Impact of Mechanical Arc Oscillation on the Microstructure and Durability of Welded Joints in Molten Salt Thermal Storage System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Welding Process
2.3. Procedure for Determining Welding Current and Arc Energy
2.4. Welding Filler Material
2.5. Preparation of Welded Specimens
2.6. Metallography and Analysis, Microstructural and Compositional Characterisation
3. Results and Discussion
3.1. Metallography and Material Mass Loss
3.2. Microhardness Measurement
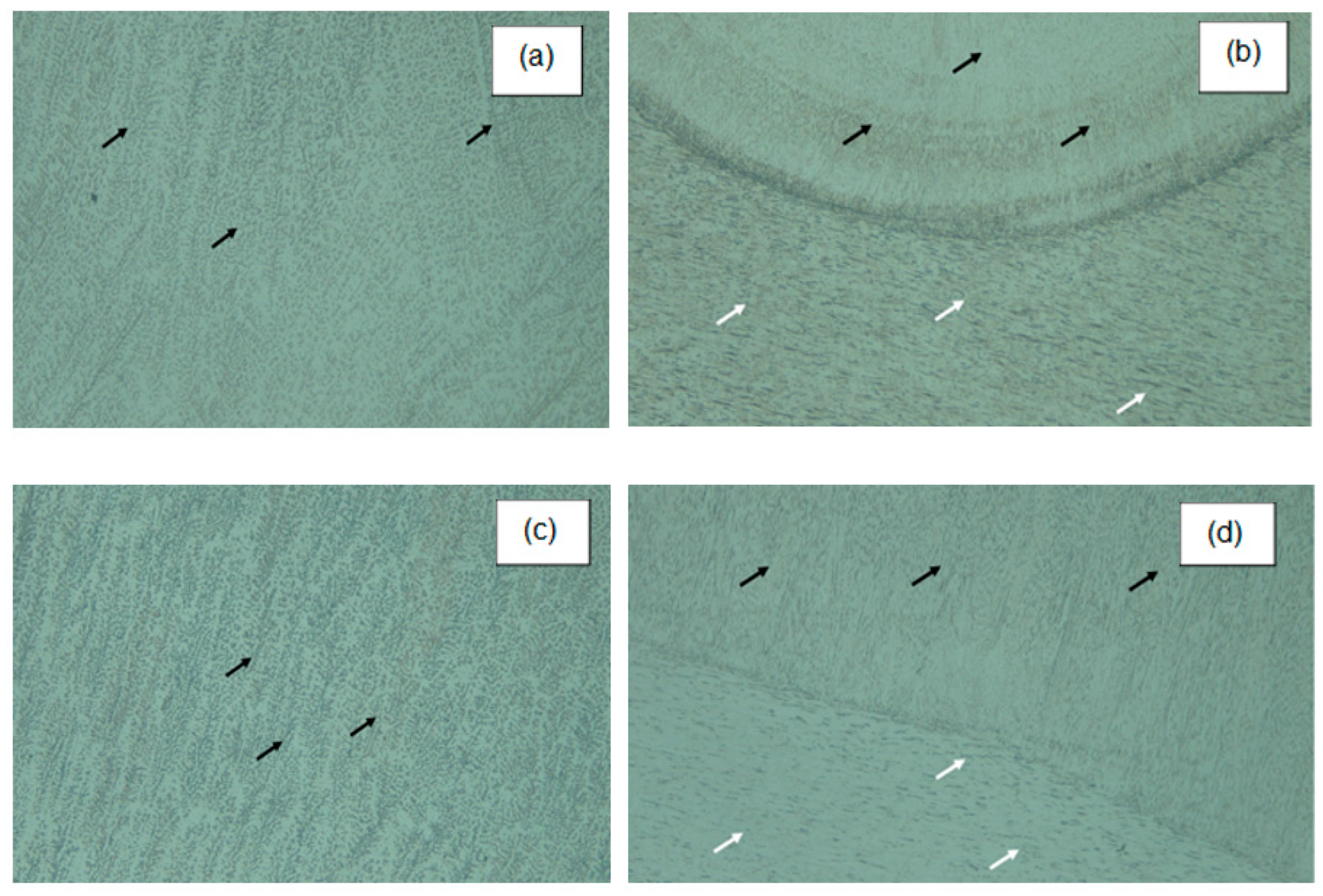
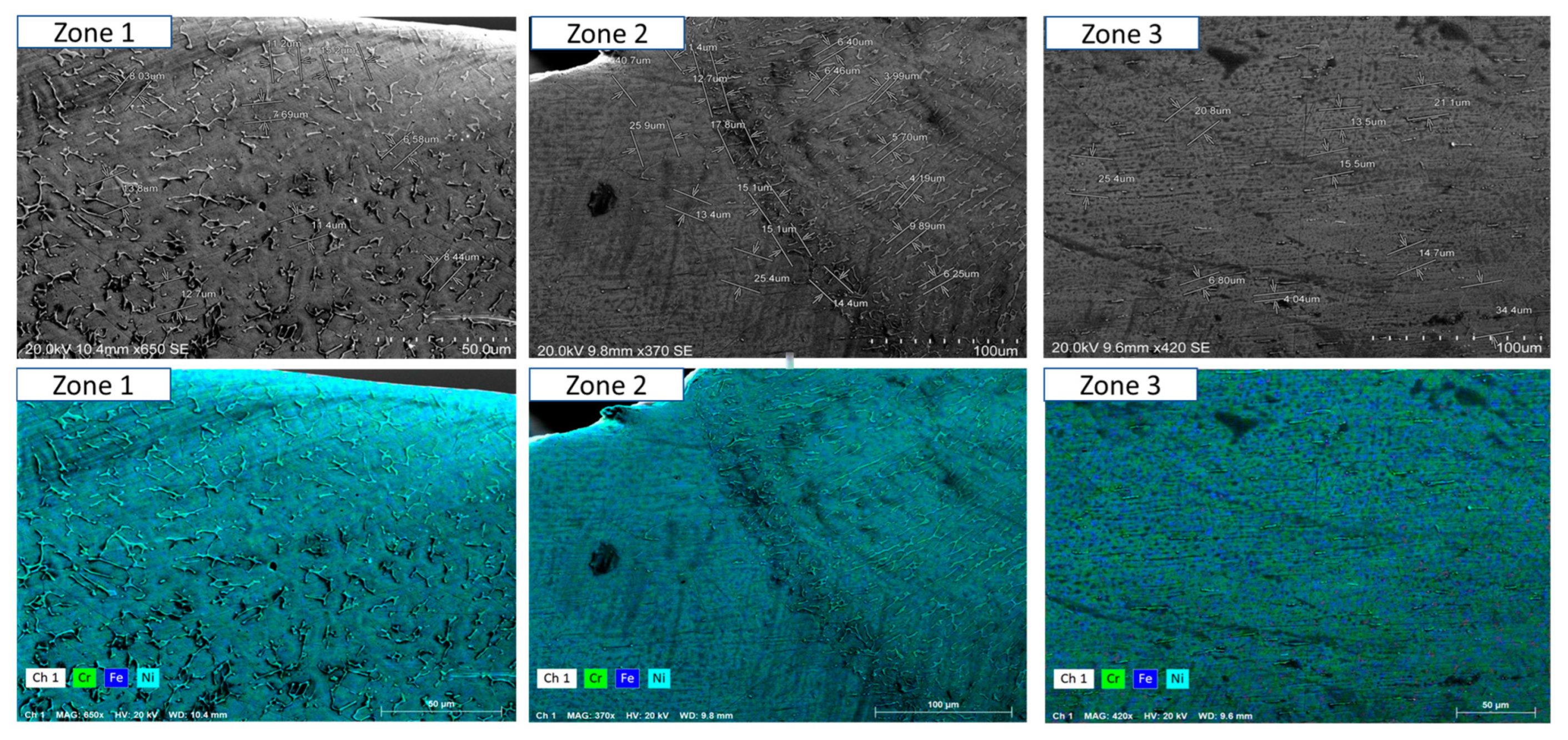
3.3. Morphology and Microstructure of Welding
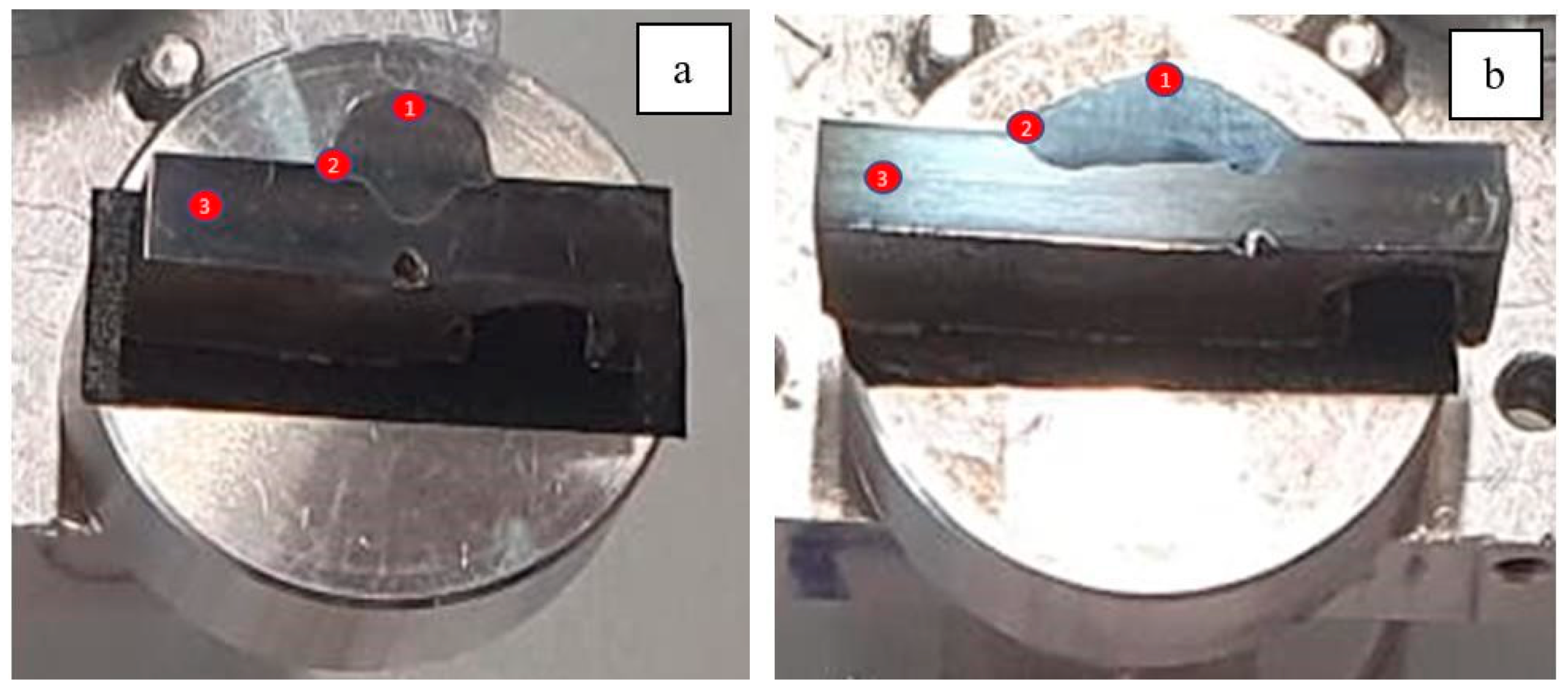
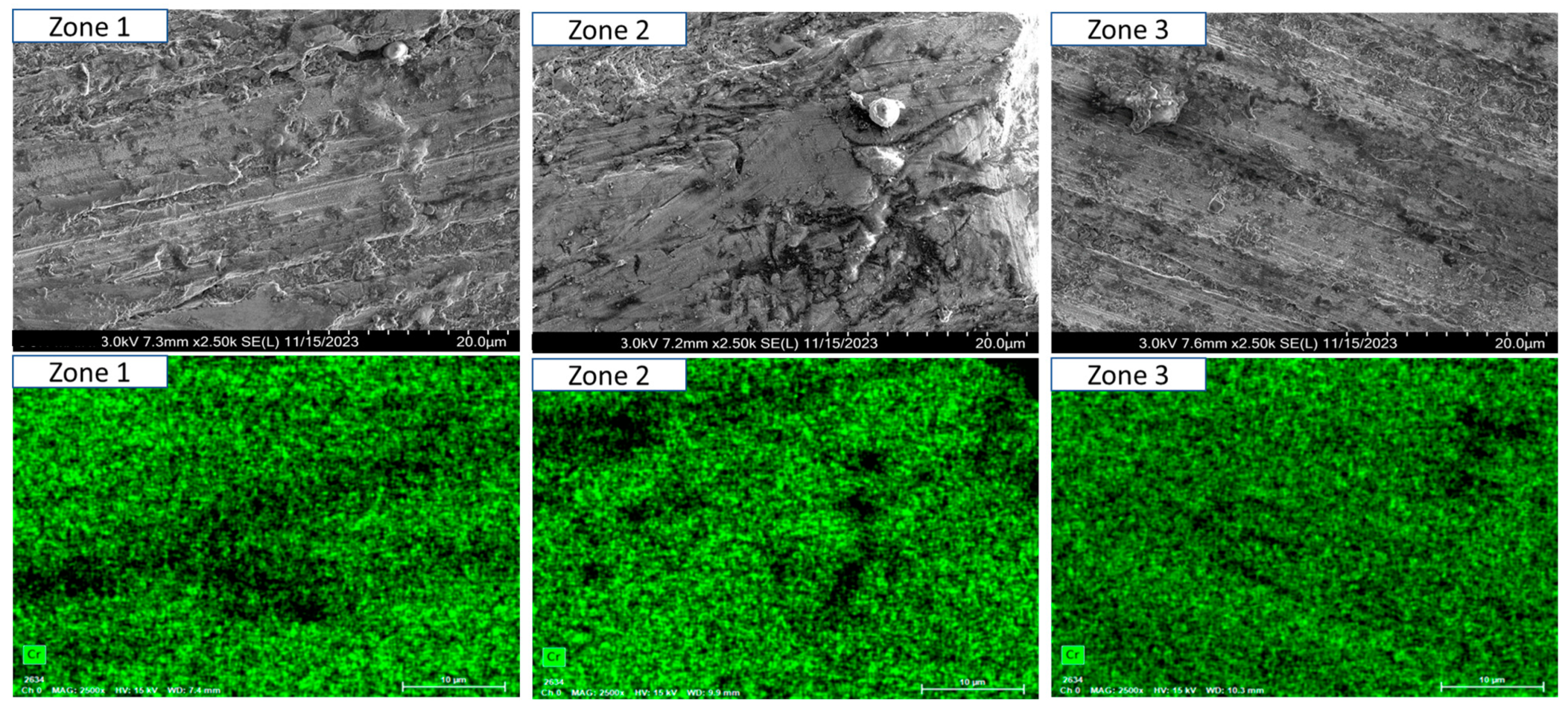
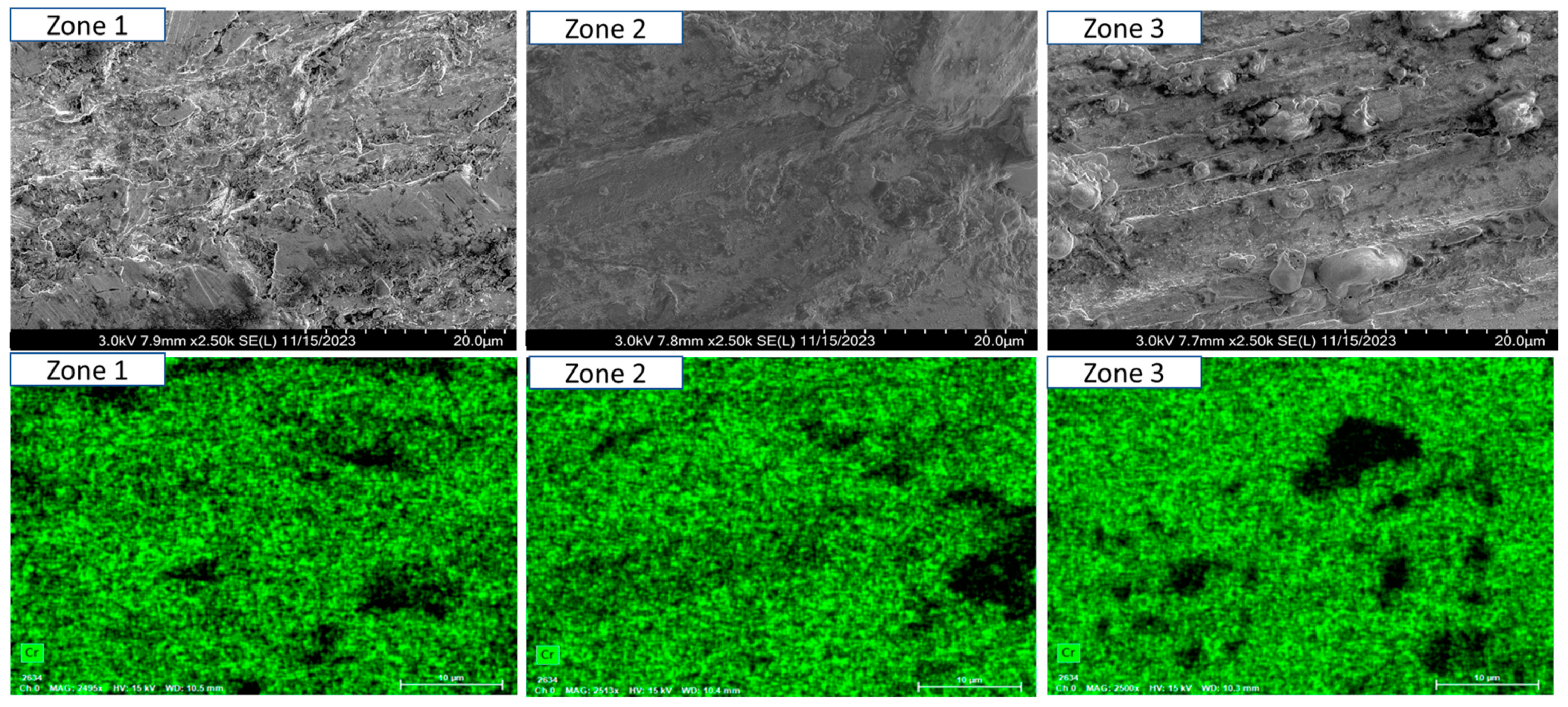
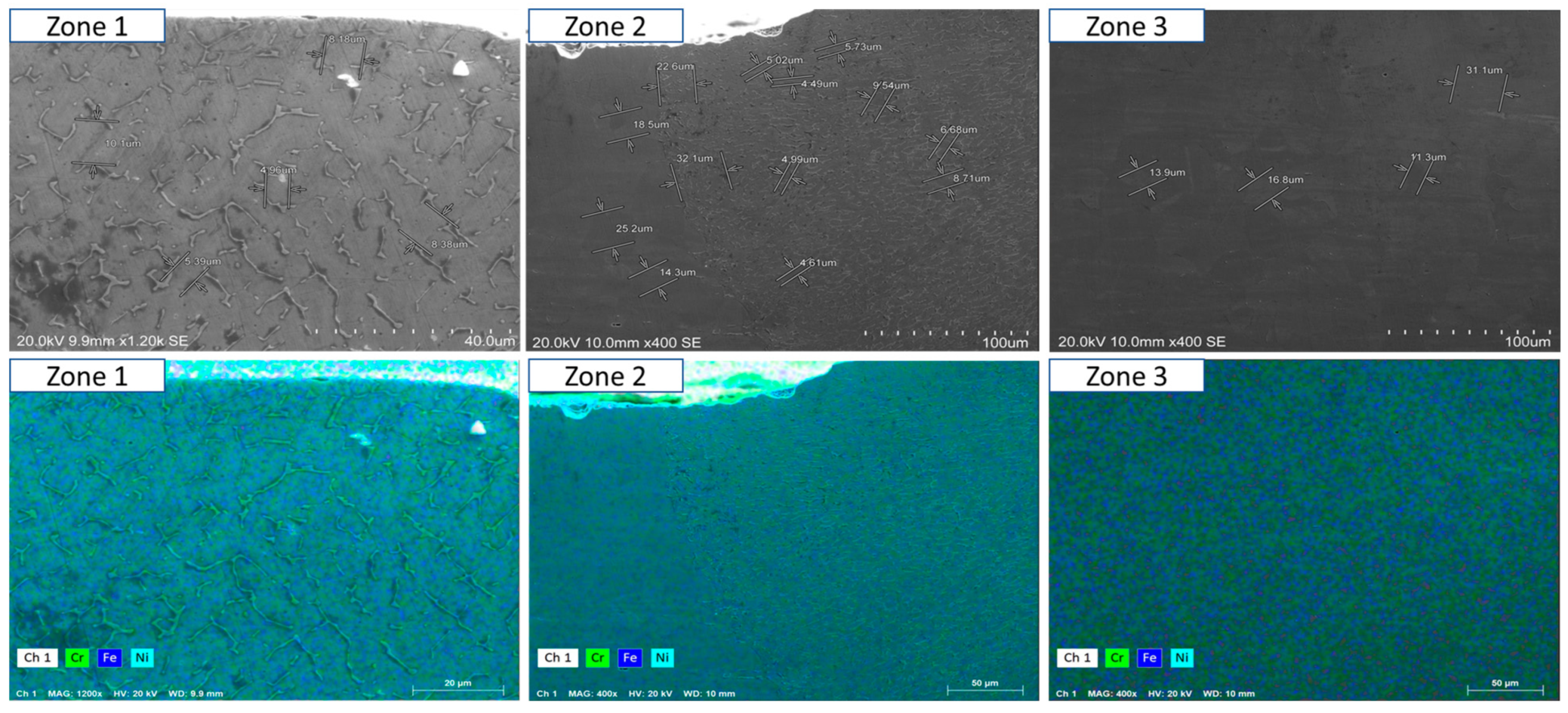
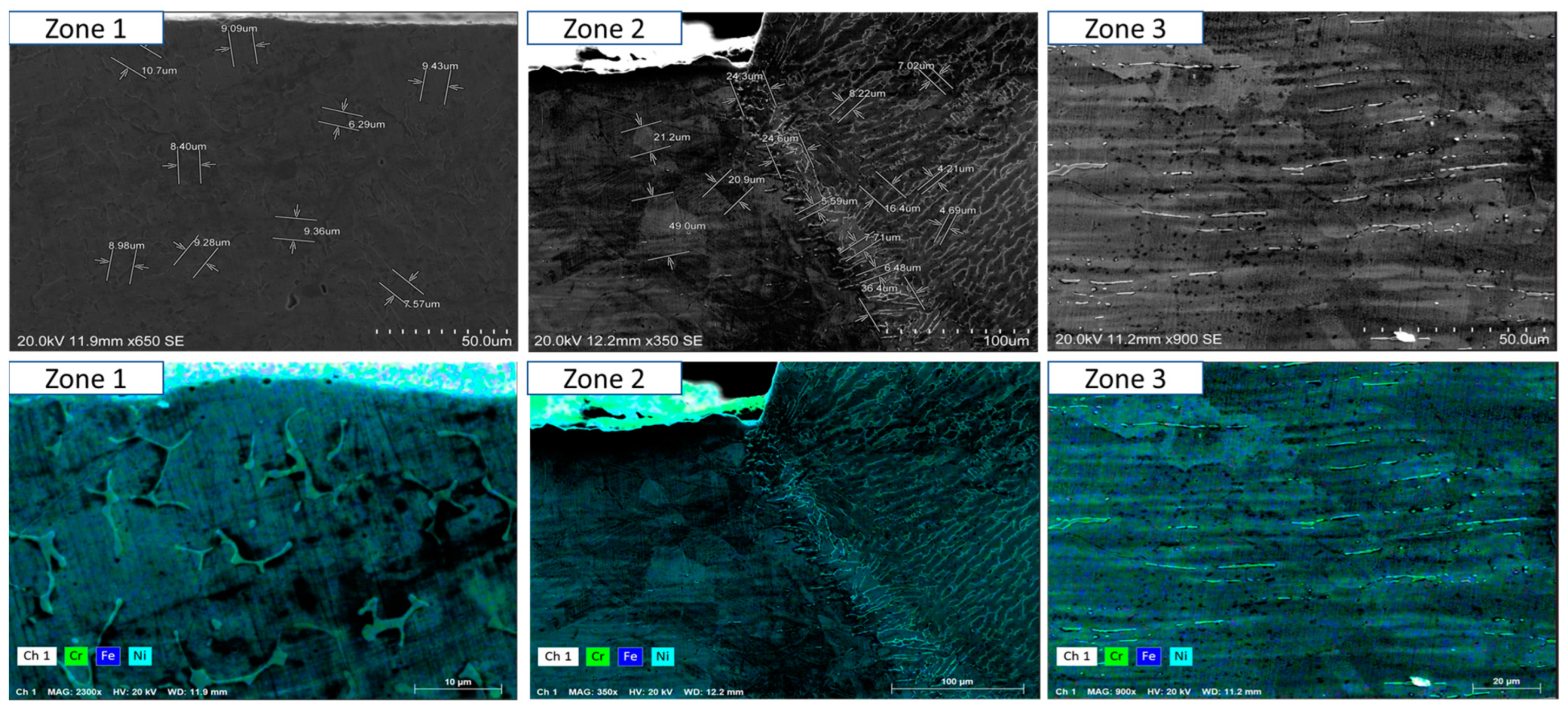
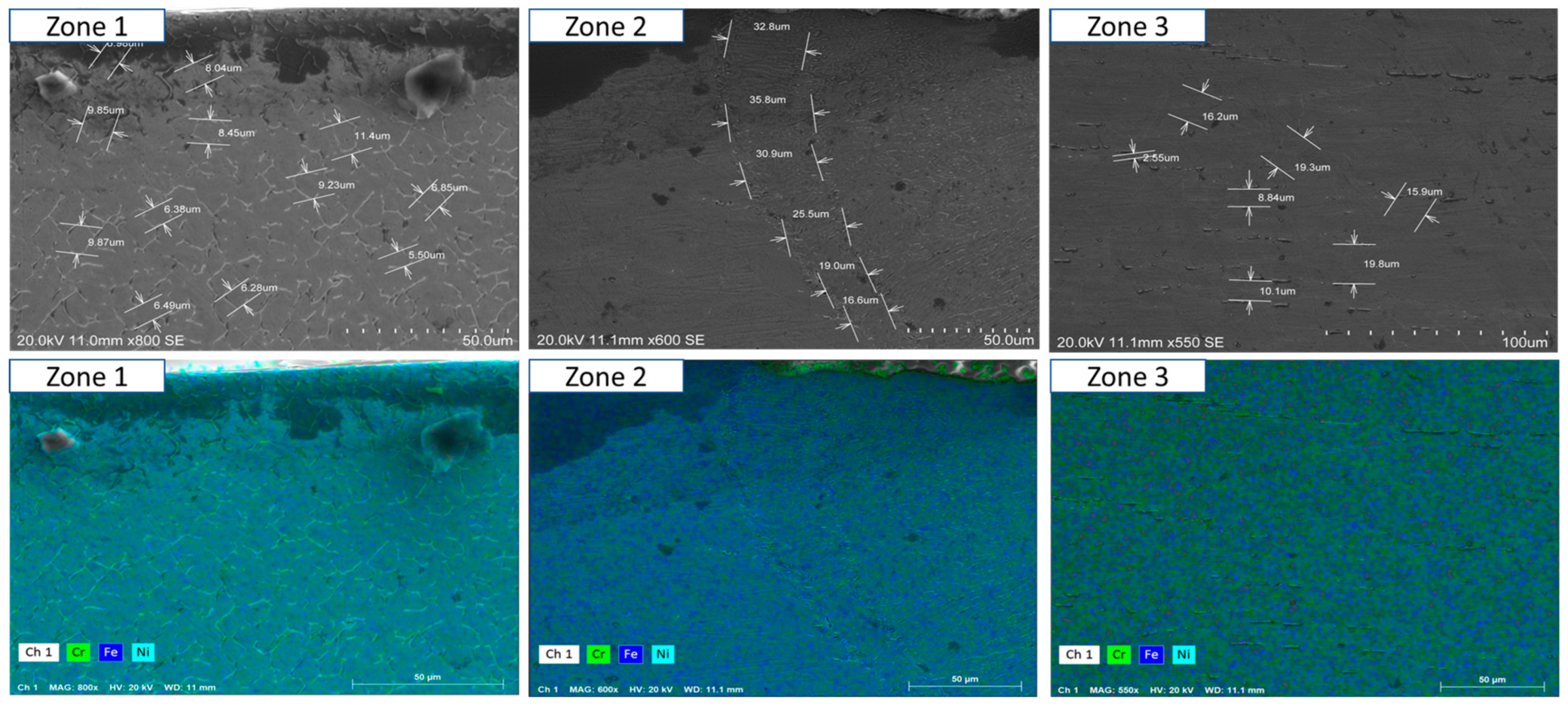
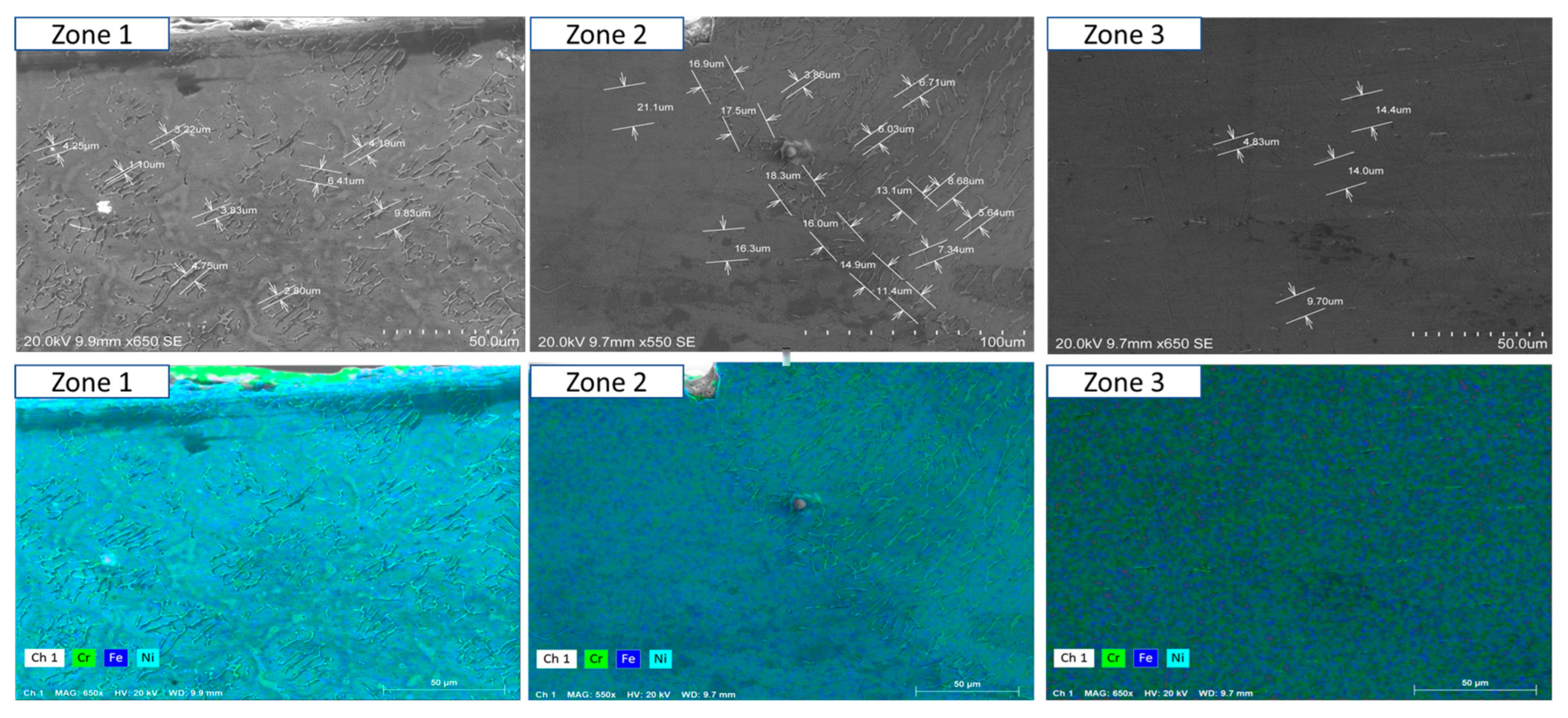
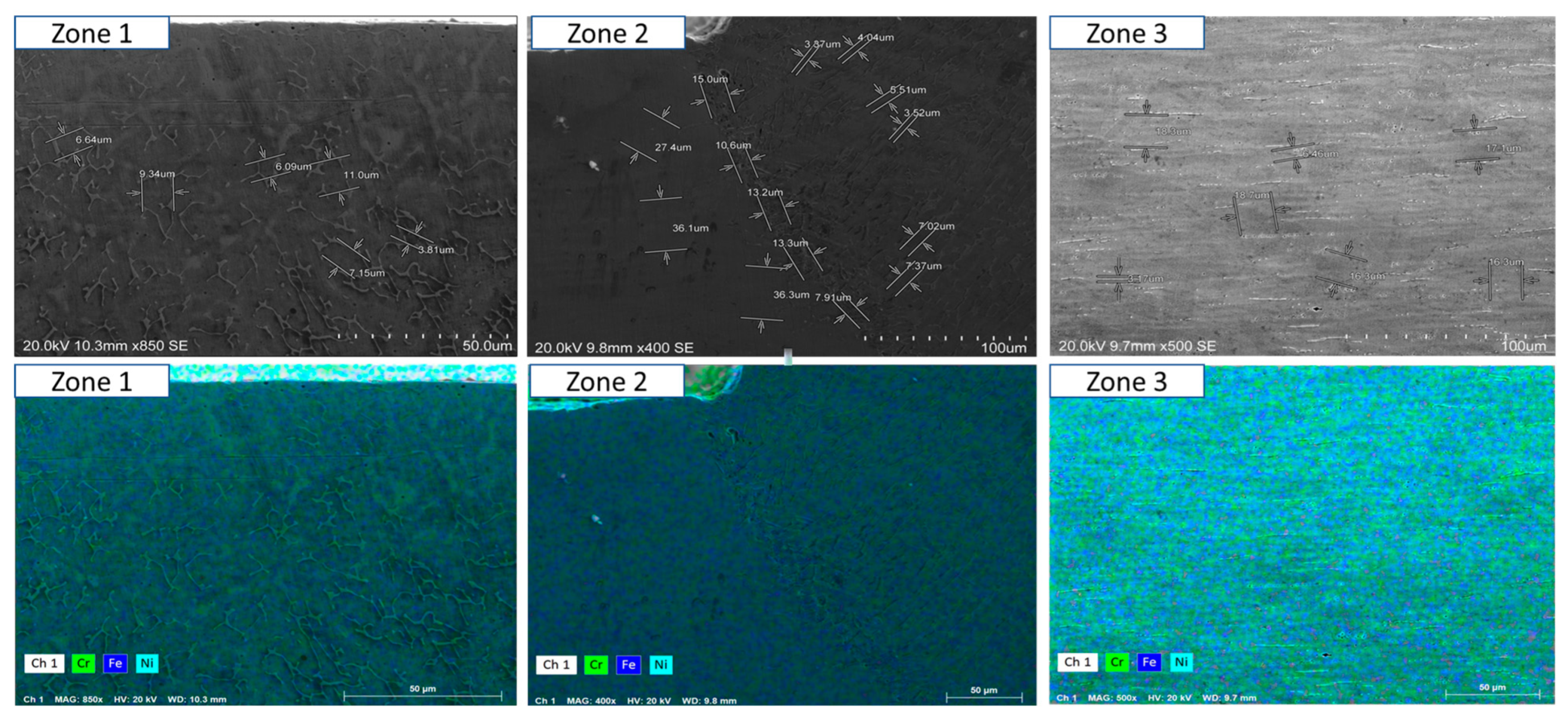
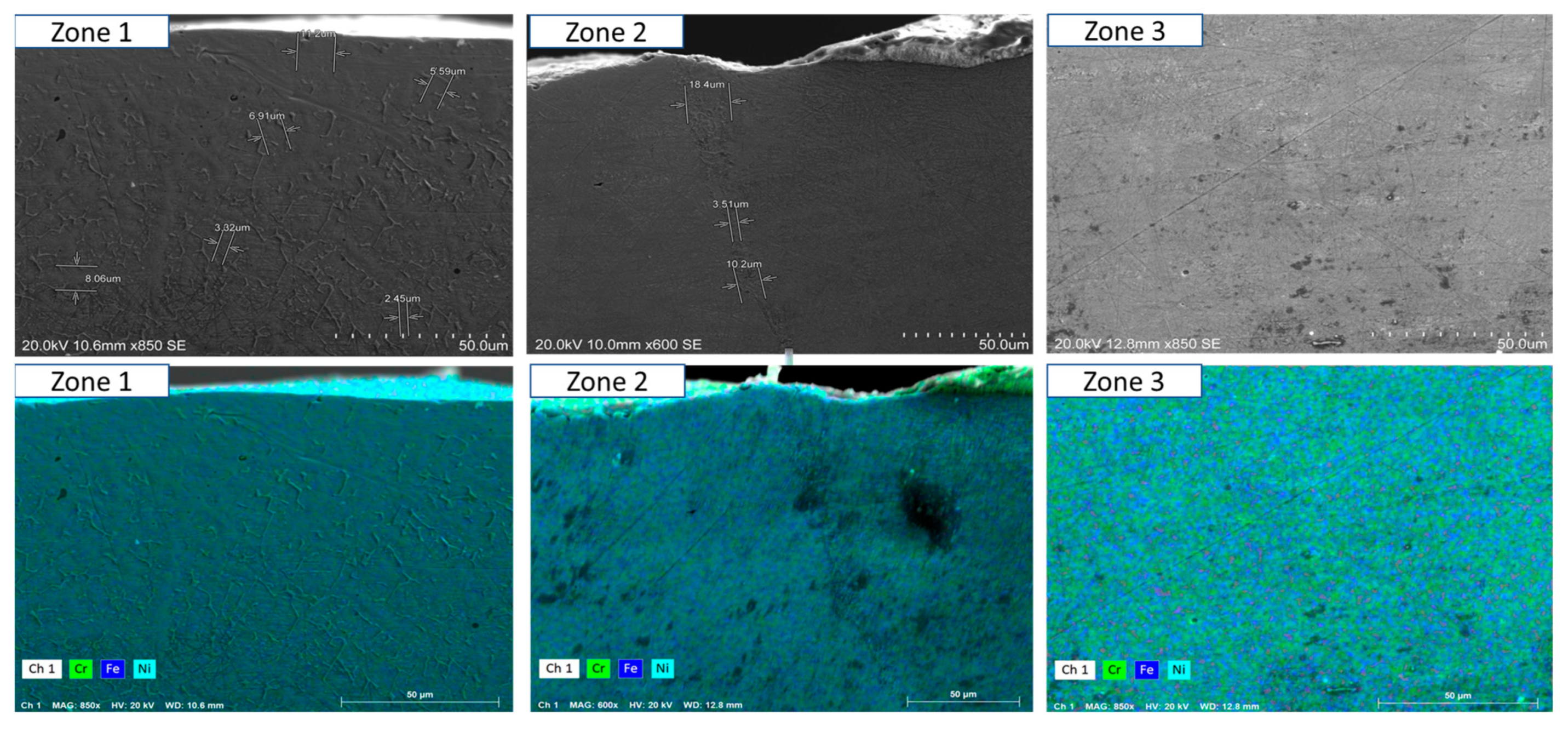
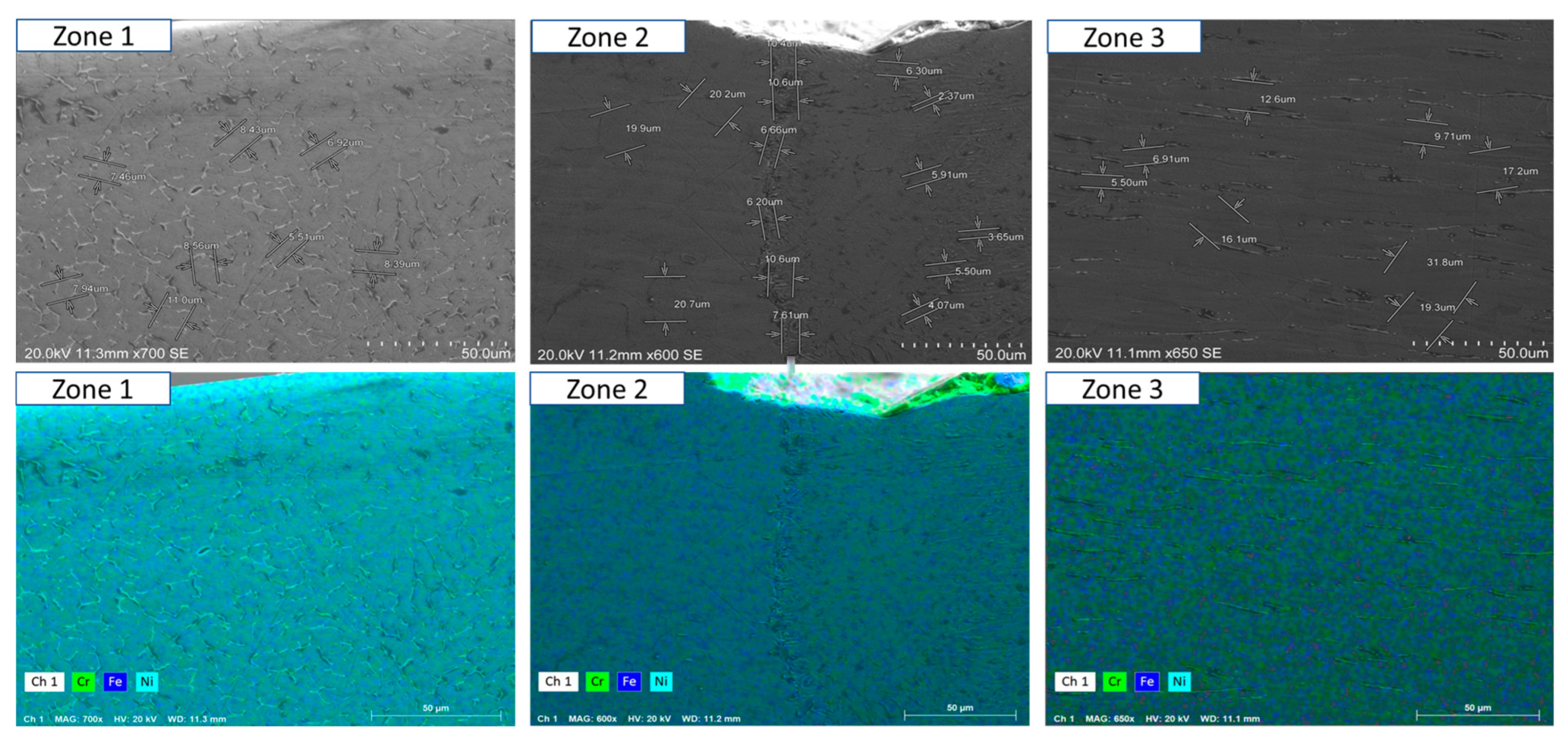
- Short-term exposure (120 h and 500 h): The differences between oscillated and non-oscillated welds become apparent in Figure 13 and Figure 15 for oscillated samples and Figure 14 and Figure 16 for non-oscillated samples. Oscillations effectively limit the initial formation of corrosion products and reduce surface irregularities, particularly in Zones 1 and 2.
