Assessment of Quality in Antimicrobial Calcium Phosphate Research (AQUACAP): A Systematic Review
Abstract
1. Introduction
- To develop a novel checklist for assessing essential quality items of preclinical studies on antimicrobial ion-substituted CaPs by a panel of subject matter experts.
- Using this checklist to assess the quality of existing literature and identify areas for improvement.
2. Materials and Methods
2.1. Design of the Checklist
- Use and reporting of positive and negative control groups.
- Material characterisation, especially phase characterisation and elemental analysis.
- Reproducibility and validity of the antimicrobial tests.
- Reporting and discussion of the results of antimicrobial tests.
- Use of replicate experiments and statistics.
- Testing of material toxicity.
- Rationales for experiment design choices.
2.2. Implementation of the Checklist
2.3. Statistical Analysis
3. Results
3.1. Design of the Checklist
3.2. Implementation of the Checklist
- The reporting of the starting number of microorganisms;
- Reporting the antimicrobial test results on a logarithmic scale;
- Reporting a rationale for the chosen microorganisms.
- Reporting of negative control groups;
- Material characterisation using X-ray diffraction (XRD);
- Material characterisation using other methods;
- Reporting the used microbial species.
- Reporting rationales for the design of antimicrobial tests;
- Reporting a measure of variance for the antimicrobial test results;
- Discussing the clinical relevance of the reported results.
- Testing the elemental composition of the material;
- Testing for ion release;
- Performing more replicate experiments;
- Testing for material toxicity.
3.3. Statistical Analysis
4. Discussion
4.1. Areas of Improvement in Reporting Quality
4.2. Areas of Improvement in Methodological Quality
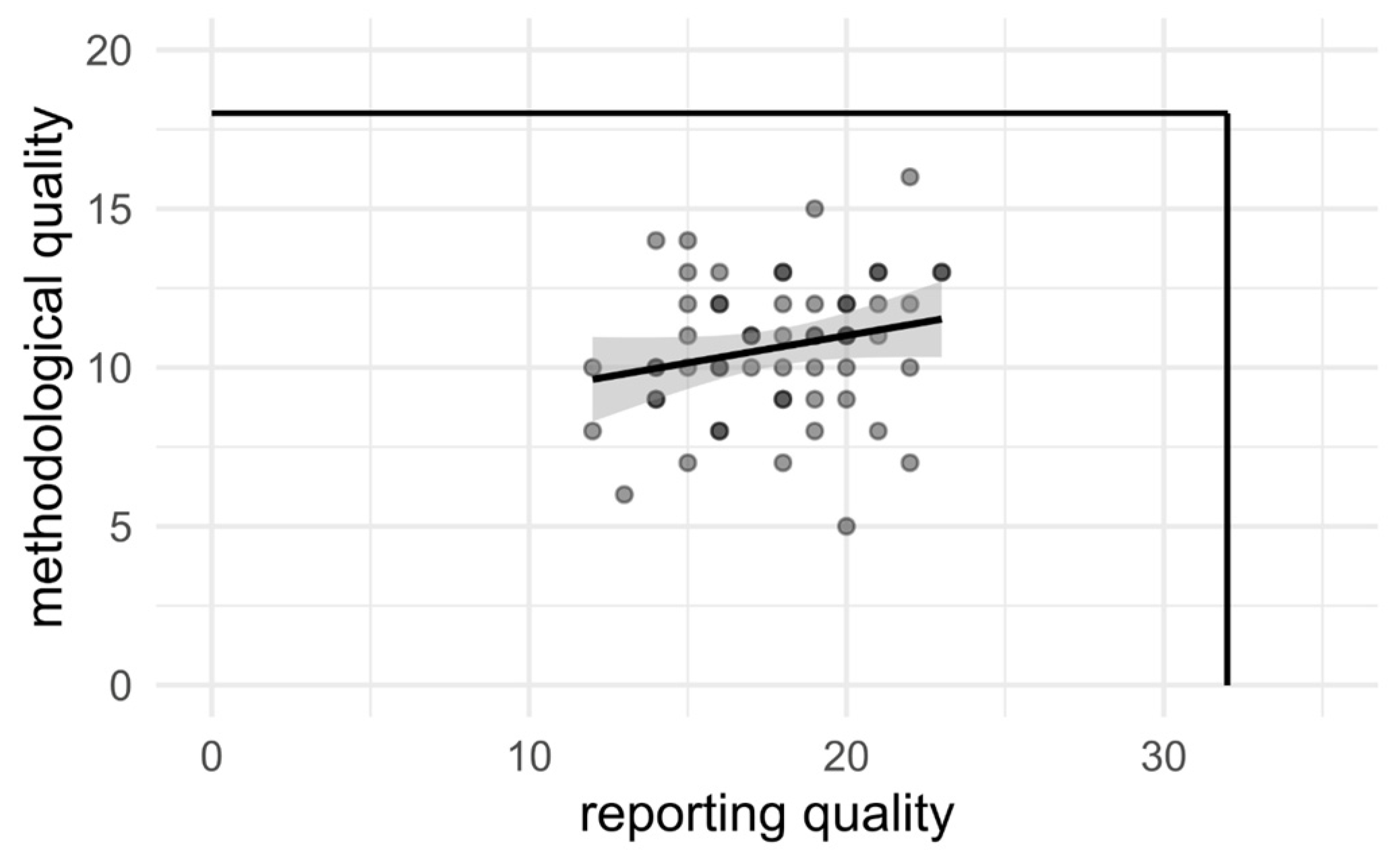
4.3. Analysis of Quality Versus Study Age and Citations
4.4. Limitations & Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CaP | calcium phosphate |
| XRD | X-ray diffraction |
| FTIR | Fourier-transform infrared spectroscopy |
References
- Postler, A.; Lützner, C.; Beyer, F.; Tille, E.; Lützner, J. Analysis of Total Knee Arthroplasty Revision Causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef]
- Karachalios, T.; Komnos, G.; Koutalos, A. Total Hip Arthroplasty: Survival and Modes of Failure. EFORT Open Rev. 2018, 3, 232–239. [Google Scholar] [CrossRef]
- Klouche, S.; Sariali, E.; Mamoudy, P. Total Hip Arthroplasty Revision Due to Infection: A Cost Analysis Approach. Orthop. Traumatol. Surg. Res. 2010, 96, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Danko, D.; Bezdan, D.; Afshin, E.E.; Zhu, S.; Mso, C.E.; International, T. A Global Metagenomic Map of Urban Microbiomes and Antimicrobial Resistance. Cell 2021, 184, 3376–3393. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Rózycka, D. Substituted Hydroxyapatites with Antibacterial Properties. Biomed. Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- Dornelas, J.; Dornelas, G.; Rossi, A.; Piattelli, A.; Di Pietro, N.; Romasco, T.; Mourão, C.F.; Alves, G.G. The Incorporation of Zinc into Hydroxyapatite and Its Influence on the Cellular Response to Biomaterials: A Systematic Review. J. Funct. Biomater. 2024, 15, 178. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic Substituted Hydroxyapatite for Bone Regeneration Applications: A Review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Kamphof, R.; Lima, R.N.O.; Schoones, J.W.; Arts, J.J.; Nelissen, R.G.H.H.; Cama, G.; Pijls, B.G.C.W. Antimicrobial Activity of Ion-Substituted Calcium Phosphates: A Systematic Review. Heliyon 2023, 9, e16568. [Google Scholar] [CrossRef] [PubMed]
