process: An ATR-FTIR Study
3. Results
3.1. pH Analysis
3.2. Microbial Growth
3.3. Emulsifying Activity and Esterase Activity Changes
3.4. Mass Reduction
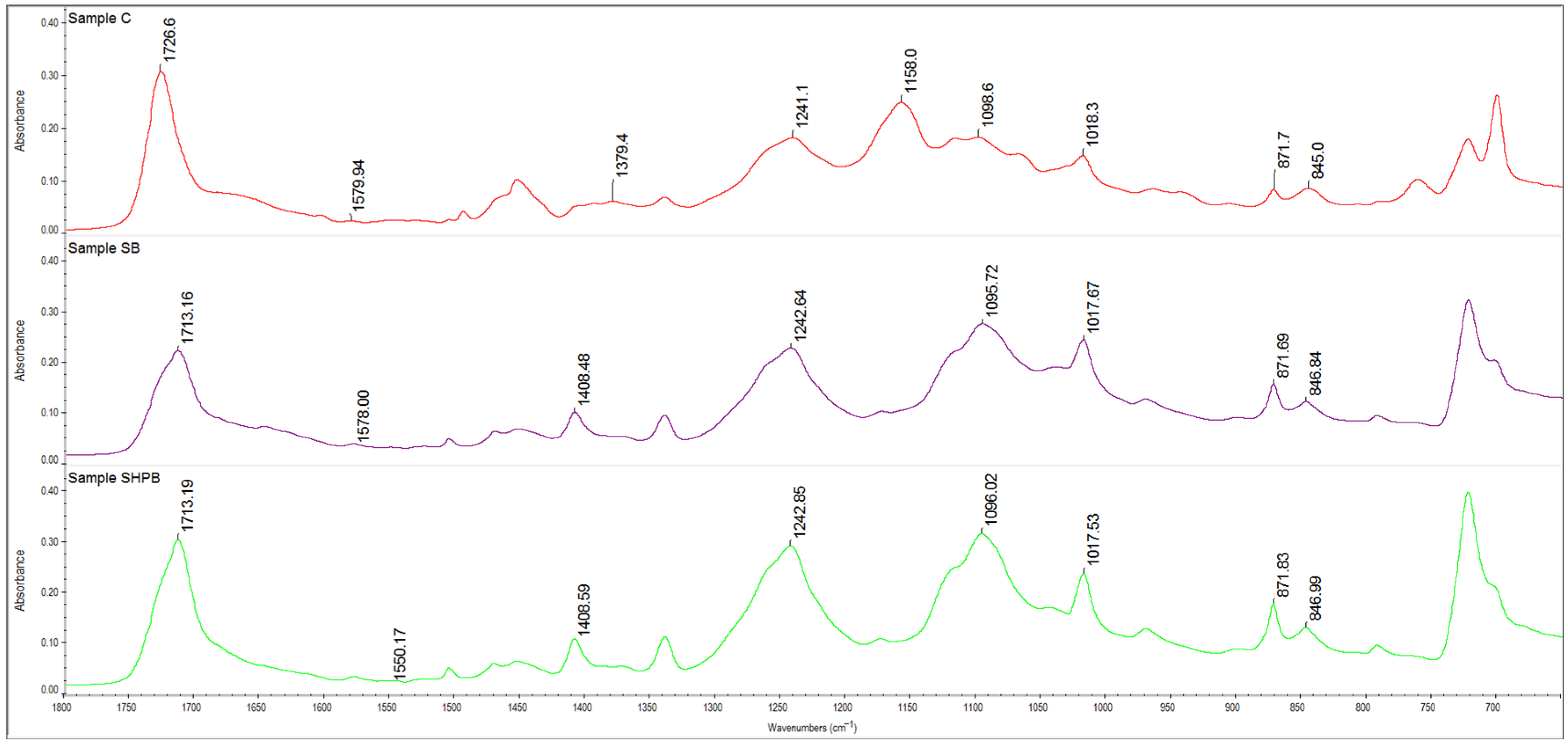
3.5. ATR-FTIR Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, S.; Bhat, G. Environmentally-friendly thermal and acoustic insulation materials from recycled textiles. J. Environ. Manag. 2019, 251, 109536. [Google Scholar] [CrossRef]
- Deckers, J.; Manshoven, S.; Mortensen, L.F. The Role of Bio-Based Textile Fibres in a Circular and Sustainable Textiles System; ETC-CE Report 2023/5; European Environment Agency: Copenhagen, Denmark, 2023. [Google Scholar]
- Mazotto, A.M.; Silva, J.R.; de Brito, L.A.A.; Rocha, N.U.; Soares, A.S. How can microbiology help to improve sustainability in the fashion industry? Environ. Technol. Innov. 2021, 23, 101760. [Google Scholar] [CrossRef]
- Bikash, J.; Bishnu Priya, D.; Khandual, A.; Sanjay, S.; Lingaraj, B. Ecofriendly Processing of Textiles. Mater. Today Proc. 2015, 2, 1776–1791. [Google Scholar]
- Mazibuko, M.; Ndumo, J.; Low, M.; Ming, D.; Harding, K. Investigating the natural degradation of textiles under controllable and uncontrollable environmental conditions. Procedia Manuf. 2019, 35, 719–724. [Google Scholar] [CrossRef]
- Pensupa, N. Recycling of end-of-life clothes. In Sustainable Technologies for Fashion and Textiles; Woodhead Publishing Series in Textiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–309. [Google Scholar]