- Mid and long-term exposure (1000 h to 2300 h): As shown in Figure 18, Figure 19, Figure 20, Figure 21, Figure 22 and Figure 23, the advantages of oscillations become more pronounced. Oscillated welds demonstrate better structural integrity and resistance to corrosion, while the non-oscillated samples exhibit significant degradation in the HAZ and weld pool.
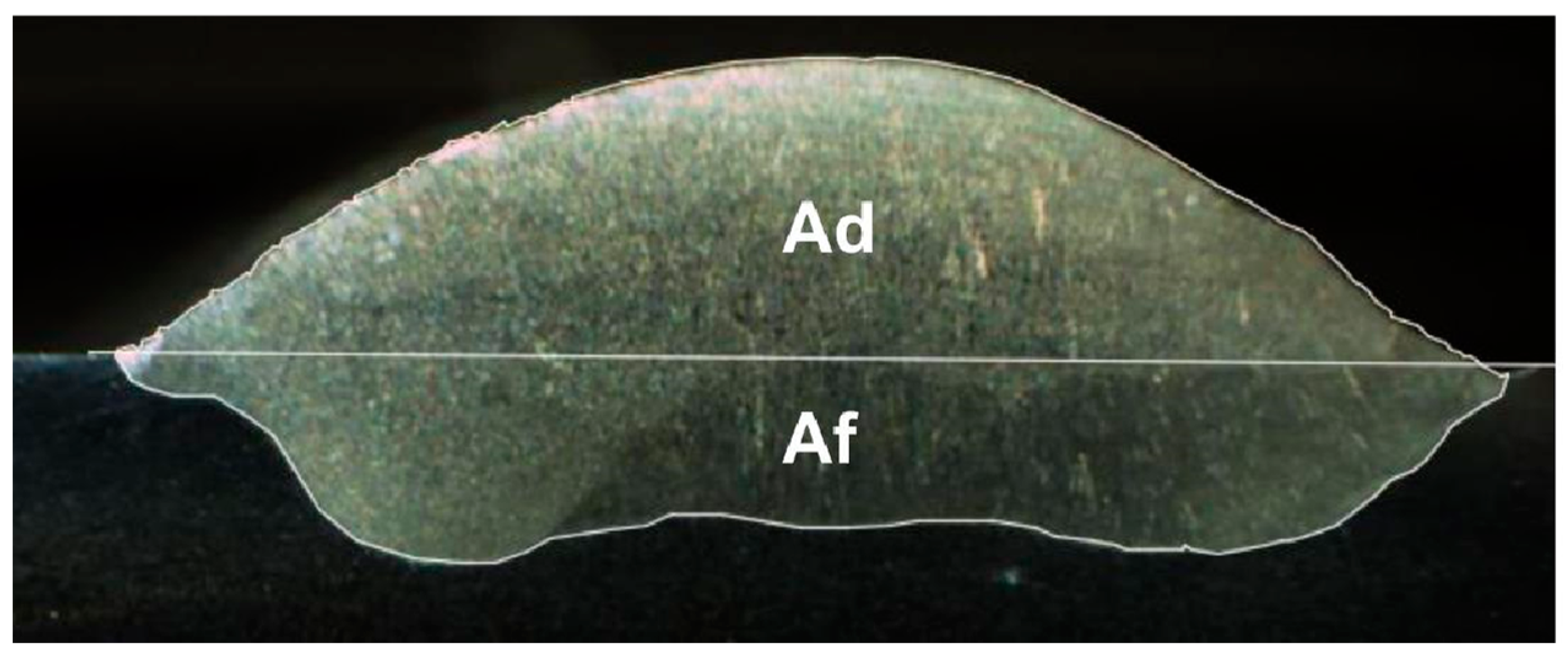
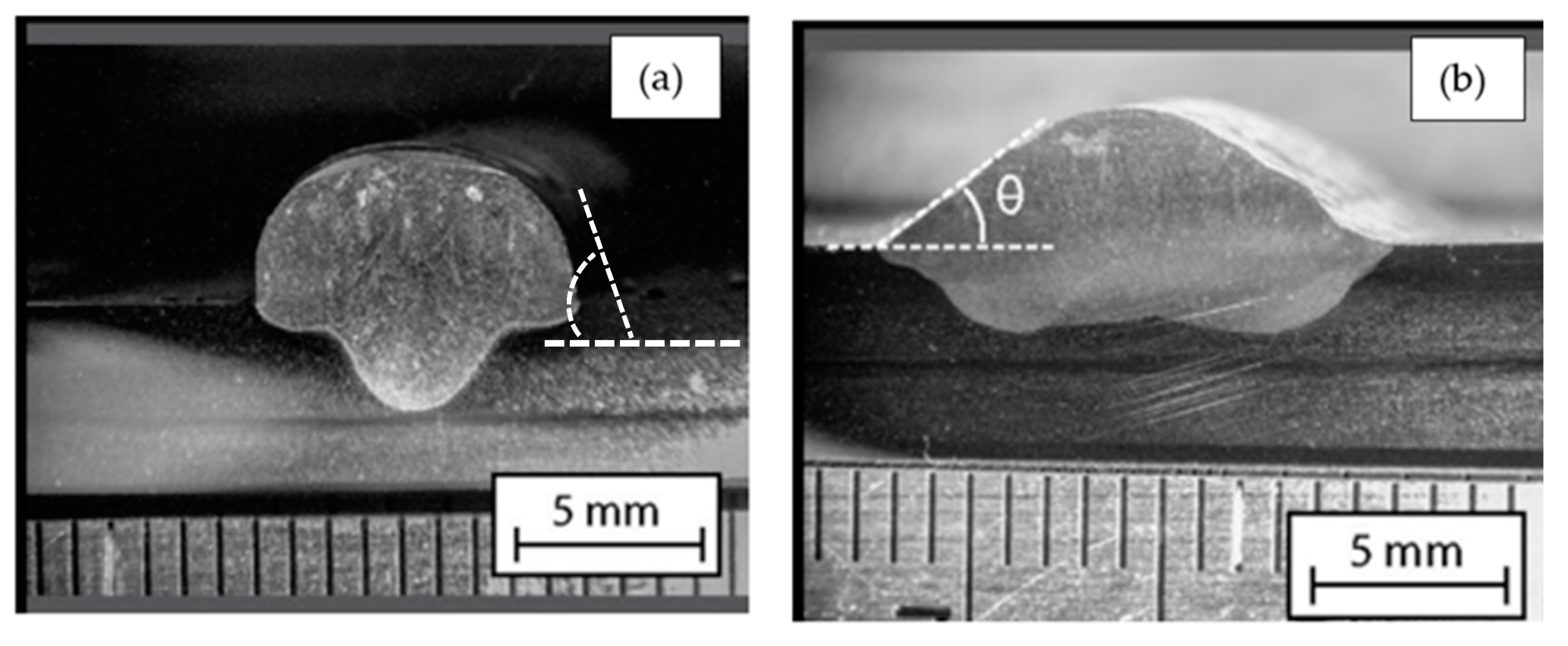
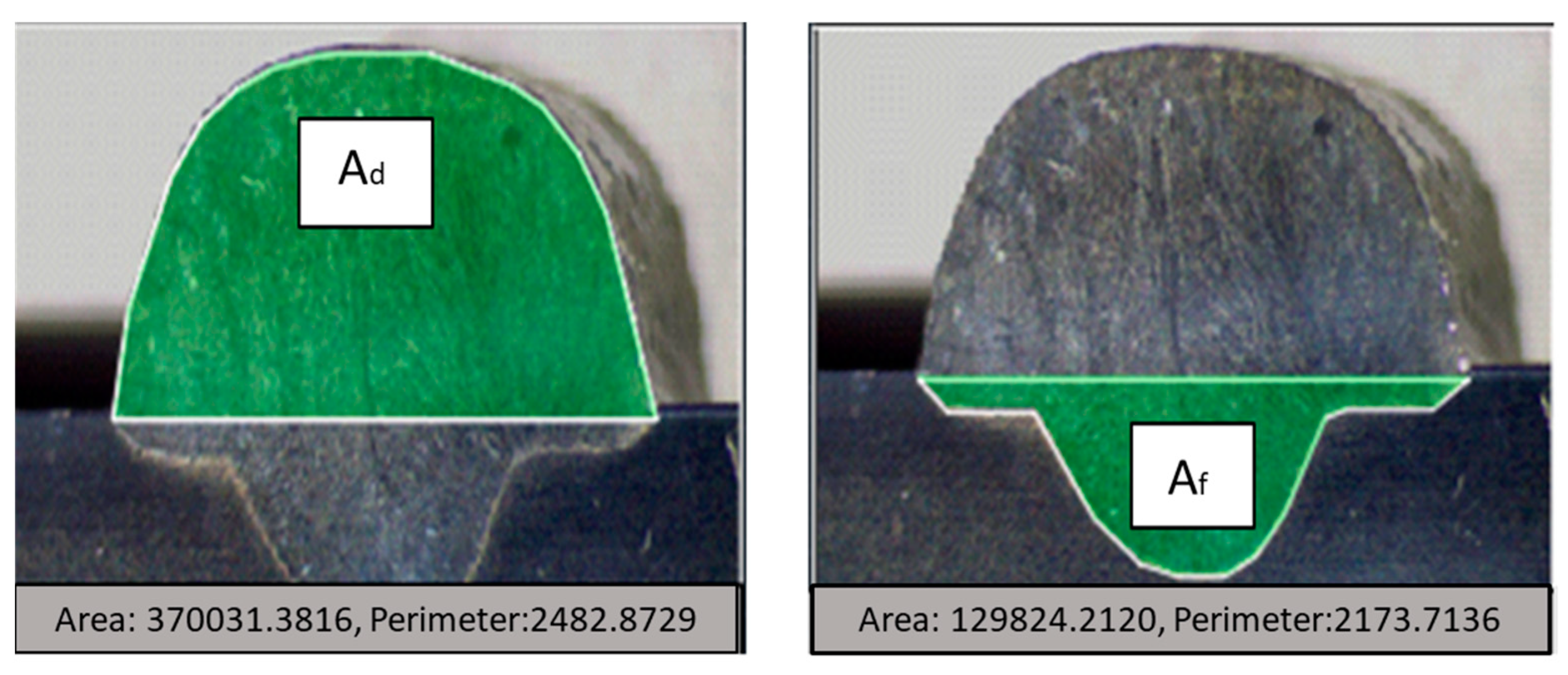
3.4. Geometric Analysis of Welds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peiró, G.; Prieto, C.; Gasia, J.; Jové, A.; Miró, L.; Cabeza, L.F. Two-tank molten salts thermal energy storage system for solar power plants at pilot plant scale: Lessons learnt and recommendations for its design, start-up and operation. Renew. Energy 2018, 121, 236–248. [Google Scholar] [CrossRef]
- Palacios, A.; Barreneche, C.; Navarro, M.; Ding, Y. Thermal energy storage technologies for concentrated solar power—A review from a materials perspective. Renew. Energy 2019, 156, 1244–1265. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Medrano, M.; Gil, A.; Martorell, I.; Potau, X.; Cabeza, L.F. State of the art on high-temperature thermal energy storage for power generation. Part 2—Case studies. Renew. Sustain. Energy Rev. 2010, 14, 56–72. [Google Scholar] [CrossRef]
- Prieto, C.; Rodríguez, A.; Patiño, D.; Cabeza, L.F. Thermal energy storage evaluation in direct steam generation solar plants. Sol. Energy 2018, 159, 501–509. [Google Scholar] [CrossRef]
- Vilchez, F.; Pineda, F.; Walczak, M.; Ramos-Grez, J. The effect of laser surface melting of stainless steel grade AISI 316L welded joint on its corrosion performance in molten Solar Salt. Sol. Energy Mater. Sol. Cells 2020, 213, 110576. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Osorio, J.D.; Mehos, M.; Imponenti, L.; Kelly, B.; Price, H.; Torres-Madronero, J.; Martinek, J. Failure Analysis for Molten Salt Thermal Energy Storage Tanks for In-Service CSP Plants Failure Analysis for Molten Salt Thermal Energy Storage Tanks for In-Service CSP Plants; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2024. [Google Scholar]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Suárez, C.; Iranzo, A.; Pino, F.; Guerra, J. Transient analysis of the cooling process of molten salt thermal storage tanks due to standby heat loss. Appl. Energy 2015, 142, 56–65. [Google Scholar] [CrossRef]
