Comprehensive Characterization of the Molecular Structure and Properties of Pitch-like Products from Coal Dissolution at Mild Temperature Using Heavy Solvents of Coal and Petroleum Origin
Abstract
1. Introduction
2. Material and Methods
2.1. Coal and Solvents Used
2.2. Reactor Unit and Dissolution Procedure
2.3. Analytical Techniques
3. Results
3.1. Characterization of Coal and Solvents
3.2. Coal Dissolution
3.2.1. The Composition of the Pitch-like Products
3.2.2. Molecular Structure of the Pitch-like Products
4. Discussion
5. Conclusions
- The liquid-phase reaction of bituminous coal with commercially available heavy hydrocarbon fractions of coal- and petroleum origin at moderate temperature of 380 °C and autogenous pressure of 1.4 to 2.5 MPa with no catalyst and hydrogen resulted in deep and selective coal dissolution into quinoline-soluble substances (to more than 80%), the yield of gaseous products being no more than 0.5%. The binary blend of highly aromatic coal tar and aliphatic petroleum-derived heavy gasoil fraction exhibited some synergistic effect resulting in improved coal dissolution.
- The resultant products represented typical pitch-like matter with the softening points of 82 to 90 °C. Comprehensive characterization by FTIR, 1H NMR, 13C NMR, XPS spectroscopy and liquid phase chromatography showed the products to consist of predominantly polycondensed aromatics, which structural parameters strongly depended on the solvent type.
- The product obtained using coal tar as solvent had highly developed aromatic structure, its polycondensed nuclei consisted of predominantly protonated and pericondensed cycles sparsely substituted by CH3 and occasionally CH2 groups. The product obtained using petroleum-derived solvent was less aromatic, its aromatic nuclei contained protonated and highly alkylated catacondensed chains. The intermediate structural parameters were characteristic of the product obtained using binary solvent.
- All the pitch-like products obtained had a reduced BaP concentration, the smallest concentration showing the product obtained using petroleum-derived solvent (40 times less than in typical coal-tar pitch). An increase in coal dissolution duration further reduced BaP concentration.
- The product obtained using petroleum-derived solvent was prone to autogenous surface oxidation by the atmospheric oxygen at room temperature. The surface of the product obtained using coal tar was much less oxidized, just like a commercial coal-tar pitch sample.
- In terms of molecular composition, the pitch-like products obtained by low-temperature dissolution of coal can serve as polyaromatic feedstock with a reduced carcinogenicity for the production of valuable carbon materials. By selecting solvents, it is possible to optimize the molecular-structural characteristics of the dissolved products in order to obtain favorable feedstock.
- The CT product with highly developed polycondensed aromatic structure can serve as a preferred feedstock for the production of needle coke. The GO product, which is characterized by catacondensed and highly substituted aromatic units, can serve as a feedstock for carbon fiber. Its increased oxidation ability contributes to production of pitch with controlled viscoelastic properties for fiber spinning.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Predel, H. Petroleum Coke. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2014; pp. 1–21. [Google Scholar] [CrossRef]
- Steppich, D. Graphite Electrodes for Electric Arc Furnaces. In Industrial Carbon and Graphite Materials, Volume I; Jäger, H., Frohs, W., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 281–319. [Google Scholar] [CrossRef]
- Wachtler, M.; Öttinger, O.; Schweiss, R. Carbon and Graphite for Electrochemical Power Sources. In Industrial Carbon and Graphite Materials, Volume I; Jäger, H., Frohs, W., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 379–455. [Google Scholar] [CrossRef]
- Global Needle Coke Industry Trends Analysis Report 2024, Forecast to 2032 (Broken Down by Type, End User, Regional Analysis, and Competitive Landscape). Available online: https://www.marketgrowthreports.com/global-needle-coke-industry-25826630 (accessed on 27 January 2025).
- Needle Coke Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/needle-coke-market (accessed on 27 January 2025).
- Gabdulkhakov, R.R.; Rudko, V.A.; Pyagay, I.N. Methods for modifying needle coke raw materials by introducing additives of various origin (review). Fuel 2022, 310, 122265. [Google Scholar] [CrossRef]
- Mondal, S.; Yadav, A.; Pandey, V.; Sugumaran, V.; Bagai, R.; Kumar, R.; Pradeep, P.R.; Das, S.K.; Christopher, J.; Kapur, G.S.; et al. Dissecting the cohesiveness among aromatics, saturates and structural features of aromatics towards needle coke generation in DCU from clarified oil by analytical techniques. Fuel 2021, 304, 121459. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, H.; Guo, S.; Lou, B.; Yu, R.; Gong, X.; Li, Z.; Li, M.; Duan, Y.; Yuan, H.; et al. Probing the effect of molecular structure and compositions in extracted oil on the characteristics of needle coke. Fuel 2021, 301, 120984. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Cai, X.; Gou, Q.; Jiang, L.; Chen, K.; Chen, Z.; Jiang, S. Current status and future trends of in situ catalytic upgrading of extra heavy oil. Energies 2023, 16, 4610. [Google Scholar] [CrossRef]
- Pham, D.D.; Nguyen, T.M.; Ho, T.H.; Le, Q.V.; Nguyen, D.L.T. Advancing hydrodesulfurization in heavy oil: Recent developments, challenges, and future prospects. Fuel 2024, 372, 132082. [Google Scholar] [CrossRef]
- Kozlov, A.P.; Cherkasova, T.G.; Frolov, S.V.; Subbotin, S.P.; Solodov, V.S. Innovative coal-tar products at PAO Koks. Coke Chem. 2020, 63, 344–350. [Google Scholar] [CrossRef]
- Stompel, D.Z. Eastern European Coal Tar Market A.D. 2023; International Tar Association: Cocoa Beach, FL, USA, 2023; Available online: https://www.itaorg.com/conf-presentations.php?year=2023 (accessed on 31 March 2025).
