The Influence of Anhydrite on the Mechanical Performance of Calcium Sulfoaluminate Cement-Based Grouting Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportions
2.3. Test Methods
2.3.1. Compressive Strength Test
2.3.2. pH Test
2.3.3. Setting Time Test
2.3.4. Expansion Test
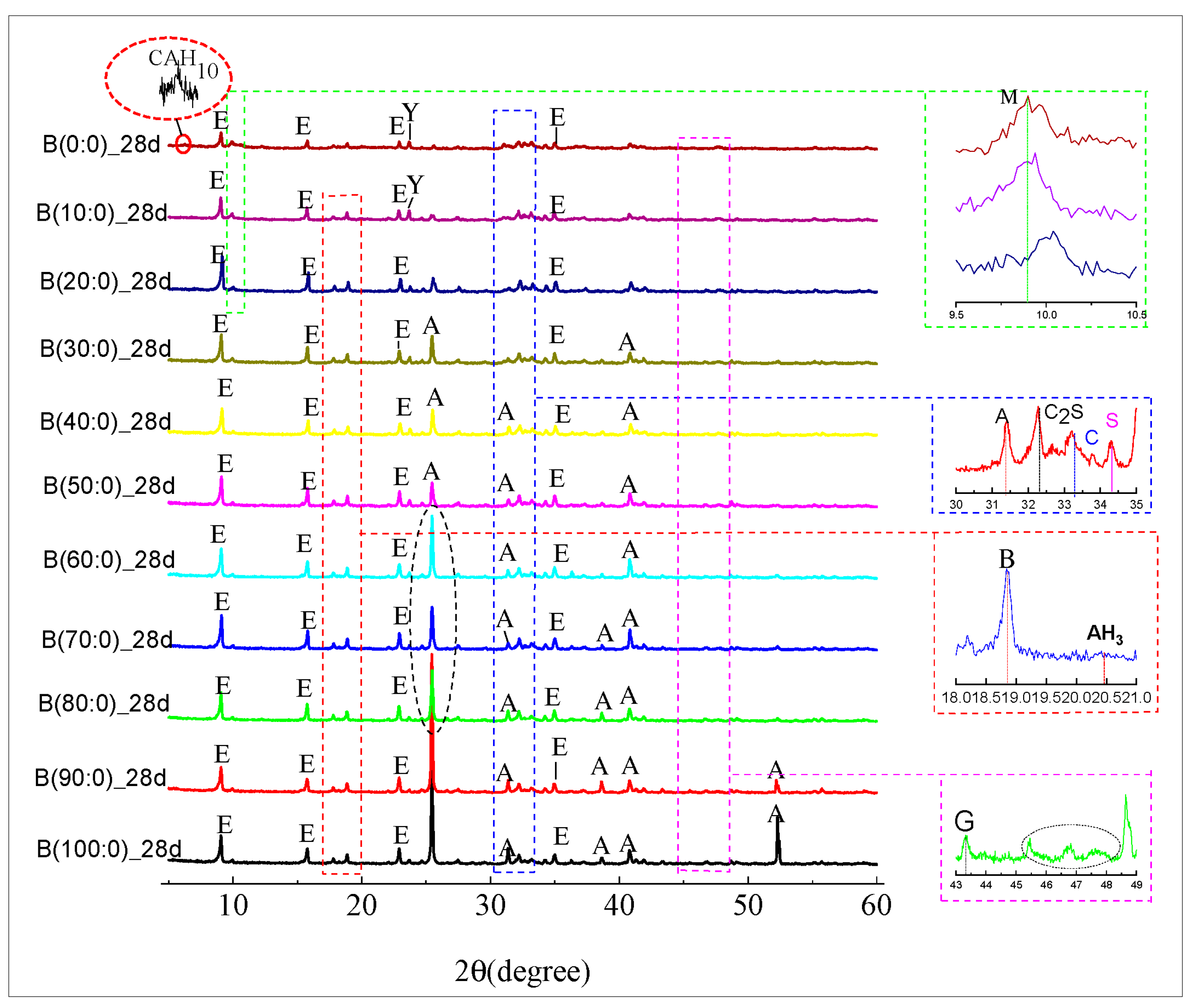
2.3.5. X-Ray Diffraction (XRD)
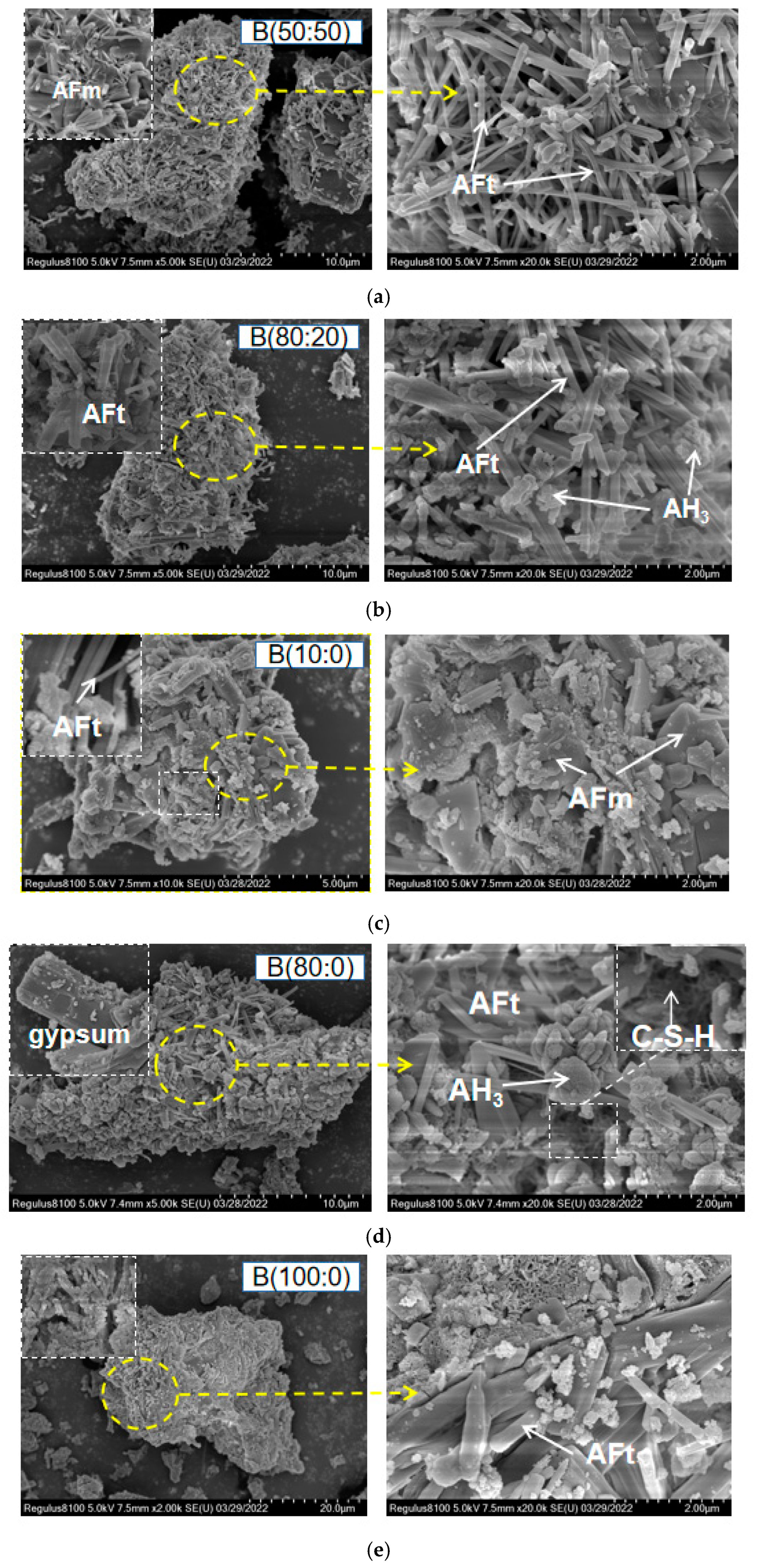