- Prieto, C.; Osuna, R.; Fernández, A.I.; Cabeza, L.F. Thermal storage in a MW scale. Molten salt solar thermal pilot facility: Plant description and commissioning experiences. Renew. Energy 2016, 99, 852–866. [Google Scholar] [CrossRef]
- Prieto, C.; Blindu, A.; Cabeza, L.F.; Valverde, J.; García, G. Molten Salts Tanks Thermal Energy Storage: Aspects to Consider during Design. Energies 2023, 17, 22. [Google Scholar] [CrossRef]
- Society, A.W.; Hong, Y.; Pickle, T.; Yu, Z.; Vidal, J.; Augustine, C. Impact of Plate Thickness and Joint Geometry on Residual Stresses in 347H Stainless Steel Welds. Weld. J. 2023, 102, 279S–292S. [Google Scholar] [CrossRef]
- Osorio, C.; Julian, D.; Mark, M.; Martinek, J.; Hamilton, B.; Imponenti, L.; Kelly, B.; Price, H.; Yu, Z.; Ni, C.; et al. Modeling of Stress Distribution in Molten Salt Thermal Energy Storage Tanks for In-Service Central Receiver Power Plants. In Proceedings of the 5th Therm, Energy Storage Work, San Antonio, TX, USA, 2–3 August 2023; p. 627281636. [Google Scholar]
- Austin Pérez, J.; Ruiz, V. Influencia de la Aplicación del Tratamiento Térmico Post Soldadura en el Acero SAE 4130; Universidad Libre Sede Bogota: Bogota, Colombia, 2014. [Google Scholar]