- Tiwari, H.P.; Saxena, V.K. Industrial perspective of the cokemaking technologies. In New Trends in Coal Conversion; Suárez-Ruiz, I., Diez, M.A., Rubiera, F., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 203–246. [Google Scholar] [CrossRef]
- Rahman, M.; Pudasainee, D.; Gupta, R. Review on chemical upgrading of coal: Production processes, potential applications and recent developments. Fuel Process. Technol. 2017, 158, 35–56. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kuznetsova, L.I.; Buryukin, F.A.; Marakushina, E.N.; Frizorger, V.K. Methods for the preparation of coal-tar pitch. Solid Fuel Chem. 2015, 49, 213–225. [Google Scholar] [CrossRef]
- Sharma, D.K.; Dhawan, H. Separative refining of coals through solvolytic extraction under milder conditions: A review. Ind. Eng. Chem. Res. 2018, 57, 8361–8380. [Google Scholar] [CrossRef]
- Kolak, J.J.; Burruss, R.C. The use of solvent extractions and solubility theory to discern hydrocarbon associations in coal, with application to the coal–supercritical CO2 system. Org. Geochem. 2014, 73, 56–69. [Google Scholar] [CrossRef]
- Kang, H.; Wang, J.; Chen, F.; Rong, T.; Yu, Y.; Ding, W.; Zuo, H. Investigation on the mechanism of solvothermal extraction of coals by macromolecular models. Fuel 2024, 355, 129547. [Google Scholar] [CrossRef]
- Li, Z.-H.; Fan, X.; Hou, R.-R.; Xu, J.-F.; Zhang, G.-Z.; Zhao, G.-M.; Liu, Z.-Q.; Liang, P. Classification of soluble proportions derived from coals and their correlation with coal type: Conjoint analyses of extraction, thermal dissolution and machine learning. J. Energy Inst. 2025, 119, 102010. [Google Scholar] [CrossRef]
- Yoo, P.; Jung, G.S.; Ryder, M.R.; Vautard, F.; Cakmak, E.; Wi, S.; Weisenberger, M.C.; Lara-Curzio, E.; Mathews, J.P.; Irle, S. Large-scale atomistic model construction of subbituminous and bituminous coals for solvent extraction simulations with reactive molecular dynamics. Carbon 2024, 222, 118939. [Google Scholar] [CrossRef]
- Cheng, X.; Li, G.; Peng, Y.; Song, S.; Shi, X.; Wu, J.; Xie, J.; Zhou, M.; Hu, G. Obtaining needle coke from coal liquefaction residue. Chem. Technol. Fuels Oils 2012, 48, 349–355. [Google Scholar] [CrossRef]
- Shui, H.; Zhou, Y.; Li, H.; Wang, Z.; Lei, Z.; Ren, S.; Pan, C.; Wang, W. Thermal dissolution of Shenfu coal in different solvents. Fuel 2013, 108, 385–390. [Google Scholar] [CrossRef]
- Cao, X.; Yan, J.; Wang, Z.; Lei, Z.; Ren, S.; Kang, S.; Li, Z.; Shui, H. Comparative study on coal blending and coke-making property of two kinds of thermal dissolution soluble fractions from lignite and coking coal. J. Anal. Appl. Pyrolysis 2022, 166, 105585. [Google Scholar] [CrossRef]
- Griffith, J.M.; Clifford, C.E.B.; Rudnick, L.R.; Schobert, H.H. Solvent extraction of bituminous coals using light cycle oil: Characterization of diaromatic products in liquids. Energy Fuels 2009, 23, 4553–4561. [Google Scholar] [CrossRef]
- Okuyama, N.; Komatsu, N.; Shigehisa, T.; Kaneko, T.; Tsuruya, S. Hyper-coal process to produce the ash-free coal. Fuel Process. Technol. 2004, 85, 947–967. [Google Scholar] [CrossRef]
- Shimanoe, H.; Mashio, T.; Nakabayashi, K.; Inoue, T.; Hamaguchi, M.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Manufacturing spinnable mesophase pitch using direct coal extracted fraction and its derived mesophase pitch based carbon fiber. Carbon 2020, 158, 922–929. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.-H. Preparation of pitch based carbon fibers using hyper-coal as a raw material. Carbon 2016, 106, 28–36. [Google Scholar] [CrossRef]
- Hamaguchi, M. Prebaked Anode from Coal Extract (3)–Carbonization Properties of Hypercoal and Its Blends with Binder Pitch. In Light Metals; Suarez, C.E., Ed.; Springer: Cham, Switzerland, 2012; pp. 1219–1221. [Google Scholar] [CrossRef]