- Pijls, B.; Dekkers, O.; Middeldorp, S.; Valstar, E.; Van Der Heide, H.; Van Der Linden-Van Der Zwaag, H.; Nelissen, R. AQUILA: Assessment of Quality in Lower Limb Arthroplasty. An Expert Delphi Consensus for Total Knee and Total Hip Arthroplasty. BMC Musculoskelet. Disord. 2011, 12, 173. [Google Scholar] [CrossRef]
- Chalmers, I.; Glasziou, P. Avoidable Waste in the Production and Reporting of Research Evidence. Lancet 2009, 374, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.R.; Cevallos, M.; Altman, D.G.; Rutjes, A.W.S.; Egger, M. Uses and Misuses of the STROBE Statement: Bibliographic Study. BMJ Open 2011, 1, 1–6. [Google Scholar] [CrossRef]
- Altman, D.G.; Simera, I. A History of the Evolution of Guidelines for Reporting Medical Research: The Long Road to the EQUATOR Network. J. R. Soc. Med. 2016, 109, 67–77. [Google Scholar] [CrossRef]
- Plint, A.C.; Moher, D.; Morrison, A.; Schulz, K.; Altman, D.G.; Hill, C.; Gaboury, I. Does the CONSORT Checklist Improve the Quality of Reports of Randomised Controlled Trials? A Systematic Review. Med. J. Aust. 2006, 185, 263–267. [Google Scholar] [CrossRef]
- Equator Network. Available online: https://www.equator-network.org/ (accessed on 5 December 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Grynyuk, I.I.; Vasyliuk, O.M.; Prylutska, S.V.; Strutynska, N.Y.; Livitska, O.V.; Slobodyanik, M.S. Influence of Nanoscale-Modified Apatite-Type Calcium Phosphates on the Biofilm Formation by Pathogenic Microorganisms. Open Chem. 2021, 19, 39–48. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Goldberg, M.A.; Preobrazhensky, I.I.; Mamin, G.V.; Davidova, G.A.; Agafonova, N.V.; Fosca, M.; Russo, F.; Barinov, S.M.; Cavalu, S.; et al. Improved Cytocompatibility and Antibacterial Properties of Zinc-Substituted Brushite Bone Cement Based on β-Tricalcium Phosphate. J. Mater. Sci. Mater. Med. 2021, 32, 99. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, S.J.; Shi, L.; Ma, W.; Liu, C.W. Fabrication and Characterization of Suspension Plasma-Sprayed Fluoridated Hydroxyapatite Coatings for Biomedical Applications. J. Therm. Spray. Technol. 2018, 27, 1322–1332. [Google Scholar] [CrossRef]
- Ballardini, A.; Montesi, M.; Panseri, S.; Vandini, A.; Balboni, P.G.; Tampieri, A.; Sprio, S. New Hydroxyapatite Nanophases with Enhanced Osteogenic and Anti-Bacterial Activity. J. Biomed. Mater. Res. Part A 2018, 106, 521–530. [Google Scholar] [CrossRef]
- Agalya, P.; Kumar, G.S.; Srinivasan, R.; Prabu, K.M.; Karunakaran, G.; Cholan, S.; Kolesnikov, E.; Kim, M. Hydroxyapatite-Based Antibacterial Bio-Nanomaterials: An Insight into the Synthesis Using Mussel Shell as a Calcium Source, Physicochemical Properties, and Nanoindentation Characteristics. Appl. Phys. 2021, 127, 12. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ergun, C.; Webster, T.J.; Bahadir, A.; Ueda, K.; Narushima, T.; Nakano, T. Effect of Precursor Deficiency Induced Ca/P Ratio on Antibacterial and Osteoblast Adhesion Properties of Ag-Incorporated Hydroxyapatite: Reducing Ag Toxicity. Materials 2021, 14, 3158. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; O’Neill, L.; O’Leary, N.D.; O’Gara, J.P.; Crean, A.M.; Ryan, K.B. Osteointegration, Antimicrobial and Antibiofilm Activity of Orthopaedic Titanium Surfaces Coated with Silver and Strontium-Doped Hydroxyapatite Using a Novel Blasting Process. Drug Deliv. Transl. Res. 2021, 11, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Fang, Y.; Hooper, T.J.N.; Kelly, N.L.; Gupta, D.; Balani, K.; Manna, I.; Baikie, T.; Bishop, P.T.; White, T.J.; et al. Crystal Chemistry and Antibacterial Properties of Cupriferous Hydroxyapatite. Materials 2019, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ustriyana, P.; Moore, F.; Sahai, N. Biological Response of and Blood Plasma Protein Adsorption on Silver-Doped Hydroxyapatite. ACS Biomater. Sci. Eng. 2019, 5, 561–571. [Google Scholar] [CrossRef]
- Hwang, K.S.; Hwangbo, S.; Kim, J.T. Silver-Doped Calcium Phosphate Nanopowders Prepared by Electrostatic Spraying. J. Nanopart. Res. 2008, 10, 1337–1341. [Google Scholar] [CrossRef]
- Padmanabhan, V.P.; Kulandaivelu, R.; Venkatachalam, V.; Veerla, S.C.; Mohammad, F.; Al-Lohedan, H.A.; Oh, W.C.; Schirhagl, R.; Obulapuram, P.K.; Hoque, M.E.; et al. Influence of Sonication on the Physicochemical and Biological Characteristics of Selenium-Substituted Hydroxyapatites. New J. Chem. 2020, 44, 17453–17464. [Google Scholar] [CrossRef]
- Putra, W.A.; Jamarun, N.; Agustien, A.; Zilfa; Septiani, U. Hydroxyapatite and Zn-Hydroxyapatite Synthesis Using Calcium from Lake Maninjau Pensi Shells and Resistance Test on Bacteria. Int. J. Pharm. Sci. Res. 2019, 10, 2993–2997. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Khalil, A.M.; Ahmed, M.K.; Shoueir, K.R. Tuning the Compositional Configuration of Hydroxyapatite Modified with Vanadium Ions Including Thermal Stability and Antibacterial Properties. J. Mol. Struct. 2021, 1242, 12. [Google Scholar] [CrossRef]
- Anwar, A.; Akbar, S.; Sadiqa, A.; Kazmi, M. Novel Continuous Flow Synthesis, Characterization and Antibacterial Studies of Nanoscale Zinc Substituted Hydroxyapatite Bioceramics. Inorganica Chim. Acta 2016, 453, 16–22. [Google Scholar] [CrossRef]
- Bir, F.; Khireddine, H.; Touati, A.; Sidane, D.; Yala, S.; Oudadesse, H. Electrochemical Depositions of Fluorohydroxyapatite Doped by Cu2+, Zn2+, Ag+ on Stainless Steel Substrates. Appl. Surf. Sci. 2012, 258, 7021–7030. [Google Scholar] [CrossRef]
- Chambard, M.; Remache, D.; Balcaen, Y.; Dalverny, O.; Alexis, J.; Siadous, R.; Bareille, R.; Catros, S.; Fort, P.; Grossin, D.; et al. Effect of Silver and Strontium Incorporation Route on Hydroxyapatite Coatings Elaborated by Rf-SPS. Materialia 2020, 12, 12. [Google Scholar] [CrossRef]
- Chen, H.J.; Wan, L. Antibacterial Property of Hydroxyapatite with Ag+ Dopant Produced by Hydrothermal Process. Asian J. Chem. 2013, 25, 10315–10318. [Google Scholar] [CrossRef]
- Ciobanu, G.; Bargan, A.M.; Luca, C. New Cerium(IV)-Substituted Hydroxyapatite Nanoparticles: Preparation and Characterization. Ceram. Int. 2015, 41, 12192–12201. [Google Scholar] [CrossRef]