- Rodriguez, R.M.H.P.; Paredes, R.S.; Wido, S.H.; Calixto, A. Comparison of aluminum coatings deposited by flame spray and by electric arc spray. Surf. Coat. Technol. 2007, 202, 172–179. [Google Scholar] [CrossRef]
- Buchanan, V.; McCartney, D.; Shipway, P. A comparison of the abrasive wear behaviour of iron-chromium based hardfaced coatings deposited by SMAW and electric arc spraying. Wear 2007, 264, 542–549. [Google Scholar] [CrossRef]
- Amushahi, M.H.; Ashrafizadeh, F.; Shamanian, M. Characterization of boride-rich hardfacing on carbon steel by arc spray and GMAW processes. Surf. Coat. Technol. 2010, 204, 2723–2728. [Google Scholar] [CrossRef]
- Xu, R.; Song, G. Post-heat treatment of arc-sprayed coating prepared by the wires combination of Mg-cathode and Al-anode to form protective intermetallic layers. Appl. Surf. Sci. 2011, 257, 3097–3102. [Google Scholar] [CrossRef]
- Kondaiah, P.; Pitchumani, R. Novel textured surfaces for superior corrosion mitigation in molten carbonate salts for concentrating solar power. Renew. Sustain. Energy Rev. 2022, 170, 112961. [Google Scholar] [CrossRef]