- Muthu, S.S. End-of-life management of textile products. In Assessing the Environmental Impact of Textiles and the Clothing Supply Chain; Woodhead Publishing: Swaston, UK, 2014; pp. 144–162. [Google Scholar]
- Volmajer Valh, J.; Majcen Le Marechal, A.; Vajnhandl, S.; Jerič, T.; Šimon, E. Water in the Textile Industry. Treatise Water Sci. 2011, 4, 685–706. [Google Scholar]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Goller, C.C.; Venditti, R.A. Aerobic biodegradation in freshwater and marine environments of textile microfibers generated in clothes laundering: Effects of cellulose and polyester-based microfibers on the microbiome. Mar. Pollut. Bull. 2020, 151, 110826. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.J.; Wiggin, K.; Kogler, M.; Deheyn, D.D. Degradation of synthetic and wood-based cellulose fabrics in the marine environment: Comparative assessment of field, aquarium, and bioreactor experiments. Sci. Total Environ. 2021, 791, 148060. [Google Scholar] [CrossRef]
- Gaylarde, C.; Baptista-Neto, J.A.; da Fonseca, E.M. Plastic microfibre pollution: How important is clothes’ laundering? Heliyon 2021, 7, e07105. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Sustainable chemical technologies for textile production. In Sustainable Fibres and Textiles; The Textile Institute Book Series; Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–322. [Google Scholar]
- Mishra, S.; Singh, R.P.; Rath, C.C.; Das, A.P. Synthetic microfibers: Source, transport and their remediation. J. Water Process Eng. 2020, 38, 101612. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/PL/ALL/?uri=LEGISSUM:ev0010 (accessed on 20 July 2021).