2.3.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
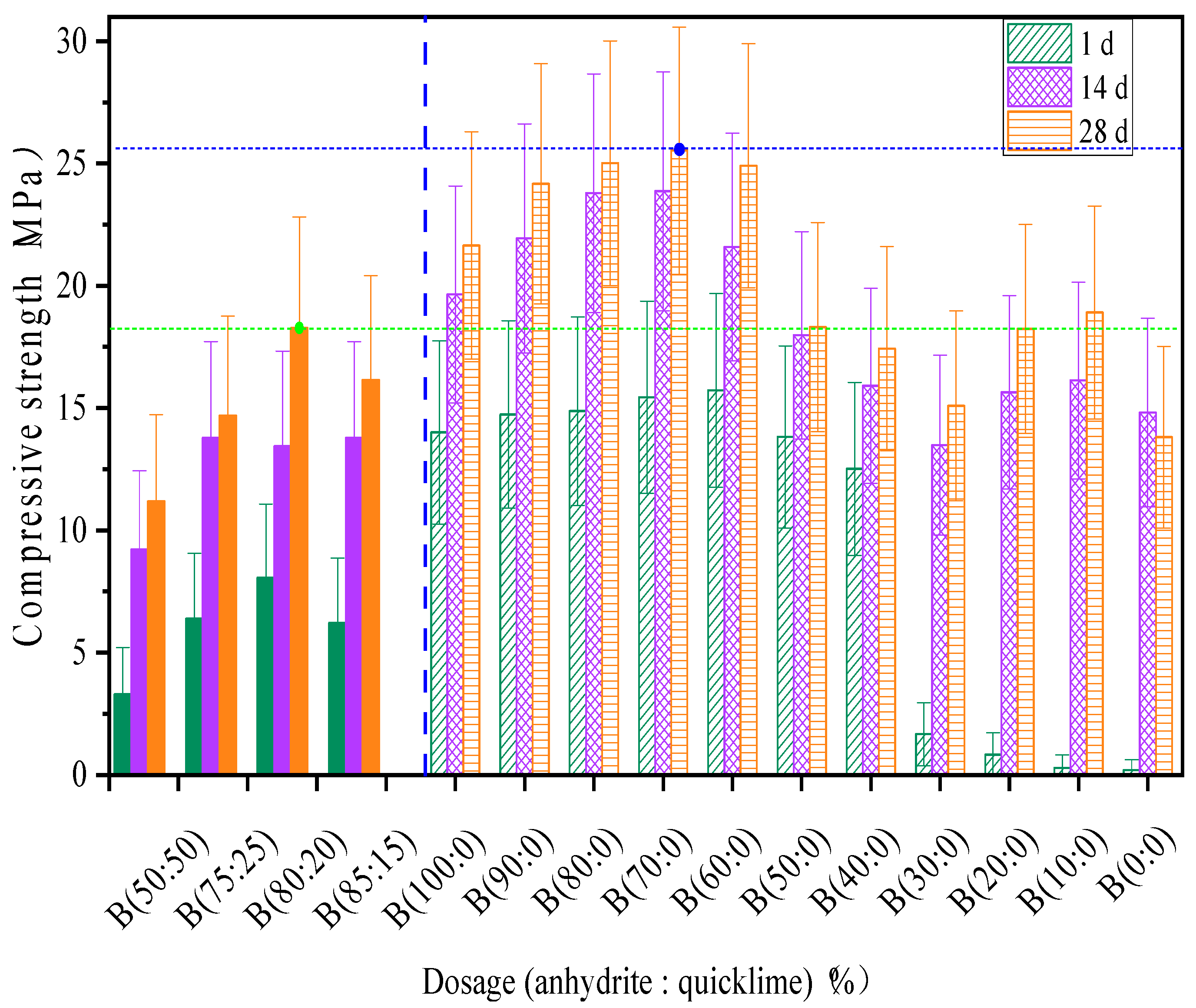
3.1. Compressive Strength
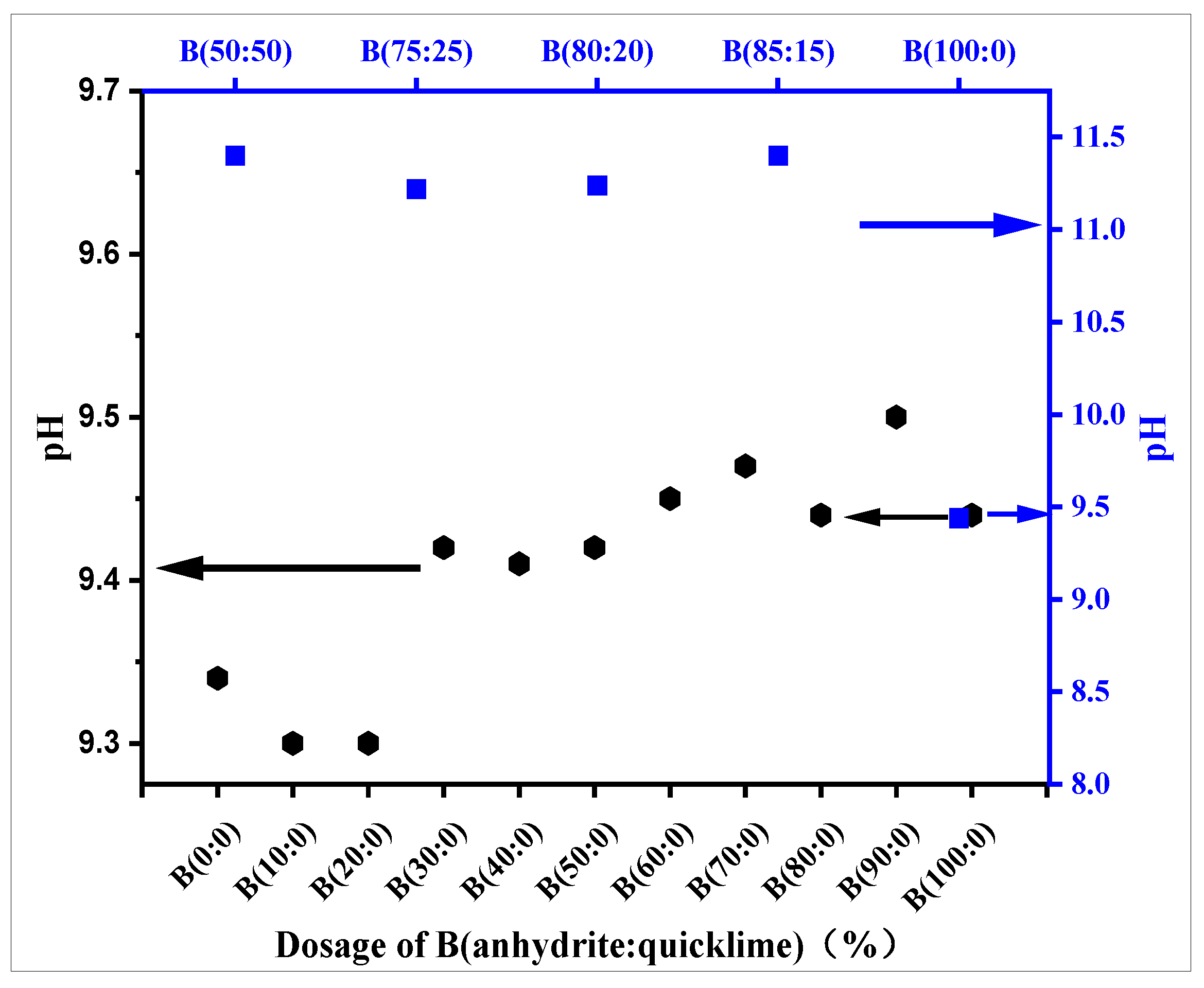
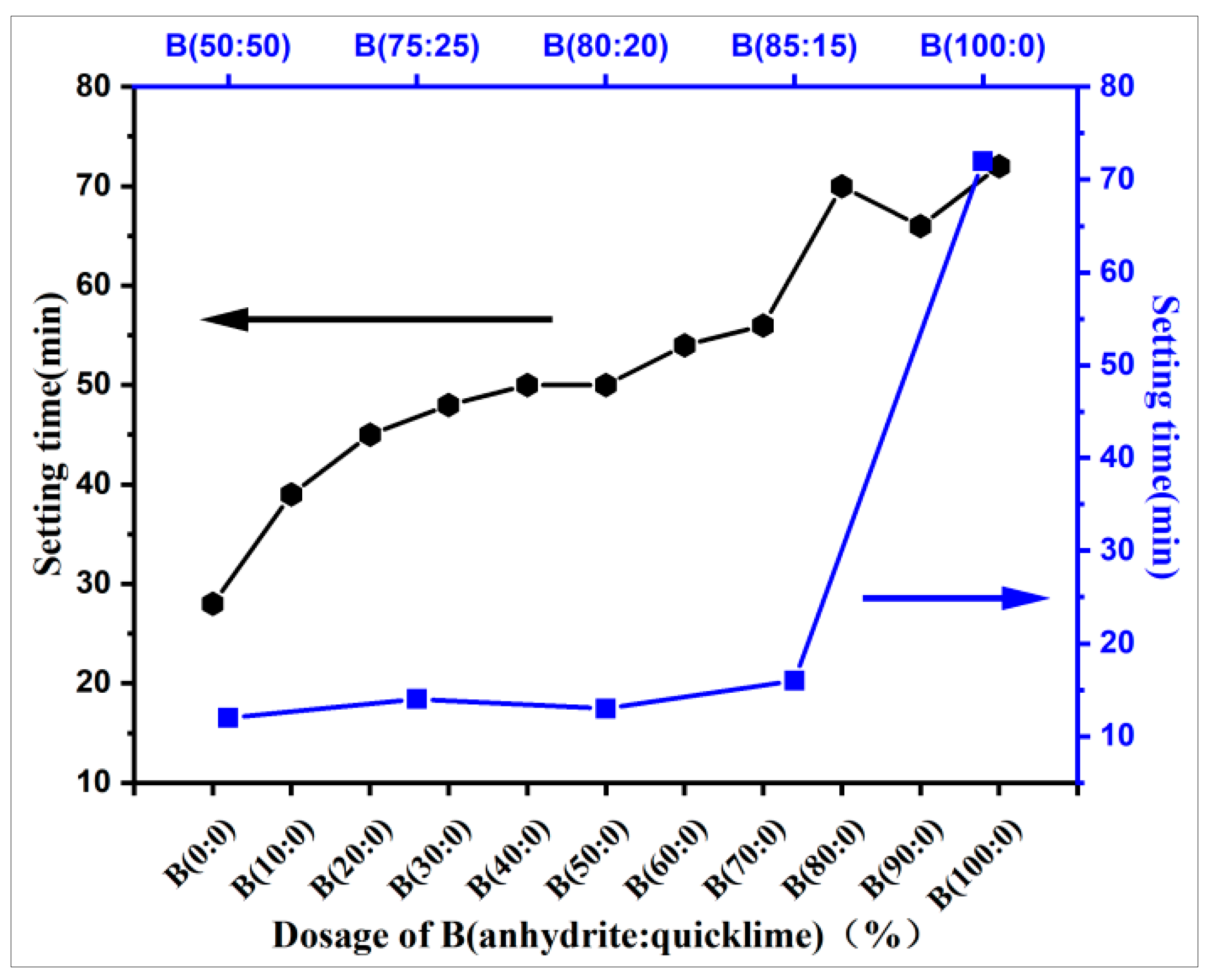
3.2. pH and Setting Time
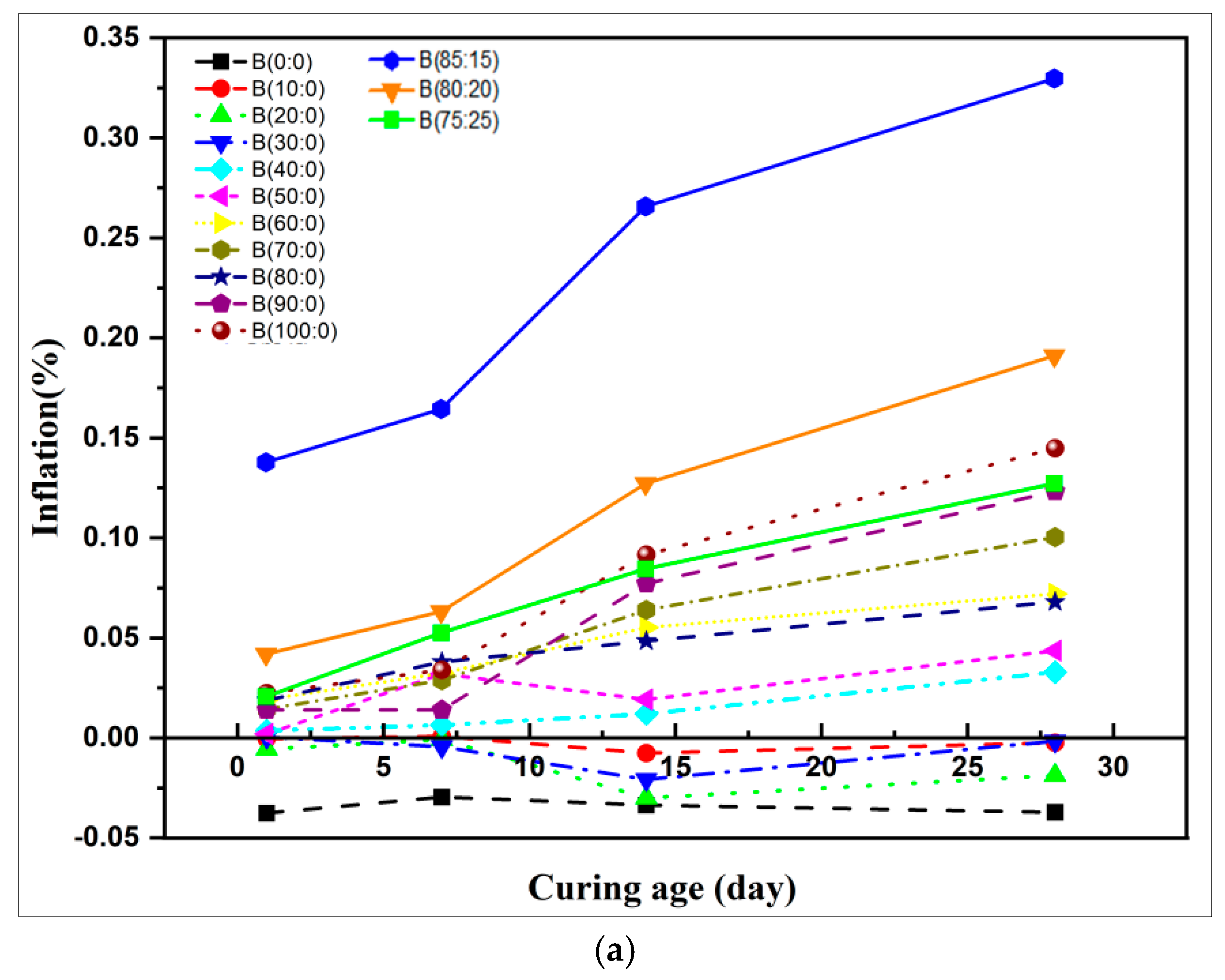
3.3. Expansion Properties
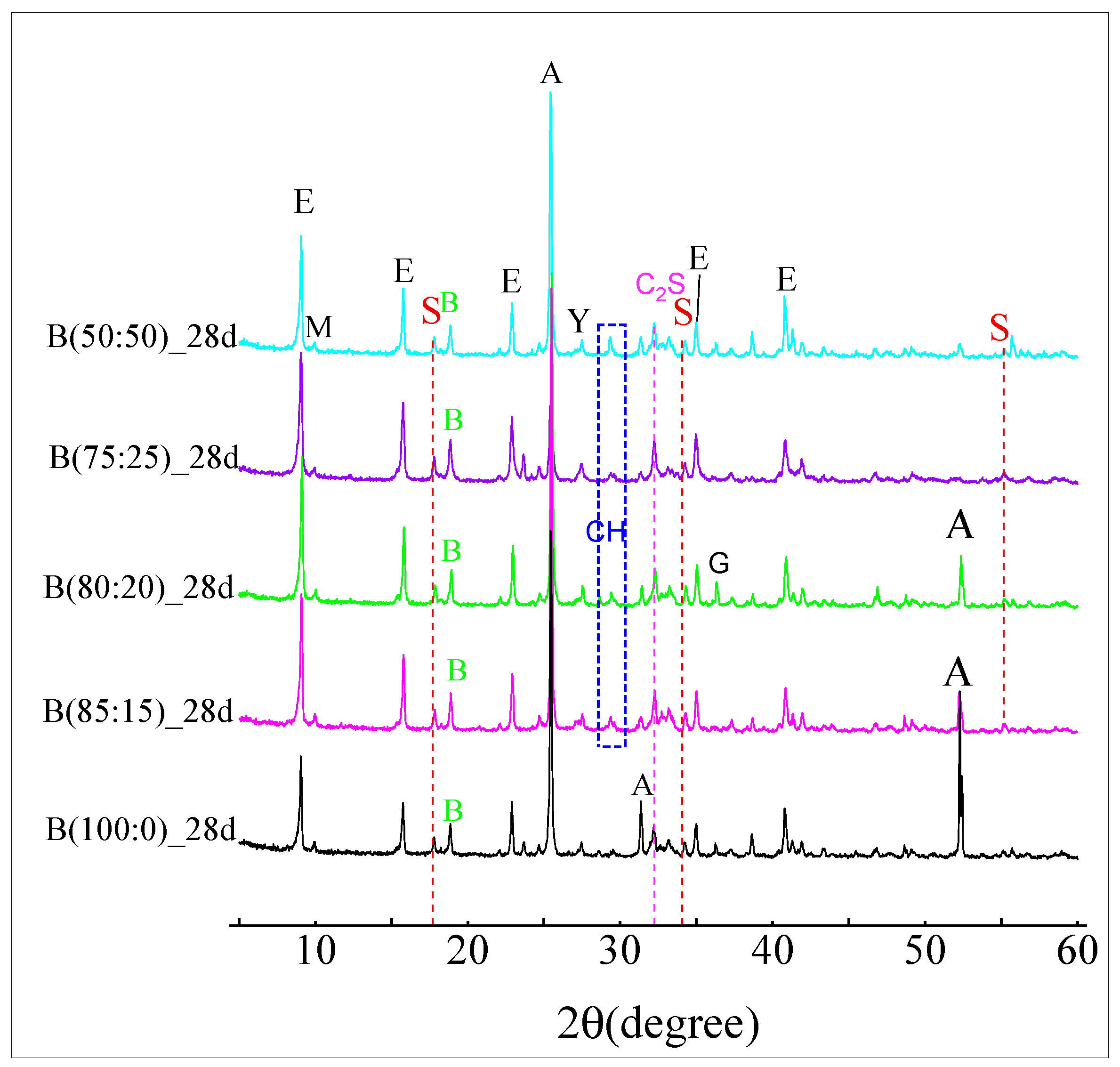
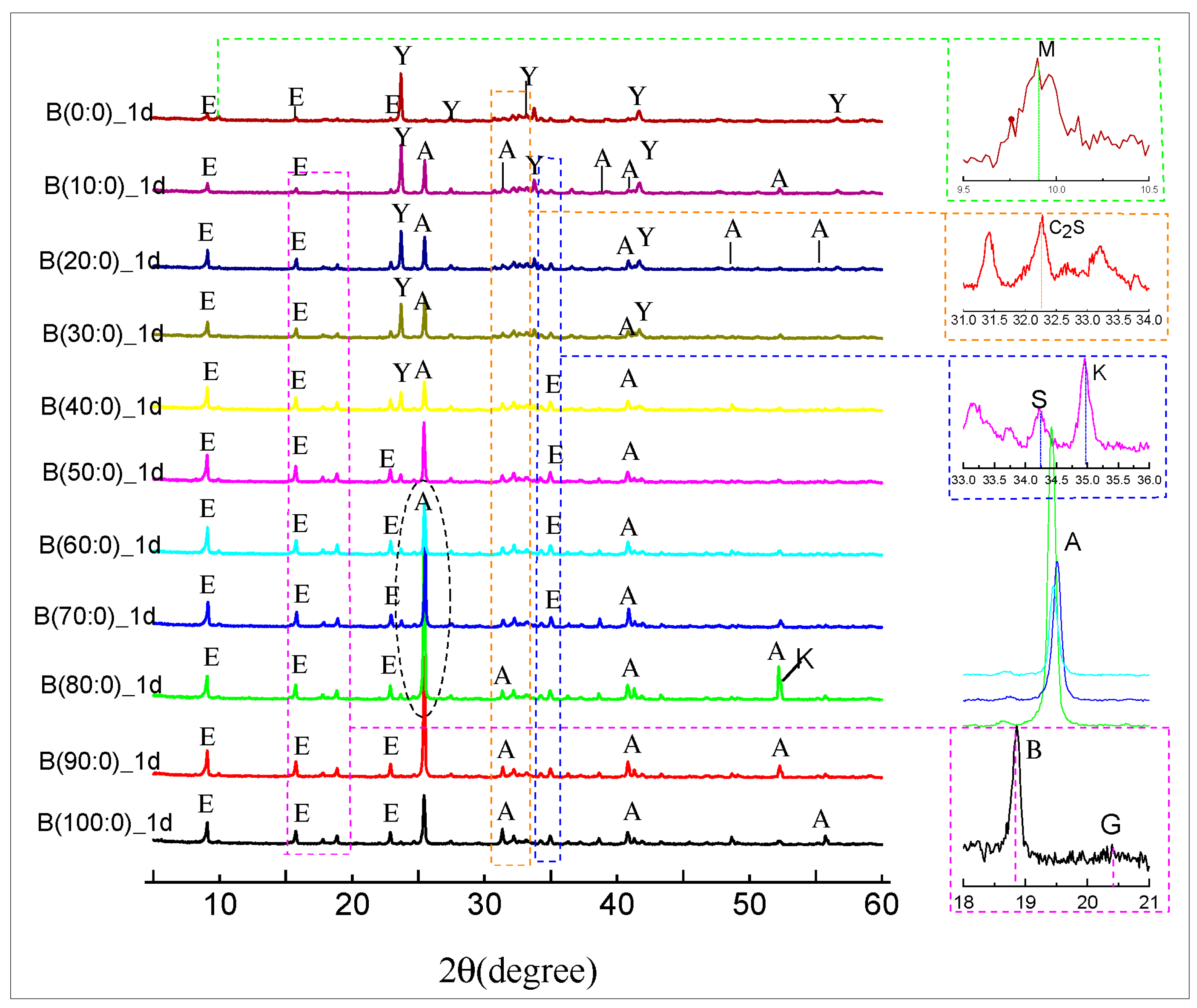
3.4. XRD Material Phase Hydration of the CSAGM