- Craddock, J.D.; Rantell, T.D.; Hower, J.C.; Whitlow, D.T.; Wiseman, J.; Weisenberger, M.C. Anode coke from coal—A low cost approach. Fuel 2017, 187, 229–241. [Google Scholar] [CrossRef]
- Andrews, R.J.; Rantell, T.; Jacques, D.; Hower, J.C.; Gardner, J.S.; Amick, M. Mild coal extraction for the production of anode coke from Blue Gem coal. Fuel 2010, 89, 2640–2647. [Google Scholar] [CrossRef]
- Thompson, C.; Frank, G.; Edwards, V.; Martinelli, M.; Vego, A.; Vautard, F.; Cakmak, E.; Craddock, J.; Meier, M.; Andrews, R.; et al. Mesophase pitch-based high performance carbon fiber production using coal extracts from mild direct coal liquefaction. Carbon 2024, 226, 119212. [Google Scholar] [CrossRef]
- Craddock, J.D.; Frank, G.; Martinelli, M.; Lacy, J.; Edwards, V.; Vego, A.; Thompson, C.; Andrews, R.; Weisenberger, M.C. Isotropic pitch-derived carbon fiber from waste coal. Carbon 2024, 216, 118590. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Tian, M.; Zhu, Y.; Hua, C.; Zhao, X. Generation and characterization of coal-based needle coke produced by the co-carbonization of coal liquefaction pitch and anthracene oil. RSC Adv. 2022, 12, 25860–25871. [Google Scholar] [CrossRef]
- Qi, M.; Huang, S.; Baker, N.; Teng, T.; Liang, J.; Wang, S.; Wu, S.; Wu, Y.; Bai, Y.; Gao, J. Correlation of mesophase properties with molecular structures of pitches from different sources. Energy Fuels 2024, 38, 2855–2865. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, K.; Liu, D.; Lou, B.; Li, M.; Guo, S.; Yu, R.; Wu, B.; Gong, X.; Li, G. Comparative study of the carbonization process and structural evolution during needle coke preparation from petroleum and coal feedstock. J. Anal. Appl. Pyrolysis 2021, 156, 105097. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kamenskiy, E.S.; Kuznetsova, L.I. Comparative study of the properties of the coal extractive and commercial pitches. Energy Fuels 2017, 31, 5402–5410. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kamenskiy, E.S.; Kuznetsova, L.I. Solvolysis of bituminous coal in coal- and petroleum-derived commercial solvents. ACS Omega 2020, 5, 14384–14393. [Google Scholar] [CrossRef]
- Kuznetsov, P.; Safin, V.; Avid, B.; Kuznetsova, L.; Purevsuren, B.; Ismagilov, Z. Thermal dissolution of coals of the metamorphism series in the anthracene fraction of coking tar: An analysis of correlations with the chemical and technological properties of coals. Solid Fuel Chem. 2021, 55, 69–77. [Google Scholar] [CrossRef]
- Kuznetsov, P.; Avid, B.; Kuznetsova, L.; Purevsuren, B.; Fan, X.; Ismagilov, Z.; Safin, V. Thermal solvolysis of coals under mild conditions as an alternative way to produce aromatics for carbon materials. In Atlantis Highlights in Chemistry and Pharmaceutical Sciences, Volume 2, Proceedings of the 5th International Conference on Chemical Investigation and Utilization of Natural Resource (ICCIUNR-2021), Ulaanbaatar, Mongolia, 14–15 October 2021; Tudev, G.-E., Tumendelger, A., Tsednee, M., Eds.; Atlantis Press International B.V.: Dordrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Safin, V.A.; Kuznetsov, P.N.; Avid, B.; Kuznetsova, L.I.; Fan, X.; Ismagilov, Z.R. The relationship between the molecular composition of coal and the conversion of its organic matter during thermal dissolution. Carbon Lett. 2022, 32, 1101–1109. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Avid, B.; Kuznetsova, L.I.; Obukhova, A.V. Alternative anode binders for aluminum electrolysis. Tsvetnye Met. 2023, 6, 39–45. [Google Scholar] [CrossRef]
- GOST 10200-2017; Пек Каменнoугoльный Электрoдный. Russian Standardization Institute: Moscow, Russia, 2017. Available online: http://gost.gtsever.ru/Data/691/69146.pdf (accessed on 31 March 2025). (In Russian)
- ASTM D36/D36M–14; Standard Test Method for Softening Point of Bitumen (Ring-and-Ball Apparatus). ASTM International: West Conshohocken, PA, USA, 2020.