- Elmi, F.; Yousefi, B.; Elmi, M.M.; Alinezhad, H.; Moulana, Z. Thermal Decomposition Synthesis of Zn-HAP (Extracted from Fish Scale) Nanopowder and Its Photocatalytic and Antibacterial Activities under Visible Light. Ceram. Int. 2021, 47, 21862–21872. [Google Scholar] [CrossRef]
- Gopi, D.; Sathishkurnar, S.; Karthika, A.; Kavitha, L. Development of Ce3+/Eu3+ Dual-Substituted Hydroxyapatite Coating on Surgical Grade Stainless Steel for Improved Antimicrobial and Bioactive Properties. Ind. Eng. Chem. Res. 2014, 53, 20145–20153. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P.; Lopez-Alvarez, M.; Azevedo, A.S.; Dorado, J.; et al. Pulsed Laser Deposition of Copper and Zinc Doped Hydroxyapatite Coatings for Biomedical Applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Ciobanu, S.C.; Chapon, P.; Predoi, D. Antimicrobial Properties of Samarium Doped Hydroxyapatite Suspensions and Coatings. Coatings 2020, 10, 1124. [Google Scholar] [CrossRef]
- Irfan, M.; Sultana, S.N.; Venkateswarlu, B.; Jagannatham, M.; Dumpala, R.; Sunil, B.R. Zinc-Substituted Hydroxyapatite: Synthesis, Structural Analysis, and Antimicrobial Behavior. Trans. Indian. Inst. Met. 2021, 74, 2335–2344. [Google Scholar] [CrossRef]
- Kamonwannasit, S.; Futalan, C.M.; Khemthong, P.; Butburee, T.; Karaphun, A.; Phatai, P. Synthesis of Copper-Silver Doped Hydroxyapatite via Ultrasonic Coupled Sol-Gel Techniques: Structural and Antibacterial Studies. J. Sol-Gel Sci. Technol. 2020, 96, 452–463. [Google Scholar] [CrossRef]
- Koizhaiganova, M.; Yasa, I.; Gulumser, G. Characterization and Antimicrobial Activity of Silver Doped Hydroxyapatite Obtained by the Microwave Method. Mater. Sci. 2016, 22, 403–408. [Google Scholar] [CrossRef]
- Lin, Y.G.; Yang, Z.R.; Cheng, J.; Wang, L.S. Synthesis, Characterization and Antibacterial Property of Strontium Half and Totally Substituted Hydroxyapatite Nanoparticles. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 475–479. [Google Scholar] [CrossRef]
- Livitska, O.V.; Strutynska, N.Y.; Vasyliuk, O.M.; Grynyuk, I.I.; Prylutska, S.V.; Slobodyanik, N.S. Synthesis, Characterization and Antimicrobial Properties of Chemically Modified Apatite-Related Calcium Phosphates. Funct. Mater. 2020, 27, 184–191. [Google Scholar] [CrossRef]
- Li, Y.; Ho, J.H.; Ooi, C.P. Antibacterial Efficacy and Cytotoxicity Studies of Copper (II) and Titanium (IV) Substituted Hydroxyapatite Nanoparticles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2010, 30, 1137–1144. [Google Scholar] [CrossRef]
- Panneerselvam, R.; Anandhan, N.; Gopu, G.; Amali Roselin, A.; Ganesan, K.P.; Marimuthu, T.; Roselin, A.A.; Ganesan, K.P.; Marimuthu, T. Impact of Different Transition Metal Ions in the Structural, Mechanical, Optical, Chemico-Physical and Biological Properties of Nanohydroxyapatite. Appl. Surf. Sci. 2020, 506, 13. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Bioceramic Layers with Antifungal Properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Buton, N.; Megier, C. Obtaining and Characterizing Thin Layers of Magnesium Doped Hydroxyapatite by Dip Coating Procedure. Coatings 2020, 10, 510. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Anjaneyulu, U.; Vijayalakshmi, U. Preparation and Characterization of Sol-Gel Derived Ce-4 + Doped Hydroxyapatite and Its in Vitro Biological Evaluations for Orthopedic Applications. Mater. Des. 2017, 119, 446–455. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Copper Substituted Hydroxyapatite and Fluorapatite: Synthesis, Characterization and Antimicrobial Properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Shi, X.Y.; Zhou, J.; Liu, G.Z.; Wang, L. The Physical and Antimicrobial Properties of Silver Doped Hydroxyapatite Sintered by Microwave and Conventional Sintering. J. Inorg. Organomet. Polym. Mater. 2017, 27, 955–961. [Google Scholar] [CrossRef]