- Salerno-Kochan, R. Analysis of the Susceptibility of Synthetic Fibres to Decomposition Processes Occurring in Waste Management Methods. Crakow Rev. Econ. Manag. 2002, 599, 73–84. [Google Scholar]
- Slater, K. Environmental impact of polyester and polyamide textiles. In Polyesters and Polyamides; Woodhead Publishing Series in Textiles; Elsevier: Amsterdam, The Netherlands, 2008; pp. 171–199. [Google Scholar]
- Bassi, A. Biotechnology for the Management of Plastic Wastes. In Current Developments in Biotechnology and Bioengineering; Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2017; pp. 293–310. [Google Scholar]
- Luyt, A.S.; Malik, S.S. Can Biodegradable Plastics Solve Plastic Solid Waste Accumulation? In Plastics to Energy, Fuel, Chemicals, and Sustainability Implications; Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–423. [Google Scholar]
- Drelich, A.H.; Plaineld, N.J. Methods of Recovering Waste Fibers. U.S. Patent 3,843,321, 22 October 1974. [Google Scholar]
- Manfredia, L.B.; Rodrígueza, E.S.; Wladyka-Przybylak, M.; Vázquez, A. Thermal degradation and fire resistance of unsaturated polyester, modified acrylic resins and their composites with natural fibres. Polym. Degrad. Stab. 2006, 91, 255–261. [Google Scholar] [CrossRef]
- Neira, A.; Tarraga, M.; Catalan, R. Degradation of Polyacrylic Acid By Fenton’s Reagent. J. Chil. Chem. Soc. 2007, 52, 1314–1317. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Aiello, G.; Mendes, B.; Urban, V.M.; Campanha, N.H.; Jorge, J.H. Effect of storage in water and thermocycling on hardness and roughness of resin materials for temporary restorations. Mater. Res. 2010, 13, 355–359. [Google Scholar] [CrossRef]
- Pereira, C.J.; Genari, B.; Leitune, V.C.B.; Collares, F.M.; Samuel, S.M.W. Effect of immersion in various disinfectant solutions on the properties of aheat-cured acrylic resin. RGO-Rev. Gaúcha De Odontol. 2019, 67, e20190052. [Google Scholar] [CrossRef]
- Ural, Ç.; Şanal, F.A.; Cengiz, S. Effect of different denture cleansers on surface roughness of denture base materials. J. Adv. Prosthodont. 2016, 8, 333–338. [Google Scholar]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Chaillan, F.F.; Flèche, A.; Burya, E.; Phantavonga, Y.; Grimontb, P.; Saliotc, A.; Oudot, J.J. Identification and biodegradation potential of tropical aerobic hydrocarbon-degrading microorganisms. Res. Microbiol. 2004, 155, 587–595. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Cappitelli, F.; Principi, P.; Sorlini, C. Biodeterioration of modern materials in contemporary collections: Can biotechnology help? Trends Biotechnol. 2006, 24, 350–354. [Google Scholar] [CrossRef]
- Bettencourt, A.F.; Neves, C.B.; de Almeida, M.S.; Pinheiro, L.M.; Oliveira, S.A.; Lopes, L.P.; Castro, M.F. Biodegradation of acrylic based resins: A review. Dent. Mater. 2010, 26, 171–180. [Google Scholar] [CrossRef]
- Cameron, M.D. Cellobiose dehydrogenase-dependent biodegradation of polyacrylate polymers by Phanerochaete chrysosporium. Environ. Sci. Pollut. Res. 2000, 7, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Kolvenbach, B.A.; Helbling, D.E.; Kohler, H.E.; Corvini, P.F. Emerging chemicals and the evolution of biodegradation capacities and pathways in bacteria. Curr. Opin. Biotechnol. 2014, 27, 8–14. [Google Scholar] [CrossRef]
- Stahl, J.D.; Cameron, M.D.; Haselbach, J.; Aust, S.D. Biodegradation of superabsorbent polymers in soil. Environ. Sci. Pollut. Res. Int. 2000, 7, 83–88. [Google Scholar] [CrossRef]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current status on the biodegradability of acrylic polymers: Microorganisms, enzymes and metabolic pathways involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef]
- Kurowski, G.; Vogt, O.; Ogonowski, J. Paint-degrading microorganisms. Tech. Trans. 2017, 12, 81–92. [Google Scholar]
- Li, J.; Luan, Z.; Yu, L.; Ji, Z. Pretreatment of acrylic fiber manufacturing wastewater by the Fenton process. Desalination 2012, 284, 62–65. [Google Scholar] [CrossRef]
- Kwapisz, E.; Wszelaka, J.; Marchut-Mikołajczyk, O.; Bielecki, S. The effect of nitrate and ammonium ions on kinetics of diesel oil degradation by Gordonia alkanivorans S7. Int. Biodeterior. Biodegrad. 2008, 61, 214–222. [Google Scholar] [CrossRef]
- Marchut-Mikołajczyk, O.; Kwapisz, E.; Wieczorek, D.; Antczak, T. Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int. Biodeterior. Biodegrad. 2015, 104, 142–148. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Margesin, R.; Feller, G.; Hämmerle, M.; Stegner, U.; Schinner, F. A colorimetric method for the determination of lipase activity in soil. Biotechnol. Lett. 2002, 24, 27–33. [Google Scholar] [CrossRef]
- PN-EN ISO 2286-2:2016-11; Rubber- or plastics-coated fabrics—Determination of roll characteristics Part 2: Methods for determination of total mass per unit area, mass per unit area of coating and mass per unit area of substrate. The Polish Committee for Standardization: Warsaw, Poland, 2016.