3.5. SEM Material Phase Microstructure of the CSAGM
4. Conclusions
- (1)
- Due to the relatively fast dissolution rate of anhydrite, the high strong and ettringite formation can be completed before 3 h of hydration when anhydrite is selected at a ratio (≥40%), causing non-expansion characteristic on the specimen hardened body in the later stage of hydration. The optimal dose of anhydrite in the CSA grouting material paste is 80%. (80% when it is incorporated alone, and 80:20 when it is incorporated with quicklime). This dose increases the ultimate strength at all ages and makes up 42.5% and 38.5% of the compressive strength independent or dependent on quicklime.
- (2)
- The incorporation of anhydrite alone does not affect the pH of the grouting material paste compared to anhydrite–quicklime dosage. It increases the pH value of the grouting material paste and releases a large amount of hydroxide (OH−), which increases the alkalinity of the system This significantly prolongs the setting time of the CSA grouting material paste but does not have a direct effect on the ettringite production, nor the early or final strength.
- (3)
- The equilibrium increase in the incorporation of the anhydrite–quicklime ratio slows the dissolution rate of the paste, leads to the expansion rate, and constrains the strength, thus leading to unified ettringite production. Increasing the anhydrite dosage inhibited the expansion performance and increased the strength. Besides, a uniformly distributed ettringite (AFt), calcium silicate hydrate, (C-S-H), and aluminum hydroxide (AH3) were produced.