- Sikder, P.; Koju, N.; Ren, Y.F.; Goel, V.K.; Phares, T.; Lin, B.R.; Bhaduri, S.B. Development of Single-Phase Silver-Doped Antibacterial CDHA Coatings on Ti6Al4V with Sustained Release. Surf. Coat. Technol. 2018, 342, 105–116. [Google Scholar] [CrossRef]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Abdullah, H. Synthesis and Characterizations of Natural Limestone-Derived Nano-Hydroxyapatite (HAp): A Comparison Study of Different Metals Doped HAps on Antibacterial Activity. RSC Adv. 2021, 11, 15896–15904. [Google Scholar] [CrossRef]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Krstić, A.; Jovanović, D.; Raičević, S.; Stanic, V.; et al. Synthesis of Antimicrobial Monophase Silver-Doped Hydroxyapatite Nanopowders for Bone Tissue Engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and Zinc Doped Nano-Hydroxyapatite: Synthesis, Characterization, Antimicrobial and Hemolytic Studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Temprom, L.; Seet, S.L.; Tippayawat, P.; Suwanna, P. Bioactivity, Cytotoxicity and Antibacterial Evaluation of Undoped, Zn-Doped, Sr-Doped, and Zn/Sr-Codoped Hydroxyapatites Synthesized by a Sol-Gel Method. Chiang Mai J. Sci. 2017, 44, 630–639. [Google Scholar]
- Ullah, I.; Siddiqui, M.A.; Kolawole, S.K.; Liu, H.; Zhang, J.; Ren, L.; Yang, K. Synthesis, Characterization and in Vitro Evaluation of Zinc and Strontium Binary Doped Hydroxyapatite for Biomedical Application. Ceram. Int. 2020, 46, 14448–14459. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, B.B.; Ma, L.F.; Xie, L.; Yang, H.; Li, Y.C.; Wang, S.S.; Qiao, H.X.; Lin, H.; Lan, J.P.; et al. Chemical Stability, Antibacterial and Osteogenic Activities Study of Strontium-Silver Co-Substituted Fluorohydroxyapatite Nanopillars: A Potential Multifunctional Biological Coating. Ceram. Int. 2020, 46, 27758–27773. [Google Scholar] [CrossRef]
- Uskoković, V.; Iyer, M.A.; Wu, V.M. One Ion to Rule Them All: The Combined Antibacterial, Osteoinductive and Anticancer Properties of Selenite-Incorporated Hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.B.; Kumar, G.S.; Kolesnikov, E.; Janarthanan, G.; Pillai, M.M.; Rajendran, S.; Boobalan, S.; Sudha, K.G.; Rajeshkumar, M.P. Mesoporous Mg-Doped Hydroxyapatite Nanorods Prepared from Bio-Waste Blue Mussel Shells for Implant Applications. Ceram. Int. 2020, 46, 28514–28527. [Google Scholar] [CrossRef]
- Singh, R.K.; Kannan, S. Synthesis, Structural Analysis, Mechanical, Antibacterial and Hemolytic Activity of Mg2+ and Cu2+ Co-Substitutions in β-Ca3(PO4)2. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 530–538. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.H.; Hu, X.X. Efficient Destruction of Bacteria with Ti(IV) and Antibacterial Ions in Co-Substituted Hydroxyapatite Films. Appl. Catal. B-Environ. 2007, 73, 345–353. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Nowicka, J.; Pajaczkowska, M.; Szymonowicz, M.; Targonska, S.; Sobierajska, P.; Wiglusz, K.; Dobrzynski, W.; Lubojanski, A.; et al. The Influence of Ozonated Olive Oil-Loaded and Copper-Doped Nanohydroxyapatites on Planktonic Forms of Microorganisms. Nanomaterials 2020, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Shi, Z.; Neoh, K.G.; Ho, B.; Tay, B.Y.; Thian, E.S. The Effects of Silver, Silicon-Containing Apatite towards Bacteria and Cell Responses. Biomed. Mater. 2014, 9, 015010. [Google Scholar] [CrossRef]