- Sable, S.; Ahuja, S.; Bhunia, H. Biodegradation kinetic modeling of acrylic acid-grafted polypropylene during thermophilic phase of composting. Iran. Polym. J. 2020, 29, 735–747. [Google Scholar] [CrossRef]
- Grabowska, B.; Bulwan, M.; Zapotoczny, S.; Grabowski, G. Biodegradation of new polymer foundry binders composition of poly(acrylic acid)/dextrin. Polimery 2012, 57, 529–534. [Google Scholar] [CrossRef]
- Nahurira, R.; Ren, L.; Song, J.; Jia, Y.; Wang, J.; Fan, S.; Wang, H.; Yan, Y. Degradation of Di(2-Ethylhexyl) Phthalate by a Novel Gordonia alkanivorans Strain YC-RL2. Curr. Microbiol. 2017, 74, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Mahaseth, T.; Kuzminov, A. Potentiation of hydrogen peroxide toxicity: From catalase inhibition to stable DNA-iron complexes. Mutat. Res. Rev. 2017, 773, 274–281. [Google Scholar] [CrossRef]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Salman, I.A.S.; Shukur, B.N. The Influence of Biosurfactant on Hardness and Roughness of Heat Cured Acrylic Resin. Indian J. Public Health Res. Dev. 2020, 11, 423–428. [Google Scholar]
- Pavarina, A.C.; Vergani, C.E.; Machado, L.A.; Giampaolo, E.T.; Teraoka, M.T. Effects of disinfectants solutions on the hardness of acrylic resin denture teeth. J. Oral. Rehabil. 2003, 30, 749–752. [Google Scholar] [CrossRef]
- Sein, T.T.; Spurio, R.; Cecchini, C.; Cresci, A. Screening for microbial strains degrading glass fiber acrylic composite filters. Int. Biodeterior. Biodegrad. 2009, 63, 901–905. [Google Scholar] [CrossRef]
- Gitalis, R.; Zhou, L.; Marashdeh, M.Q.; Sun, C.; Glogauer, M.; Finer, Y. Human neutrophils degrade methacrylate resin composites and tooth dentin. Acta Biomater. 2019, 88, 325–331. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Y.; Gao, S.; Zhang, Z.; Zhao, H. Biodegradation of Dental Resin-Based Composite—A Potential Factor Affecting the Bonding Effect: A Narrative Review. Biomedicines 2022, 10, 2313. [Google Scholar] [CrossRef] [PubMed]