- (4)
- The incorporation of anhydrite has a positive significant effect on the dicalcium silicate (β-C2S) hydration products of CSAGM. The XRD and SEM characterizations confirmed that the hydration products of the CSAGM system are rich in needle-like AFt crystals, and plume-shaped aluminum hydroxide (AH3), which contribute to strength improvement. The hydration of dicalcium silicate (β-C2S) is inhibited by forming calcium silicate hydrate (C-S-H), due to its dissolution into calcium hydroxide, which produces trace amounts of katoite (C2ASH8) as the hydration product.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSA | Calcium sulfoaluminate |
| CSAGM | Calcium sulfoaluminate grouting mater |
| wt.% | Weight percentage |
| pH | Potential of hydrogen |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EX | Expansion rate |
| LX | Length (test block) |
| L1 | Initial length (test block) |
References
- Wang, Q.; Jiang, Z.; Jiang, B.; Gao, H.; Huang, Y.; Zhang, P. Research on an automatic roadway formation method in deep mining areas by roof cutting with high-strength bolt-grouting. Int. J. Rock Mech. Min. Sci. 2020, 128, 104264. [Google Scholar] [CrossRef]
- Ding, W.; Wang, Z.; Huang, X.; Chen, L.; Zheng, Y. Influence of corrosion on anchoring bond behavior of jointed rock mass. KSCE J. Civ. Eng. 2022, 26, 1914–1928. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Z.; Nie, W.; Gui, Y. A numerical model of fully grouted bolts considering the tri-linear shear bond-slip model. Tunn. Undergr. Space Technol. 2016, 54, 73–80. [Google Scholar] [CrossRef]
- Dai, H.L.; Wang, X.; Xie, G.X.; Wang, X.Y. Theoretical model, and solution for the rheological problem of anchor-grouting a soft rock tunnel. Int. J. Press. Vessel. Pip. 2004, 81, 739–748. [Google Scholar] [CrossRef]
- Bobet, A.; Einstein, H.H. Tunnel reinforcement with rockbolts. Tunn. Undergr. Space Technol. 2011, 26, 100–123. [Google Scholar] [CrossRef]
- He, S.; Lai, J.; Wang, L.; Wang, K. A literature review on properties and applications of grouts for shield tunnel. Constr. Build. Mater. 2020, 239, 117782. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Yang, X.; Ren, S.; Song, Z. Study on a high-strength ternary blend containing calcium sulfoaluminate cement/calcium aluminate cement/ordinary Portland cement. Constr. Build. Mater. 2018, 191, 544–553. [Google Scholar] [CrossRef]
- Gu, P.; Beaudoin, J.J.; Quinn, E.G.; Myers, R.E. Early strength development and hydration of ordinary Portland cement/calcium aluminate cement pastes. Adv. Cem.-Based Mater. 1997, 6, 53–58. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.-M.; Lecomte, A.; Roux, A.; Le Rolland, B. Impact of anhydrite proportion in a calcium sulfoaluminate cement and Portland cement blend. Adv. Cem. Res. 2014, 26, 325–333. [Google Scholar] [CrossRef]
- Lu, J.; Ling, S.; Wang, X.; Li, X.; Liu, D. Research on Portland Cement Grouting Material in Sandy Pebble Soil. Sci. Adv. Mater. 2019, 11, 1027–1036. [Google Scholar] [CrossRef]
- Mollamahmutoğlu, M.; Avci, E. Ultrafine Portland cement grouting performance with or without additives. KSCE J. Civ. Eng. 2015, 19, 2041–2050. [Google Scholar] [CrossRef]
- Moosavi, M.; Bawden, W.F. Shear strength of Portland cement grout. Cem. Concr. Compos. 2003, 25, 729–735. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Sabbah, A.; Zhutovsky, S. Effect of sulfate content and synthesis conditions on the phase composition of belite-ye’elimite-ferrite (BYF) clinker. Cem. Concr. Res. 2022, 155, 106745. [Google Scholar] [CrossRef]
- Fu, J.; Hu, B.; Guo, W.; Hu, Y.; Jiang, C. Effect of additive compounding on early properties of belite sulfoaluminate cement. Adv. Cem. Res. 2024, 36, 164–178. [Google Scholar] [CrossRef]
- Habert, G. 1—Environmental impact of Portland cement production. In Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V.M., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–25. [Google Scholar] [CrossRef]
- Ige, O.E.; Olanrewaju, O.A.; Duffy, K.J.; Collins, O.C. Environmental Impact Analysis of Portland Cement (CEM1) Using the Midpoint Method. Energies 2022, 15, 2708. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Giergiczny, Z.; Król, A.; Tałaj, M.; Wandoch, K. Performance of concrete with low CO2 emission. Energies 2020, 13, 4328. [Google Scholar] [CrossRef]
- Popescu, C.D.; Muntean, M.; Sharp, J.H. Industrial trial production of low energy belite cement. Cem. Concr. Compos. 2003, 25, 689–693. [Google Scholar] [CrossRef]
- Sherman, N.; Beretka, J.; Santoro, L.; Valenti, G.L. Long-term behaviour of hydraulic binders based on calcium sulfoaluminate and calcium sulfosilicate. Cem. Concr. Res. 1995, 25, 113–126. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A.; Hashim, M.H. Environmental impact of cement production and Solutions: A review. Mater. Today Proc. 2022, 48, 741–746. [Google Scholar] [CrossRef]
- Soomro, M.; Tam, V.W.; Evangelista, A.C.J. Production of cement and its environmental impact. In Recycled Concrete; Elsevier: Amsterdam, The Netherlands, 2023; pp. 11–46. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration characteristics of calcium sulfoaluminate (CSA) cement/portland cement blended pastes. J. Build. Eng. 2021, 34, 8650. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xu, Y.M.; Geng, C.L. Sulfoaluminate cement: An alternative to portland cement. Adv. Mater. Res. 2012, 368–373, 478–484. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Ben Haha, M. CSA raw mix design: Effect on clinker formation and reactivity. Mater. Struct. 2015, 48, 3895–3911. [Google Scholar] [CrossRef]