- Lim, P.N.; Wang, Z.; Tong, S.Y.; Ho, B.; Wang, W.; Aizawa, M.; Yang, Z.; Thian, E.S. Silver, Silicon Co-Substituted Hydroxyapatite Modulates Bacteria-Cell Competition for Enhanced Osteogenic Function. Biomed. Mater. 2021, 16, 055018. [Google Scholar] [CrossRef]
- Sprio, S.; Preti, L.; Montesi, M.; Panseri, S.; Adamiano, A.; Vandini, A.; Pugno, N.M.; Tampieri, A. Surface Phenomena Enhancing the Antibacterial and Osteogenic Ability of Nanocrystalline Hydroxyapatite, Activated by Multiple-Ion Doping. ACS Biomater. Sci. Eng. 2019, 5, 5947–5959. [Google Scholar] [CrossRef]
- Graziani, G.; Barbaro, K.; Fadeeva, I.V.; Ghezzi, D.; Fosca, M.; Sassoni, E.; Vadalà, G.; Cappelletti, M.; Valle, F.; Baldini, N.; et al. Ionized Jet Deposition of Antimicrobial and Stem Cell Friendly Silver-Substituted Tricalcium Phosphate Nanocoatings on Titanium Alloy. Bioact. Mater. 2021, 6, 2629–2642. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Jadalannagari, S.; Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Antimicrobial Activity of Hemocompatible Silver Doped Hydroxyapatite Nanoparticles Synthesized by Modified Sol-Gel Technique. Appl. Nanosci. 2014, 4, 133–141. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Li, W. Zinc-Containing Hydroxyapatite Enhances Cold-Light-Activated Tooth Bleaching Treatment In Vitro. Biomed. Res. Int. 2017, 2017, 6261248. [Google Scholar] [CrossRef] [PubMed]
- Andrés, N.C.; Sieben, J.M.; Baldini, M.; Rodríguez, C.H.; Famiglietti, Á.; Messina, P.V. Electroactive Mg2+-Hydroxyapatite Nanostructured Networks against Drug-Resistant Bone Infection Strains. ACS Appl. Mater. Interfaces 2018, 10, 19534–19544. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Kalita, V.I.; Komlev, D.I.; Radiuk, A.A.; Fomin, A.S.; Davidova, G.A.; Fursova, N.K.; Murzakhanov, F.F.; Gafurov, M.R.; Fosca, M.; et al. In Vitro Properties of Manganese-Substituted Tricalcium Phosphate Coatings for Titanium Biomedical Implants Deposited by Arc Plasma. Materials 2020, 13, 4411. [Google Scholar] [CrossRef] [PubMed]
- Gyotoku, H.; Azuma, Y.; Furuzono, T. Evaluation of Fluorinated Hydroxyapatite Nanoparticles as an Antibacterial Material for Catheter Coating. Ren. Replace. Ther. 2020, 6, 8. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Hassan, R.; Gupta, A.; Verma, M.; Murugan, P.A.; Sengupta, P.; Saravanan, M.; Manna, I.; Balani, K. Effect of Zn and Co Doping on Antibacterial Efficacy and Cytocompatibility of Spark Plasma Sintered Hydroxyapatite. J. Am. Ceram. Soc. 2020, 103, 4090–4100. [Google Scholar] [CrossRef]
- OHAT Risk of Bias Rating Tool for Human and Animal Studies. January 2015. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 5 December 2024).
- ASTM E2149-20; Standard Test Method for Determining the Antimcirobial Activity of Antimcirobial Agents Under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2020.