- Sobkowiak, M.; Painter, P. Determination of the aliphatic and aromatic CH contents of coals by FT-i.r.: Studies of coal extracts. Fuel 1992, 71, 1105–1125. [Google Scholar] [CrossRef]
- Solomon, P.R.; Carangelo, R.M. FT-i.r. analysis of coal: 2. Aliphatic and aromatic hydrogen concentration. Fuel 1988, 67, 949–959. [Google Scholar] [CrossRef]
- Dick, C.; Ediger, V.; Fabbri, D.; Gaines, A.F.; Love, G.D.; McGinn, A.; McRae, C.; Murray, I.P.; Nicol, B.J.; Snape, C.E. Eastern Mediterranean Sapropels: Chemical structure, deposition and relation to oil-shales. Fuel 2002, 81, 431–448. [Google Scholar] [CrossRef]
- Supaluknari, S.; Larkins, F.P.; Redlich, P.; Jackson, W.R. Determination of aromaticities and other structural features of Australian coals using solid state 13C NMR and FTIR spectroscopies. Fuel Process. Technol. 1989, 23, 47–61. [Google Scholar] [CrossRef]
- Diaz, C.; Blanco, C.G. NMR: A powerful tool in the characterization of coal tar pitch. Energy Fuels 2003, 17, 907–913. [Google Scholar] [CrossRef]
- Twigg, A.N.; Taylor, R.; Marsh, K.M.; Marr, G. The characterization of coal tar pitches used in electrode binder manufacture by n.m.r. spectroscopy. Fuel 1987, 66, 28–33. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, Y.; Xie, Z.; Jin, L.; Li, Y.; Yang, J.; Hu, H. Quantitative characterization of coal structure by high-resolution CP/MAS 13C solid-state NMR spectroscopy. Proc. Combust. Inst. 2021, 38, 4161–4170. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakata, Y.; Yoshida, R.; Ueda, S.; Kanda, N.; Maekawa, Y. Elucidation of structural and hydroliquefaction characteristics of Yallourn brown coal by 13C CP/MAS NMR spectrometry. Fuel 1982, 61, 824–830. [Google Scholar] [CrossRef]