- Ward, A.L.; Blackburn, L.D. Creep and Rupture Behavior of Weld-Deposited Type 16-8-2 Stainless Steel at 593 °C; Westinghouse Hanford Co., Ltd.: Richland, WA, USA, 1976. [Google Scholar]
- Olsson, C.-O.; Landolt, D. Passive films on stainless steels—chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Héctor, R.-P.O. Welding of chromium-nickel alloyed austenitic stainless steels. Technological recommen-dations. Cienc. Holguín 2017, 23, 47–56. [Google Scholar]
- Lara, M.; Díaz, V.V.; Camus, M.; Da Cunha, T.V. Effect of transverse arc oscillation on morphology, dilution and microstructural aspects of weld beads produced with short-circuiting transfer in GMAW. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–13. [Google Scholar] [CrossRef]
- Sindo, K. Welding Metallurgy, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- De Miranda, E.C.; Silva, C.C.; Motta, M.F.; de Miranda, H.C.; Farias, J.P. Avaliação do uso do tecimento sobre o nível de diluição e geometria do cordao de solda na soldagem TIG com alimentação automatica de arame frio. Soldag. Inspecao 2015, 20, 180–190. [Google Scholar] [CrossRef]
- ISO 5817:2023; Soldeo. Uniones Soldadas por Fusión en Acero, Níquel, Titanio y sus Aleaciones (Excluido el Soldeo por haz de Electrones). Niveles de Calidad para las Imperfecciones. UNE: Madrid, Spain, 2014.