- Marashdeh, M.Q.; Friedman, S.; Lévesque, C.; Finer, Y. Esterases affect the physical properties of materials used to seal the endodontic space. Dent. Mater. 2019, 35, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Gaytán, I.; Sánchez-Reyes, A.; Burelo, M.; Vargas-Suárez, M.; Liachko, I.; Press, M.; Sullivan, S.; Cruz-Gómez, M.J.; Loza-Tavera, H. Degradation of recalcitrant polyurethane and xenobiotic additives by a selected landfill microbial community and its biodegradative potential revealed by proximity ligation-based metagenomic analysis. Front. Microbiol. 2020, 10, 2986. [Google Scholar] [CrossRef]
- Kumagai, P.S.; Gutierrez, R.F.; Lopes, J.L.S.; Martins, J.M.; Jameson, D.M.; Castro, A.M.; Martins, L.F.; DeMarco, R.; Bossolan, N.R.; Wallace, B.A.; et al. Characterization of esterase activity from an Acetomicrobium hydrogeniformans enzyme with high structural stability in extreme conditions. Extremophiles 2018, 22, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nishiyama, T.; Sato, E.; Horibe, H. Degradation of Poly(acrylic acid) in Aqueous Solution by Using O3 Microbubble. J. Photopolym. Sci. Technol. 2018, 31, 409–412. [Google Scholar] [CrossRef]
- Bankeeree, W.; Samathayanon, C.; Prasongsuk, S.; Lotrakul, P.; Kiatkamjornwong, S. Rapid Degradation of Superabsorbent Poly (Potassium Acrylate) and its Acrylamide Copolymer Via Thermo-Oxidation by Hydrogen Peroxide. J. Polym. Environ. 2021, 29, 3964–3976. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Chaudhari, S.B. Study on structural, mechanical and functional properties of polyester silica nanocomposite fabric. Int. J. Pure Appl. Sci. 2014, 21, 43–52. [Google Scholar]
- Ghezal, I.; Jaouachi, B.; Sakli, F. Investigating the Performances of a Coated Plain Weave Fabric Designed for Producing Protective Gowns. Adv. Mater. Sci. Eng. 2021, 2021, 4260411. [Google Scholar] [CrossRef]
- Fabris, H.J.; Knauss, W.G. Synthetic Polymer Adhesives, Comprehensive Polymer Science and Supplements; Elsevier Science: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Nejman, A.; Kamińska, I.; Giesz, P.; Cieślak, M. Thermal Stability of Polyester Fabric with Polyacrylic Coatings. Fibres Text. East. Eur. 2015, 4, 73–82. [Google Scholar]

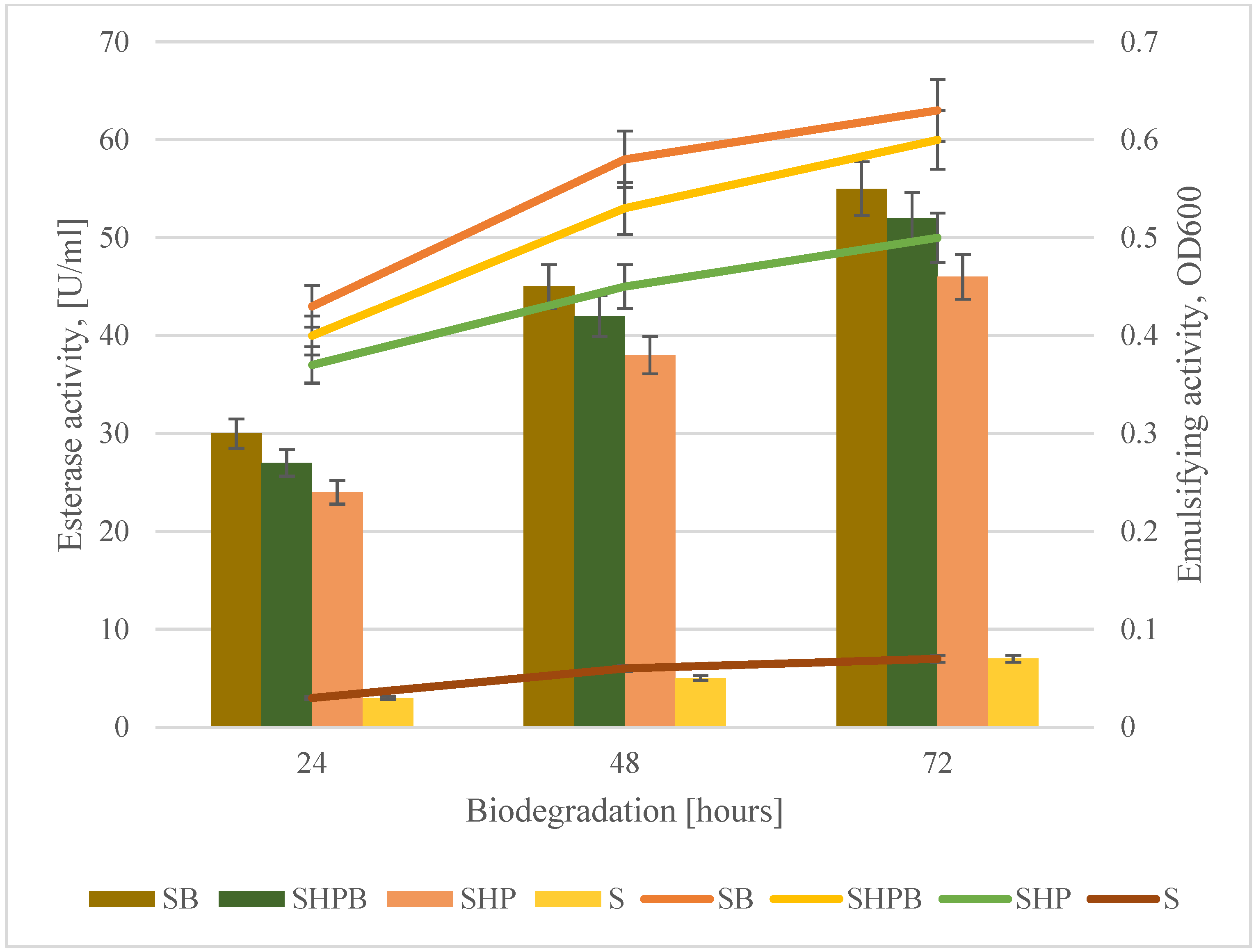

| Sample | Protein Concentration [µg/cm3] | ||
|---|---|---|---|
| Biodegradation Time [h] | |||
| 24 | 48 | 72 | |
| SB | 0.450 ± 0.013 | 0.520 ± 0.016 | 0.720 ± 0.002 |
| SHPB | 0.260 ± 0.008 | 0.320 ± 0.009 | 0.430 ± 0.013 |