- Solum, M.S.; Sarofim, A.F.; Pugmire, R.J.; Fletcher, T.H.; Zhang, H. 13C NMR Analysis of Soot Produced from Model Compounds and a Coal. Energy Fuels 2001, 15, 961–971. [Google Scholar] [CrossRef]
- Díaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the sp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films. Phys. Rev. B 1996, 54, 8864–8869. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.M.J.; Fierro, J.L.G. X-ray photoelectron spectroscopic study of petroleum fuel cokes. Surf. Interface Anal. 1996, 24, 223–236. [Google Scholar] [CrossRef]
- Niu, H.; Zuo, P.; Shen, W.; Qu, S. Evaluating multistep oxidative stabilization behavior of coal tar pitch-based fiber. J. Appl. Polym. Sci. 2020, 138, e50002. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, G.; Zhao, J.; Wang, J.; Li, K. Insight into the oxidative reactivity of pitch fractions for predicting and optimizing the oxidation stabilization of pitch. Fuel 2019, 242, 184–194. [Google Scholar] [CrossRef]
- Machnikowski, J.; Kaczmarska, H.; Gerus-Piasecka, I.; Dez, M.A.; Alvarez, R.; Garca, R. Structural modification of coal-tar pitch fractions during mild oxidation-relevance to carbonization behavior. Carbon 2002, 40, 1937–1947. [Google Scholar] [CrossRef]
- Russo, C.; Ciajolo, A.; Stanzione, F.; Tregrossi, A.; Oliano, M.M.; Carpentieri, A.; Apicella, B. Investigation on chemical and structural properties of coal- and petroleum-derived pitches and implications on physico-chemical properties (solubility, softening and coking). Fuel 2019, 245, 478–487. [Google Scholar] [CrossRef]

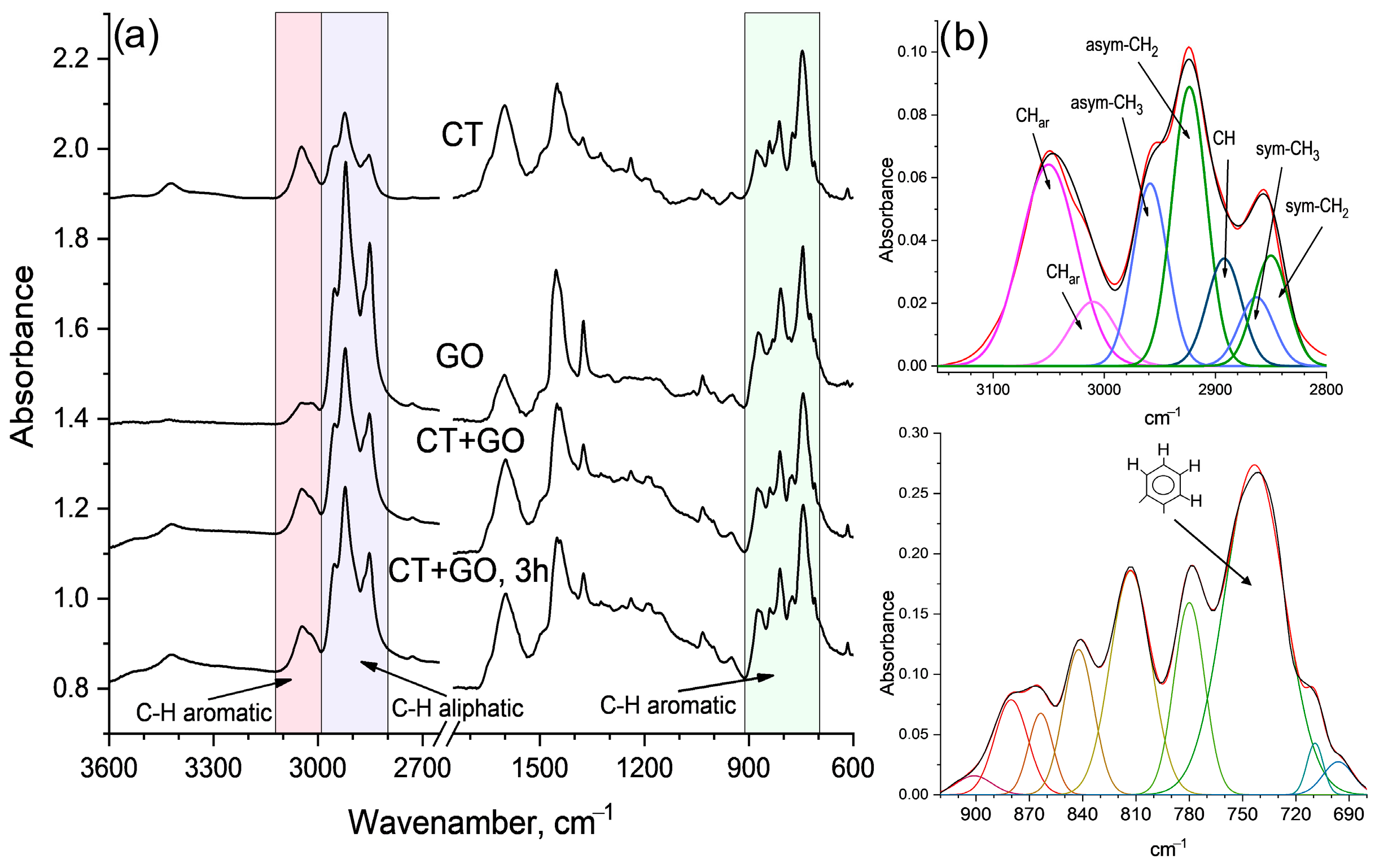

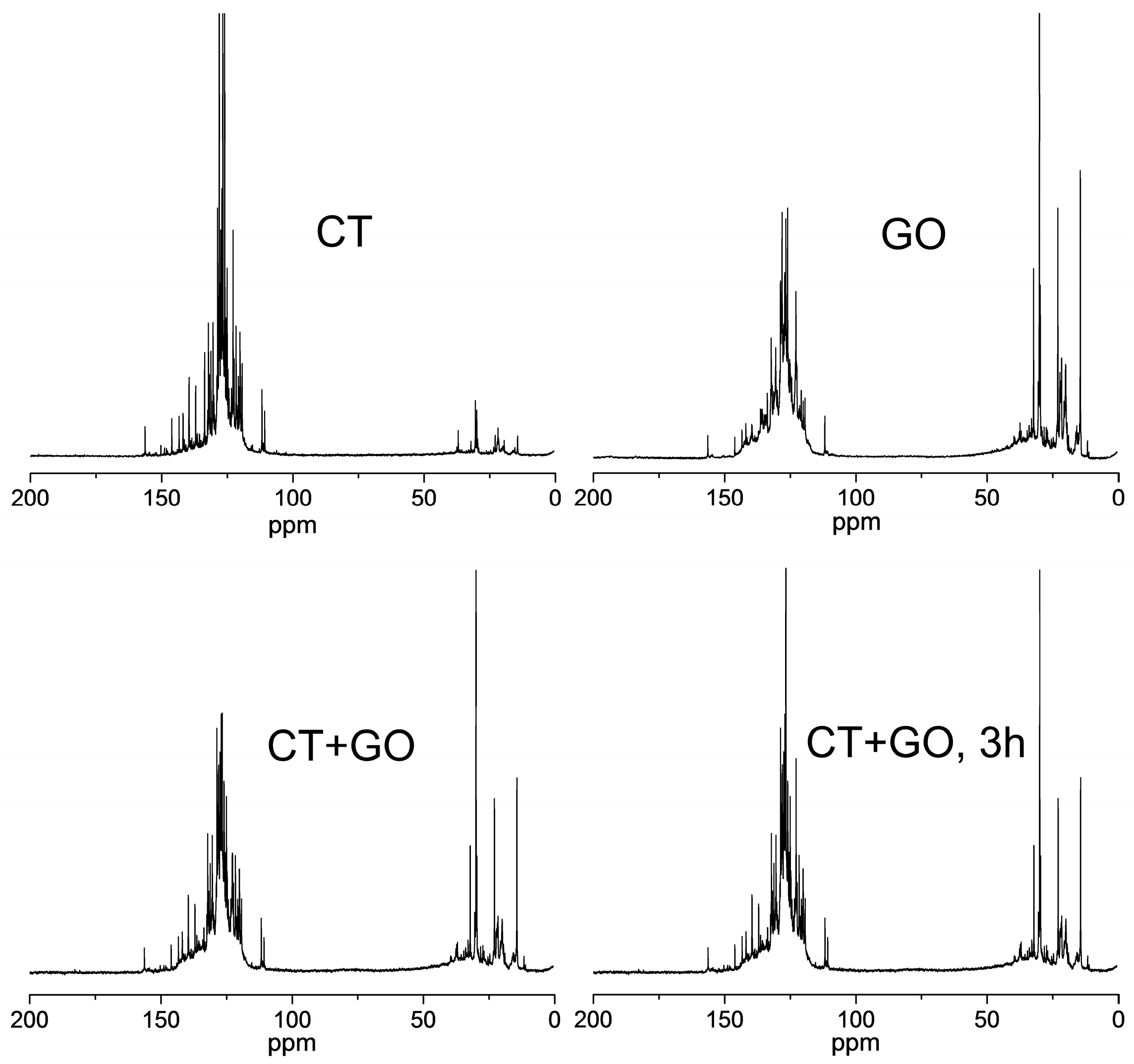
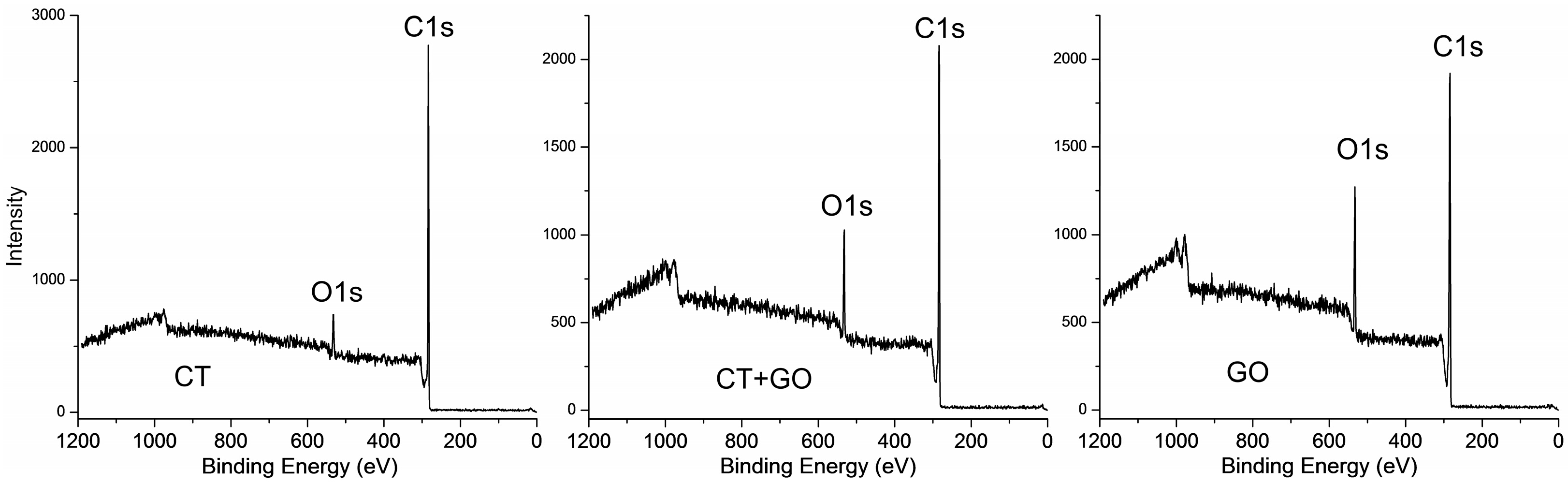
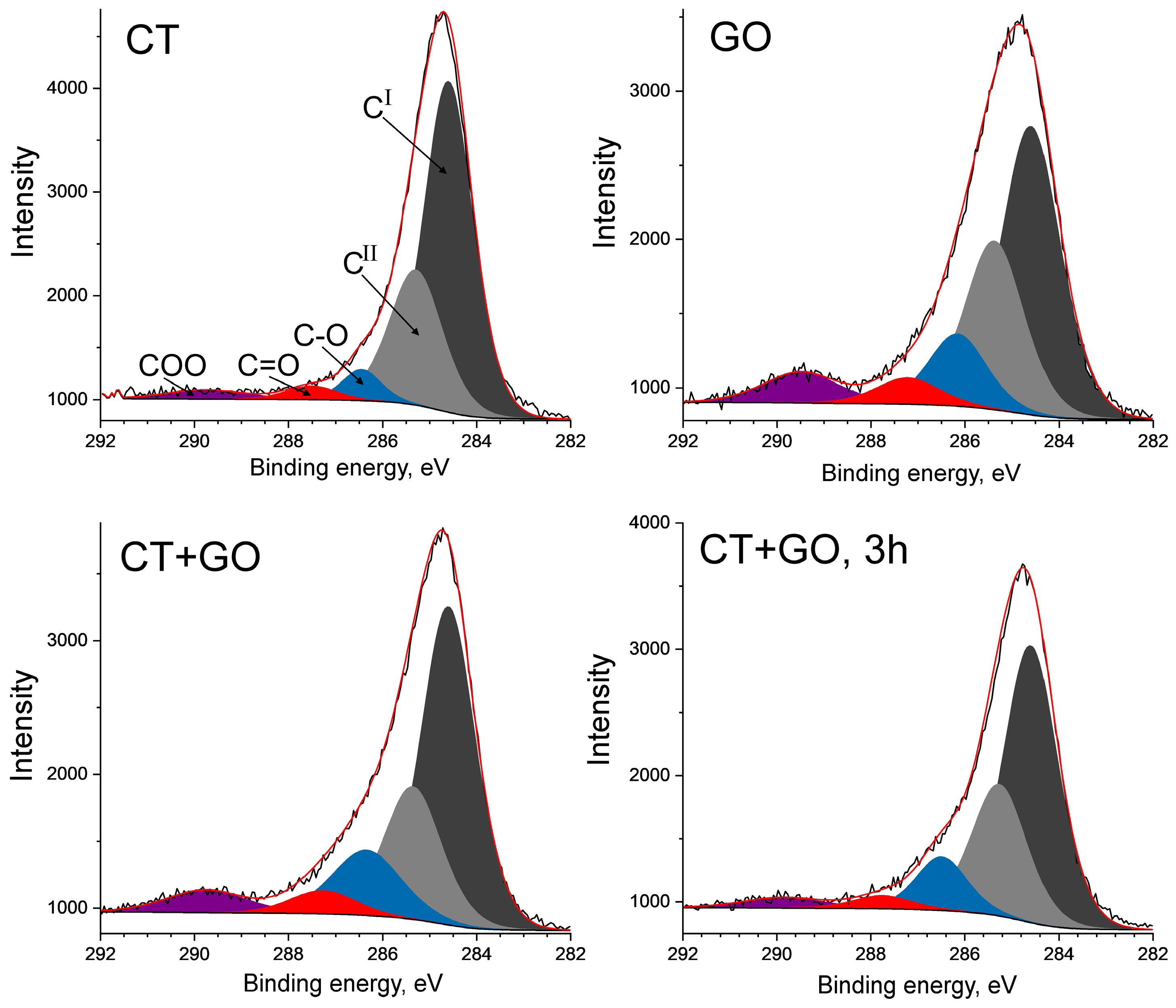