- ASTM E384-22; Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Schempp, P.C.E.; Cross, A.P.; Rethmeier, M. Solidification of GTA aluminum weld metal: Part 2—Thermal conditions and model for columnar-to-equiaxed transition. Weld. J. 2014, 93, 3. [Google Scholar]
- Nabahat, M.; Ahmadpour, K.; Saeid, T. Effect of ultrasonic vibrations in TIG welded AISI 321 stainless steel: Microstructure and mechanical properties. Mater. Res. Express 2018, 5, 096509. [Google Scholar] [CrossRef]
- Kou, S.; Le, Y. Nucleation mechanism and grain refining of weld metal. Weld J. 1986, 65, 305s–313s. [Google Scholar]

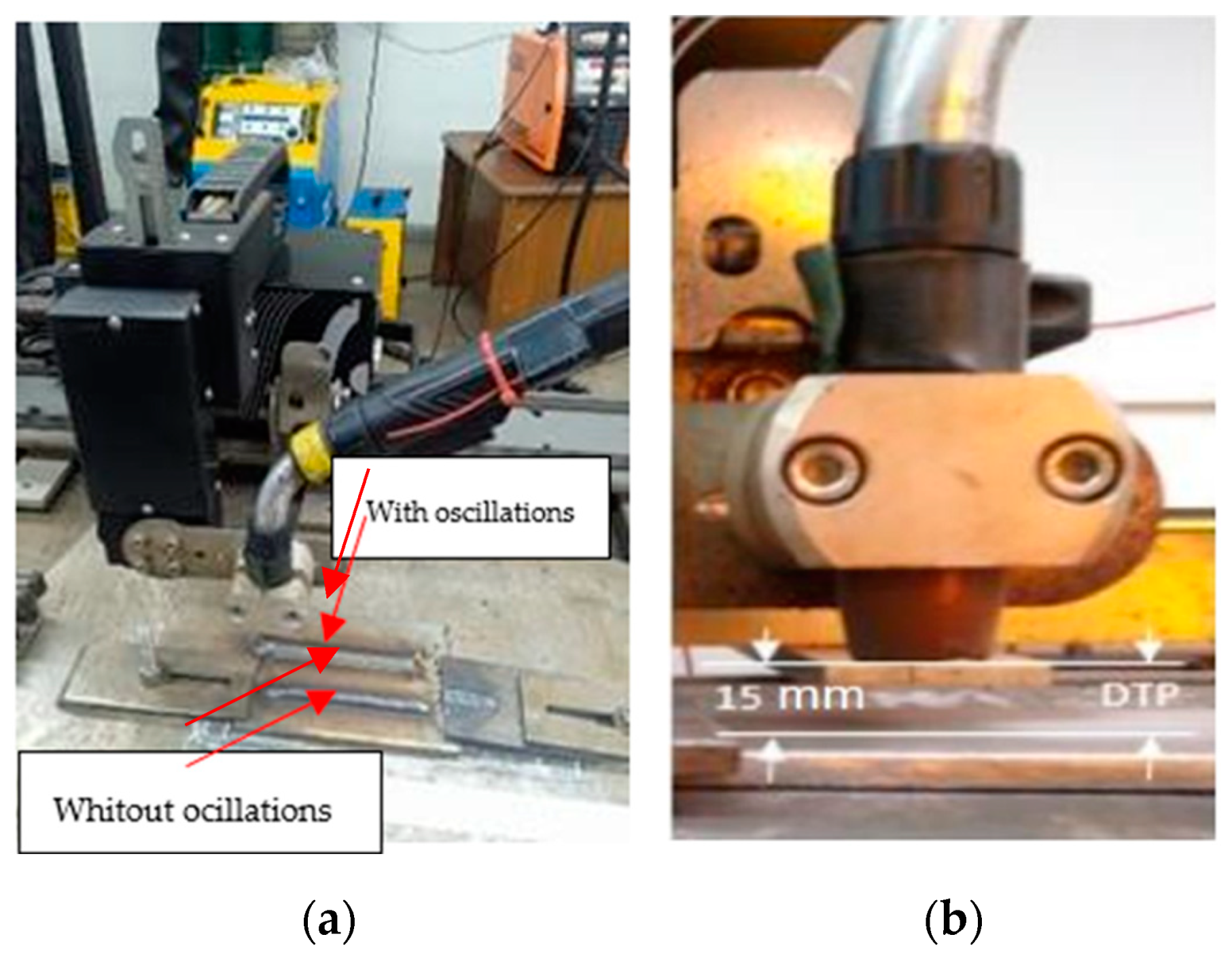
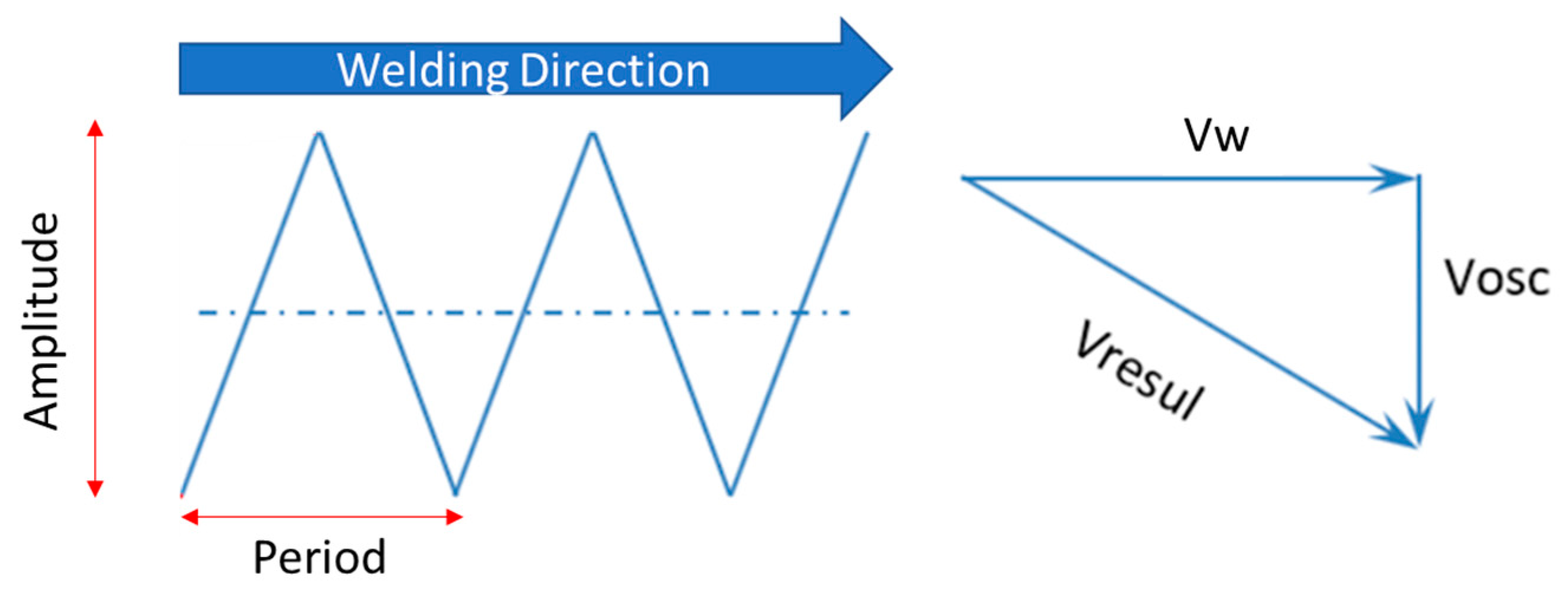


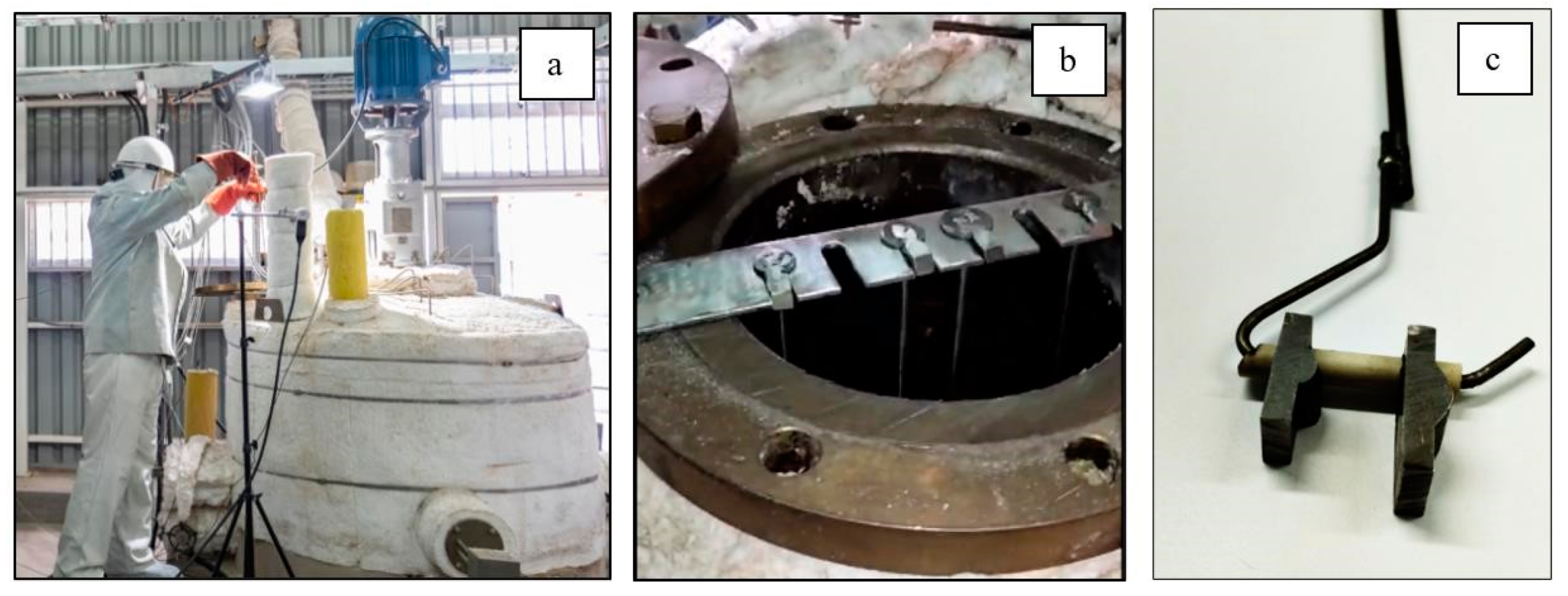
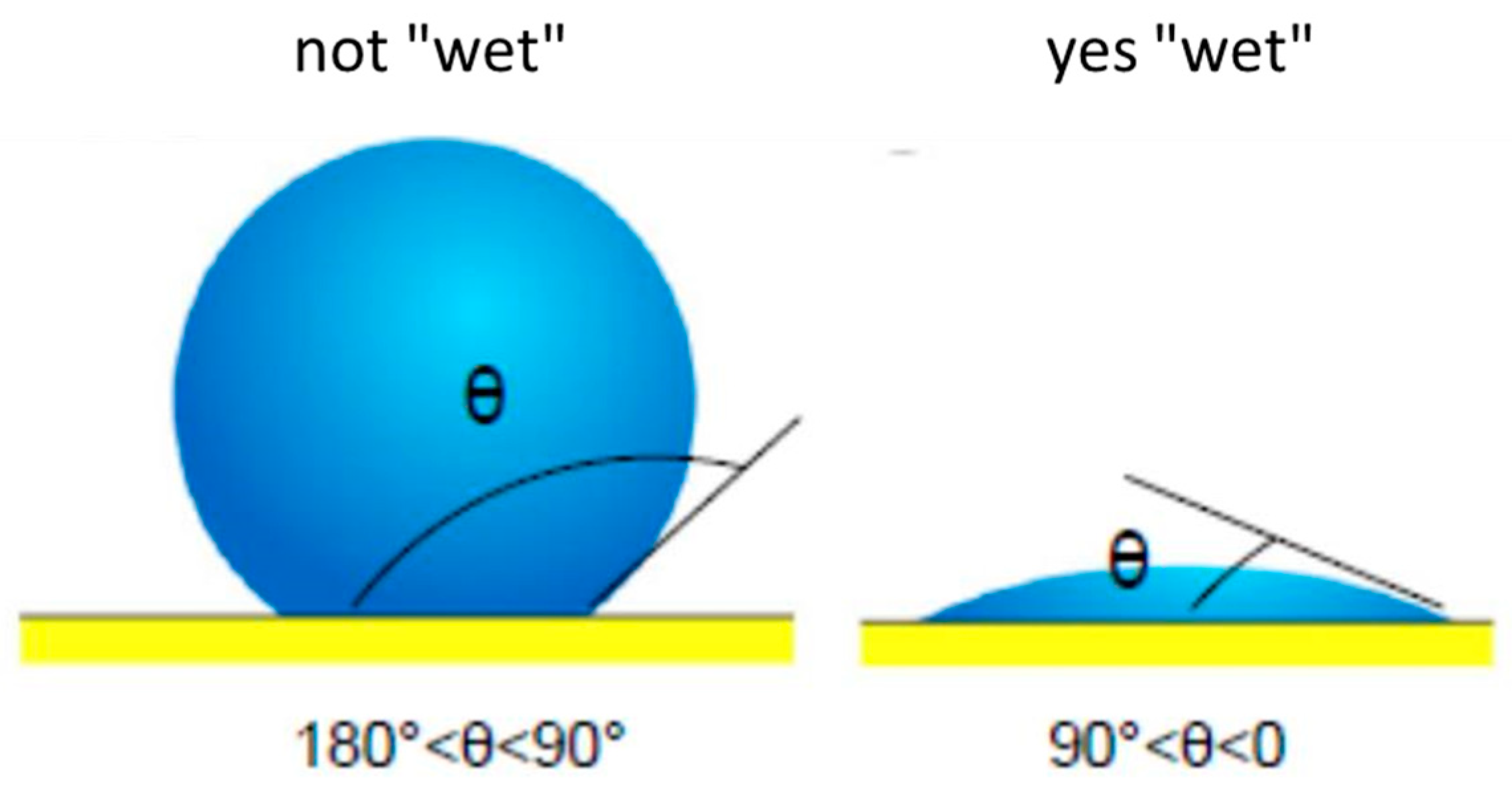




| AISI | C | Mn | Si | Cr | Ni | Mo | P | S |
|---|---|---|---|---|---|---|---|---|
| 316L | 0.039 | 1.14 | 0.393 | 16.54 | 10.30 | 1.99 | 0.024 | 0.0024 |
| Standard | 0.03 | 2.00 | 0.75 | 16–18 | 10–14 | 2–3 | 0.045 | 0.030 |
| Oscillation amplitude A (mm) | 12 |
| Reference voltage Ur (V) | 18 |
| Oscillation type | Triangular |
| Welding speed (cm/min) | 15 |
| Shielding gas type | 80% Ar + 20% CO2 |
| Flow rate (L/min) | 16 |
| Welding position | Flat |
| Transfer type | Short Circuit |
| Nozzle-to-work distance DTP (mm) | 15 |
| Wire type | AWS ER316L |
| Wire diameter (mm) | 1.2 |
| Welding Bead Type | Oscillations Type | Frequency (Hz) | Wire Speed (m/min) |
|---|---|---|---|
| Without oscillations | N/A | 0 | 4.0 |
| With oscillations | Triangular | 1 | 4.0 |
| Test | (mm/min) | (A) | (KJ/mm) |
|---|---|---|---|
| Without oscillations | 150 | 145 | 0.960 |
| With Oscillations | 735 | 143 | 0.188 |
| Description | Composition | (% wt) |
|---|---|---|
| Standard Specification | C | 0.02 |
| AWS | Mn | 1.69 |
| AWS ER-316 L | Si P | 0.38 0.03 |