- ISO 20776-1; Susceptibility Testing of Infectious Agents and Evaluating of Performance of Antimicrobial Susceptiblity Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly G. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 22196; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- Ranganathan, P.; Pramesh, C.; Buyse, M. Common Pitfalls in Statistical Analysis: Clinical versus Statistical Significance. Perspect. Clin. Res. 2015, 6, 169. [Google Scholar] [CrossRef]
- Fethney, J. Statistical and Clinical Significance, and How to Use Confidence Intervals to Help Interpret Both. Aust. Crit. Care 2010, 23, 93–97. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Grainger, D.W.; Richards, R.G. Challenges in Linking Preclinical Anti-Microbial Research Strategies with Clinical Outcomes for Device-Associated Infections. Eur. Cells Mater. 2014, 28, 112–128. [Google Scholar] [CrossRef]


| Reporting quality items |
| Use and reporting of positive and negative control groups |
| 1. How were negative control groups reported for the antimicrobial experiment? |
| Material characterisation |
| 2. Was material phase purity (by XRD) adequately reported? |
| 3. Were the results of other characterisation methods adequately reported? |
| Reproducibility and validity of the antimicrobial tests |
| 4. Were all antimicrobial experiments and material characterisations that were described in the method also reported in the results? |
| 5. Was there a study protocol? |
| 6. Was the bacterial species reported? |
| 7. Was the bacterial strain reported? |
| 8. Was the bacterial challenge dose (starting inoculum) reported? |
| 9. Was the amount of sample (e.g., mg/mL of powder or coating surface area) used reported in enough detail to reproduce the experiment? |
| 10. Are other conditions used for antimicrobial tests reported in a reproducible manner? |
| Reporting and discussion of the results of antimicrobial tests |
| 11. Were the results of antimicrobial tests reported in a readable manner? |
| 12. Was bacterial enumeration reported on a logarithmic scale? |
| 13. Was the clinical relevance of the observed antimicrobial effect discussed in relation to a desired outcome? |
| Use of replicate experiments and statistics |
| 14. Was there clear reporting of the number of technical/biological replicates of the antimicrobial tests? |
| 15. Was there a measure of variance adequately reported for of the antimicrobial tests? |
| 16. Were the applied statistics for the antimicrobial tests appropriately described? |
| Rationales for experiment design choices |
| 17. Was a clear clinical rationale provided for the chosen material formulation? |
| 18. Was a rationale provided for the clinical relevance of the used microorganisms? |
| 19. Was a clear rationale for the chosen antibacterial test method provided? |
| 20. Was a bacterial killing mechanism proposed and substantiated? |
| Methodological quality items |
| Use and reporting of positive and negative control groups |
| 1. Was an appropriate negative control group used? |
| 2. Was an appropriate positive control group used? |
| Material characterisation |
| 3. What was the formulation of the antimicrobial material? |
| 4. Was the material phase purity determined? |
| 5. Were materials characterised using other methods as well? |
| 6. Was the release of antimicrobial agents from the material investigated? |
| 7. Did the authors quantify the elemental composition of the antimicrobial product? |
| Reproducibility and validity of the antimicrobial tests |
| 8. Was the antimicrobial experiment performed on planktonic or surface-adherent bacteria? |
| 9. Were multiple species of microorganisms used for the antimicrobial studies? |
| Use of replicate experiments and statistics |
| 10. How many replicate experiments were performed? |
| Testing of material toxicity |
| 11. How was the (non-)toxicity of the materials tested? |
| Reporting Quality Item | Answers (Score) | Results N (%) |
|---|---|---|
| Use and reporting of positive and negative control groups | ||
| 1. How were negative control groups reported for the antimicrobial experiment? | A—Not reported/reported in method only (0) B—Results reported as relative versus negative control (1) C—Test samples and controls reported separately (2) | 1 (2%) 4 (7%) 53 (91%) |
| Material characterisation | ||
| 2. Was material phase purity (by XRD) adequately reported? | A—No XRD results were reported (0) B—XRD was performed, but the pattern(s) not reported (0) C—XRD patterns were provided, but not discussed (1) D—the XRD patterns were reported and discussed (2) | 7 (12%) 1 (2%) 0 (0%) 50 (86%) |
| 3. Were the results of other characterisation methods adequately reported? | A—No other characterisation results were reported (0) B—Other characterisation was performed, but the results are not shown (0) C—The results of other characterisation were reported but not discussed (1) D—The results of other characterisations were reported and discussed (2) | 2 (3%) 1 (2%) 0 (0%) 55 (95%) |
| Reproducibility and validity of the antimicrobial tests | ||
| 4. Were all antimicrobial experiments and material characterisations that were described in the method also reported in the results? | A—Not all performed experiments described in the method are reported in the results or vice versa (0) B—Some tests were only performed on a selection of the materials (1) C—All data are present and accounted for (2) | 13 (22%) 25 (43%) 20 (34%) |
| 5. Was there a study protocol? | A—No (0) B—Yes (1) | 58 (100%) 0 (0%) |