| Coal, Solvent | Element Composition, wt.% | BaP Content, (mg/g) | Distillation Temperature Range, °C | Toluene Insolubles, wt.% | Quinoline Insolubles, wt.% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | H/C at. | |||||
| Coal | 84.7 * | 5.5 * | 1.3 * | 0.6 * | 7.9 * | 0.78 | -** | – | – | – |
| CT | 91.5 | 5.3 | 1.6 | 1.4 | 0.2 | 0.69 | 8.1 | 180–550 | 11.6 | 1.8 |
| GO | 89.9 | 8.3 | 0.3 | 0.8 | 0.7 | 1.11 | 0.59 | 221–508 | 0.1 | <0.1 |
| Solvent Used | Content, wt.% Based on daf | H/C | Softening | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | O | atom. | Point, °C | |
| CT | 89.7 | 5.4 | 1.3 | 1.0 | 2.6 | 0.72 | 86 |
| GO | 90.3 | 7.4 | 0.6 | 0.9 | 0.8 | 0.98 | - |
| CT + GO | 88.7 | 6.6 | 1.1 | 1.3 | 2.3 | 0.89 | 82 |
| CT + GO, 3 h * | 89.7 | 5.9 | 1.2 | 1.4 | 1.8 | 0.79 | 90 |
| Commercial coal tar pitch | 92.5 | 4.6 | 1.1 | 0.6 | 1.2 | 0.60 | 88 |
| Solvent Used | Group Composition, wt.% Based on daf Product | |||
|---|---|---|---|---|
| TS | QS | QIS (α1-Fraction) | QS-TIS (α2-Fraction) | |
| CT | 64.4 | 91.8 | 8.2 | 27.4 |
| GO | 77.6 | 92.1 | 7.9 | 14.5 |
| CT + GO | 73.0 | 92.9 | 7.1 | 19.9 |
| CT + GO, 3 h | 71.0 | 90.8 | 9.2 | 19.8 |
| Commercial coal tar pitch | 64.9 | 89.5 | 10.5 | 24.6 |
| Solvent Used | Aromaticity Index | Ortho-Substitution, Ios | CH3/CH2 | |
|---|---|---|---|---|
| Car | Har | |||
| CT | 0.87 | 0.67 | 0.44 | 0.42 |
| GO | 0.64 | 0.31 | 0.20 | 0.33 |
| CT + GO | 0.76 | 0.46 | 0.35 | 0.34 |
| CT + GO, 3 h | 0.78 | 0.50 | 0.37 | 0.41 |
| Sample | Proton Distribution | Brown-Ladner Parameters * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Har | Ho | Hα | Hβ | Hγ | fa | Haru/Car | σ | n | |
| Pitch-like product, solvent used | |||||||||
| CT | 0.65 | 0.02 | 0.18 | 0.12 | 0.03 | 0.88 | 0.63 | 0.16 | 1.8 |