| Material | S | 0.008 |
| Stainless steel (ASS) | Cr Ni Mo | 18.1 11.1 2.1 |
| Item | Hardness HV (Without Oscillations) | Hardness HV (With Oscillations) |
|---|---|---|
| 1 | 233 | 188 |
| 2 | 222 | 181 |
| 3 | 237 | 192 |
| 4 | 241 | 188 |
| 5 | 229 | 192 |
| 6 | 237 | 183 |
| 7 | 225 | 176 |
| 8 | 230 | 173 |
| 9 | 221 | 172 |
| 10 | 206 | 180 |
| 11 | 202 | 188 |
| 12 | 200 | 190 |
| 13 | 203 | |
| 14 | 205 | |
| 15 | 210 | |
| Average | 220 | 184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastén, R.; Henríquez, M.; Nabahat, M.; Vergara, V.; Reinoso-Burrows, J.C.; Soto, C.; Durán, C.; Fuentealba, E.; Guerreiro, L. Impact of Mechanical Arc Oscillation on the Microstructure and Durability of Welded Joints in Molten Salt Thermal Storage System. Materials 2025, 18, 1619. https://doi.org/10.3390/ma18071619
Pastén R, Henríquez M, Nabahat M, Vergara V, Reinoso-Burrows JC, Soto C, Durán C, Fuentealba E, Guerreiro L. Impact of Mechanical Arc Oscillation on the Microstructure and Durability of Welded Joints in Molten Salt Thermal Storage System. Materials. 2025; 18(7):1619. https://doi.org/10.3390/ma18071619
Chicago/Turabian StylePastén, Raúl, Mauro Henríquez, Mehran Nabahat, Victor Vergara, Juan C. Reinoso-Burrows, Carlos Soto, Carlos Durán, Edward Fuentealba, and Luis Guerreiro. 2025. "Impact of Mechanical Arc Oscillation on the Microstructure and Durability of Welded Joints in Molten Salt Thermal Storage System" Materials 18, no. 7: 1619. https://doi.org/10.3390/ma18071619
APA StylePastén, R., Henríquez, M., Nabahat, M., Vergara, V., Reinoso-Burrows, J. C., Soto, C., Durán, C., Fuentealba, E., & Guerreiro, L. (2025). Impact of Mechanical Arc Oscillation on the Microstructure and Durability of Welded Joints in Molten Salt Thermal Storage System. Materials, 18(7), 1619. https://doi.org/10.3390/ma18071619