| SHP | 0.012 (1) | 0.021 (1) | 0.021 (1) |
| S | 0.015 (1) | 0.018 (1) | 0.020 (1) |
| Treatment Variant | Mass Reduction After Treatment [wt%] |
|---|---|
| SB | 6.90 ± 0.02 |
| SHPB | 5.81 ± 0.02 |
| SHP | 0.0 |
| S | 0.0 |
| Fuctional Group | C (Initial Coated Woven Fabric) | SB | SHPB |
|---|---|---|---|
| Wavenumber [cm−1] | |||
| -OH of carobxyl group | 3350 | 3390 | 3233 |
| -CH2 | 2957, 2931 | 2958, 2959 | 2959, 2909 |
| C=O carboxyl from ester group | 1726 | 1713 | 1713 |
| C=C benzene ring | 1579, 722 | 1578, 722 | 1550, 722 |
| Aromatic ring | 1379 | 1408 | 1408 |
| C-O | 1241 | 1242 | 1242 |
| C-O | 1158 | - | - |
| C-O-C | 1098 | 1095 | 1096 |
| C-OH | 1018 | 1017 | 1017 |
| Five hydrogen atoms substituted in an aromatic ring | 871, 845 | 871, 846 | 871, 847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struszczyk, M.H.; Olejnik, M.; Gutowska, A.; Chmal-Fudali, E.; Marchut-Mikołajczyk, O.; Struszczyk-Świta, K.; Drożdżyński, P. The Biodegradation of Acrylic-Coated Woven Fabrics by Gordonia alkanivorans S7: A Novel Approach for Sustainable Textile Waste Management. Materials 2025, 18, 1745. https://doi.org/10.3390/ma18081745
Struszczyk MH, Olejnik M, Gutowska A, Chmal-Fudali E, Marchut-Mikołajczyk O, Struszczyk-Świta K, Drożdżyński P. The Biodegradation of Acrylic-Coated Woven Fabrics by Gordonia alkanivorans S7: A Novel Approach for Sustainable Textile Waste Management. Materials. 2025; 18(8):1745. https://doi.org/10.3390/ma18081745
Chicago/Turabian StyleStruszczyk, Marcin Henryk, Magdalena Olejnik, Agnieszka Gutowska, Edyta Chmal-Fudali, Olga Marchut-Mikołajczyk, Katarzyna Struszczyk-Świta, and Piotr Drożdżyński. 2025. "The Biodegradation of Acrylic-Coated Woven Fabrics by Gordonia alkanivorans S7: A Novel Approach for Sustainable Textile Waste Management" Materials 18, no. 8: 1745. https://doi.org/10.3390/ma18081745
APA StyleStruszczyk, M. H., Olejnik, M., Gutowska, A., Chmal-Fudali, E., Marchut-Mikołajczyk, O., Struszczyk-Świta, K., & Drożdżyński, P. (2025). The Biodegradation of Acrylic-Coated Woven Fabrics by Gordonia alkanivorans S7: A Novel Approach for Sustainable Textile Waste Management. Materials, 18(8), 1745. https://doi.org/10.3390/ma18081745