- Gu, X.; Tan, H.; He, X.; Smirnova, O.; Zhang, J.; Luo, Z. Utilization of carbide slag by wet grinding as an accelerator in calcium sulfoaluminate cement. Materials 2020, 13, 4526. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Ma, J.; Yu, Z.; Ni, C.; Shi, H.; Shen, X. Effects of limestone powder on the hydration and microstructure development of calcium sulphoaluminate cement under long-term curing. Constr. Build. Mater. 2019, 199, 688–695. [Google Scholar] [CrossRef]
- Shen, B.; Poulsen, B. Investigation of overburden behaviour for grout injection to control mine subsidence. Int. J. Min. Sci. Technol. 2014, 24, 317–323. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Zhou, Y. Application of a novel grouting material for prereinforcement of shield tunnelling adjacent to existing piles in a soft soil area. Tunn. Undergr. Space Technol. 2022, 128, 104646. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Hu, W.; Wu, K. Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cem. Concr. Compos. 2014, 46, 26–31. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Compos. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H. A review on effects of limestone powder on the properties of concrete. Constr. Build. Mater. 2018, 192, 153–166. [Google Scholar] [CrossRef]
- Elgalhud, A.A.; Dhir, R.K.; Ghataora, G. Limestone addition effects on concrete porosity. Cem. Concr. Compos. 2016, 72, 222–234. [Google Scholar] [CrossRef]
- Tsivilis, S.; Tsantilas, J.; Kakali, G.; Chaniotakis, E.; Sakellariou, A. The permeability of Portland limestone cement concrete. Cem. Concr. Res. 2003, 33, 1465–1471. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, C. Improved ettringite stabilization by calcium carbonate and calcium nitrate additions in ternary PC-CSA-C $ systems. Cem. Concr. Res. 2024, 175, 107383. [Google Scholar] [CrossRef]
- Pelletier, L.; Winnefeld, F.; Lothenbach, B. The ternary system Portland cement–calcium sulphoaluminate clinker–anhydrite: Hydration mechanism and mortar properties. Cem. Concr. Compos. 2010, 32, 497–507. [Google Scholar] [CrossRef]
- Long, Z.; Long, G.; Tang, Z.; Shangguan, M.; Zhang, Y.; Wang, L.; Peng, L.; Yi, M. Hydration, strength, and microstructure evolution of Portland cement-calcium sulphoaluminate cement-CSH seeds ultra-early strength cementitious system. Constr. Build. Mater. 2024, 430, 136492. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Z.; Gao, X.; Liu, C.; Yang, L. Study on properties of Portland cement-sulfoaluminate cement-based grouting materials modified by in-situ polymerization of calcium acrylate. Constr. Build. Mater. 2023, 409, 134087. [Google Scholar] [CrossRef]
- GB 2002;50081:3–5; Rong, J.M.; Lu, J.W. Shape and Tolerances of Test Specimens. The Standard for Test Methods of Mechanical Properties of Ordinary Concrete. China Building Industry Press: Beijing, China, 2003.
- ASTM C191-08 2008; Standard Test Methods for the Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2008.
- Wetton, R.E. Thermomechanical methods. In Handbook of Thermal Analysis and Calorimetry; Elsevier B.V.: Amsterdam, The Netherlands, 1998; pp. 363–399. [Google Scholar] [CrossRef]
- Gabrisova, A.; Havlica, J.; Sahu, S. Stability of calcium sulphoaluminate hydrates in water solutions with various pH values. Cem. Concr. Res. 1991, 21, 1023–1027. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Mehta, P.K. Mechanism of expansion associated with ettringite formation. Cem. Concr. Res. 1973, 3, 1–6. [Google Scholar] [CrossRef]
- Odler, I.; Colán-Subauste, J. Investigations on cement expansion associated with ettringite formation. Cem. Concr. Res. 1999, 29, 731–735. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]



| Mineral | β–C2S | C2F | Fe2O3 | |
|---|---|---|---|---|
| wt.% | 58.64 | 24.43 | 6.41 | 1.92 |
| Oxide | Fe2O3 | TiO2 | Al2O3 | CaO | MgO | SiO2 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|
| wt.% | 2.21 | 1.45 | 29.54 | 45.36 | 1.94 | 10.59 | 8.45 | 0.46 |
| Oxide | SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | TiO2 | Loss |
|---|---|---|---|---|---|---|---|---|
| wt.% | 2.83 | 39.5 | 0.23 | 1.36 | 0.03 | 54.16 | 1.17 | 0.75 |
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| CSA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| anhydrite | 0 | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| Sample Number | Slurry A | Slurry B | |
|---|---|---|---|
| CSA | Anhydrite | Quicklime | |
| 1-A/B(50:50) | 100 wt.% | 50 wt.% | 50 wt.% |
| 2-A/B(75:25) | 100 wt.% | 75 wt.% | 25 wt.% |
| 3-A/B(80:20) | 100 wt.% | 80 wt.% | 20 wt.% |
| 4-A/B(85:15) | 100 wt.% | 85 wt.% | 15 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Teah, N.S.; Liao, Z.; Hao, R.; Zhao, Y.; Xue, W. The Influence of Anhydrite on the Mechanical Performance of Calcium Sulfoaluminate Cement-Based Grouting Materials. Materials 2025, 18, 1547. https://doi.org/10.3390/ma18071547
Liao L, Teah NS, Liao Z, Hao R, Zhao Y, Xue W. The Influence of Anhydrite on the Mechanical Performance of Calcium Sulfoaluminate Cement-Based Grouting Materials. Materials. 2025; 18(7):1547. https://doi.org/10.3390/ma18071547
Chicago/Turabian StyleLiao, Lin, Nathan Saye Teah, Zhiling Liao, Ruiqing Hao, Yubin Zhao, and Wanwen Xue. 2025. "The Influence of Anhydrite on the Mechanical Performance of Calcium Sulfoaluminate Cement-Based Grouting Materials" Materials 18, no. 7: 1547. https://doi.org/10.3390/ma18071547
APA StyleLiao, L., Teah, N. S., Liao, Z., Hao, R., Zhao, Y., & Xue, W. (2025). The Influence of Anhydrite on the Mechanical Performance of Calcium Sulfoaluminate Cement-Based Grouting Materials. Materials, 18(7), 1547. https://doi.org/10.3390/ma18071547