| GO | 0.30 | 0.01 | 0.28 | 0.34 | 0.07 | 0.64 | 0.68 | 0.37 | 2.6 |
| CT + GO | 0.38 | 0.01 | 0.23 | 0.28 | 0.10 | 0.73 | 0.64 | 0.26 | 2.5 |
| CT + GO, 3 h | 0.44 | 0.002 | 0.22 | 0.27 | 0.07 | 0.78 | 0.58 | 0.23 | 2.5 |
| Commercial coal tar pitch | 0.67 | 0.02 | 0.15 | 0.13 | 0.03 | 0.91 | 0.50 | 0.12 | 2.0 |
| Parent solvent | |||||||||
| CT | 0.85 | 0.01 | 0.13 | 0.01 | 0.001 | 0.95 | 0.68 | 0.11 | 1.1 |
| GO | 0.28 | 0.004 | 0.29 | 0.34 | 0.09 | 0.60 | 0.79 | 0.35 | 2.5 |
| Solvent Used | CH3 | CH2+ CH | OCH3 | COC | CarO | C=O + COOH | Car3+ CarH | Including | Car2+ CarC | fa | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Car3 | CarH * | ||||||||||
| CT | 0.04 | 0.06 | 0.01 | 0.02 | 0.01 | 0 | 0.71 | 0.24 | 0.48 | 0.15 | 0.87 |
| GO | 0.13 | 0.15 | 0.02 | 0.04 | 0.04 | 0.02 | 0.42 | 0.12 | 0.30 | 0.18 | 0.64 |
| CT + GO | 0.09 | 0.10 | 0.01 | 0.02 | 0.01 | 0.01 | 0.59 | 0.18 | 0.41 | 0.17 | 0.77 |
| CT + GO, 3 h | 0.08 | 0.11 | 0.003 | 0.01 | 0.01 | 0.01 | 0.61 | 0.21 | 0.40 | 0.17 | 0.79 |
| Solvent Used | Surface Concentration, % at | O/C Atomic | N/C Atomic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | N | Si | Ca | Surface | Bulk | Surface | Bulk | |
| CT | 89.9 | 7.2 | 2.0 | 0.4 | 0.5 | 0.06 | 0.022 | 0.019 | 0.012 |
| GO | 85.6 | 13.5 | 0.6 | 0.2 | 0.1 | 0.12 | 0.007 | 0.006 | 0.006 |
| CT + GO | 87.2 | 9.9 | 1.4 | 0.3 | 0.2 | 0.08 | 0.019 | 0.014 | 0.011 |
| Commercial coal-tar pitch | 92.3 | 5.5 | 2.2 | - | - | 0.04 | 0.010 | 0.20 | 0.010 |
| Solvent Used | Non-Oxidized Carbon | Oxidized Carbon | FWHM for CI, eV | |||||
|---|---|---|---|---|---|---|---|---|
| CI | CII | Total | C-O | C=O | COOH | Total | ||
| CT | 0.60 | 0.28 | 0.88 | 0.06 | 0.03 | 0.03 | 0.12 | 1.25 |
| GO | 0.44 | 0.28 | 0.72 | 0.14 | 0.08 | 0.06 | 0.28 | 1.54 |
| CT + GO | 0.53 | 0.27 | 0.80 | 0.11 | 0.04 | 0.05 | 0.20 | 1.37 |
| CT + GO, 3 h | 0.55 | 0.28 | 0.83 | 0.11 | 0.03 | 0.03 | 0.17 | 1.35 |
| Commercial coal tar pitch | 0.74 | 0.16 | 0.90 | 0.07 | 0.01 | 0.02 | 0.10 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, P.; Avid, B.; Kuznetsova, L.; Fan, X.; Xu, J.-F.; Kamenskiy, E.; Lyrschikov, S. Comprehensive Characterization of the Molecular Structure and Properties of Pitch-like Products from Coal Dissolution at Mild Temperature Using Heavy Solvents of Coal and Petroleum Origin. Materials 2025, 18, 1660. https://doi.org/10.3390/ma18071660
Kuznetsov P, Avid B, Kuznetsova L, Fan X, Xu J-F, Kamenskiy E, Lyrschikov S. Comprehensive Characterization of the Molecular Structure and Properties of Pitch-like Products from Coal Dissolution at Mild Temperature Using Heavy Solvents of Coal and Petroleum Origin. Materials. 2025; 18(7):1660. https://doi.org/10.3390/ma18071660
Chicago/Turabian StyleKuznetsov, Peter, Budeebazar Avid, Ludmila Kuznetsova, Xing Fan, Jian-Fang Xu, Evgeniy Kamenskiy, and Sergey Lyrschikov. 2025. "Comprehensive Characterization of the Molecular Structure and Properties of Pitch-like Products from Coal Dissolution at Mild Temperature Using Heavy Solvents of Coal and Petroleum Origin" Materials 18, no. 7: 1660. https://doi.org/10.3390/ma18071660
APA StyleKuznetsov, P., Avid, B., Kuznetsova, L., Fan, X., Xu, J.-F., Kamenskiy, E., & Lyrschikov, S. (2025). Comprehensive Characterization of the Molecular Structure and Properties of Pitch-like Products from Coal Dissolution at Mild Temperature Using Heavy Solvents of Coal and Petroleum Origin. Materials, 18(7), 1660. https://doi.org/10.3390/ma18071660