| 6. Was the bacterial species reported? | A—No (0) B—Yes (1) | 0 (0%) 58 (100%) |
| 7. Was the bacterial strain reported? | A—No (0) B—Yes (1) | 25 (43%) 33 (57%) |
| 8. Was the bacterial challenge dose (starting inoculum) reported? | A—No (0) B—Yes (1) | 23 (40%) 35 (60) |
| 9. Was the amount of sample (e.g., mg/mL of powder or coating surface area) used reported in enough detail to reproduce the experiment? | A—No (0) B—Yes (1) | 22 (38%) 36 (62%) |
| 10. Are other conditions used for antimicrobial tests reported in a reproducible manner? | A—Experimental conditions are not reported in a reproducible manner (0) B—Test were carried out according to a referenced procedure (1) C—The experiment is reported in sufficient detail that the experiment can be reproduced (2) | 8 (14%) 7 (12%) 43 (74%) |
| Reporting and discussion of the results of antimicrobial tests | ||
| 11. Were the results of antimicrobial tests reported in a readable manner? | A—Data are unintelligible (e.g., 3D graphs) (0) B—Results are reported as images (1) C—Results reported quantitatively (tables, annotated graphs or written text) (2) | 0 (0%) 37 (64%) 21 (36%) |
| 12. Was bacterial enumeration reported on a logarithmic scale? | A—No bacteria count was performed (0) B—Enumeration was reported on a linear scale (1) C—Enumeration was performed on a logarithmic scale (2) | 23 (40%) 22 (38%) 13 (22%) |
| 13. Was the clinical relevance of the observed antimicrobial effect discussed in relation to a desired outcome? | A—No (0) B –Results were compared to those of other studies (1) C—The relevance of the antimicrobial results were discussed in relation to a desired outcome (2) D—The study reports that no consensus exists on the desired outcome (2) | 49 (84%) 5 (9%) 3 (5%) 1 (2%) |
| Use of replicate experiments and statistics | ||
| 14. Was there clear reporting of the number of technical/biological replicates of the antimicrobial tests? | A—There are error bars, but the number of replicate experiments was not reported (0) B—There is no reporting of replicates (1) C—It is reported that there are replicates, but it is not clear if they are technical or biological replicates (1) D—The number of replicates is reported, and it is clear if they are technical or biological replicates (2) | 0 (0%) 13 (22%) 9 (16%) 36 (62%) |
| 15. Was there a measure of variance adequately reported for of the antimicrobial tests? | A—It is reported that there are replicate experiments, but only the mean outcome is reported (0) B—Variance is reported, but it is unclear what measure of uncertainty they represent (0) C—There is a measure of variance, and it is clear what it represents (1) D—There is no evidence of replicate experiments, and no measure of variance is provided (1) E—There are replicate measurements, and all raw data are available (2) | 14 (24%) 11 (19%) 21 (36%) 12 (21%) 0 (0%) |
| 16. Were the applied statistics for the antimicrobial tests appropriately described? | A—No statistics were applied, or results were not reported (0) B—Statistical values are used (e.g., p-values), but it is unclear by which method they were obtained (0) C—Statistical methods were applied, and it is clear which ones (1) | 35 (60%) 4 (7%) 19 (33%) |
| Rationales for experiment design choices | ||
| 17. Was a clear clinical rationale provided for the chosen material formulation? | A—No (0) B—Yes (1) | 45 (78%) 13 (22%) |
| 18. Was a rationale provided for the clinical relevance of the used microorganisms? | A—No rationale was provided (0) B—A rationale was provided other than clinical (e.g., to cover Gram+ and Gram-) (1) C—A clinical rationale was provided (2) | 22 (38%) 15 (26%) 21 (36%) |
| 19. Was a clear rationale for the chosen antibacterial test method provided? | A—No (0) B—Yes (1) | 54 (93%) 4 (7%) |
| 20. Was a bacterial killing mechanism proposed and substantiated? | A—No killing mechanism was proposed (0) B—A theoretical killing mechanism was proposed but not substantiated (0) C—A theoretical killing mechanism was proposed based on references to literature (1) D—A killing mechanism was proposed and substantiated based on experimental evidence (2) | 16 (28%) 8 (14%) 32 (55%) 2 (3%) |
| Reporting Quality | Methodological Quality | |||
|---|---|---|---|---|
| Slope | 95% CI | Slope | 95% CI | |
| Study age | 0.09 | −0.11–0.29 | −0.09 | −0.24–0.07 |
| Citation rate | 0.02 | −0.13–0.16 | 0.14 | 0.03–0.25 |
| Methodological Quality Item | Answers (Score) | Results N (%) |
|---|---|---|
| Use and reporting of positive and negative control groups | ||
| 1. Was an appropriate negative control group used? | A—No negative control was used/unclear negative control (0) B—A blank group was used as negative control (e.g., empty culture well) (1) C—A CaP material without antimicrobial effect was used as negative control (2) D—A different clinically relevant negative control was used (e.g., uncoated titanium or bone graft) (2) | 3 (5%) 4 (7%) 49 (84%) 2 (3%) |
| 2. Was an appropriate positive control group used? | A—No positive control was used (0) B—Antibiotics were used as a positive control group (1) C—A different positive control was used (1) | 49 (84%) 7 (12%) 2 (3%) |
| Material characterisation | ||
| 3. What was the formulation of the antimicrobial material? | A—(Nano)powder (0) B—Coating (1) C—Pure CaP scaffold (1) D—Composite particles (1) E—Composite scaffold (1) F—Cement (1) G—Powders pressed into pellets or disks (0) | 30 (52%) 13 (22%) 1 (2%) 0 (0%) 1 (2%) 1 (2%) 12 (21%) |
| 4. Was the material phase purity determined? | A—No (0) B—Yes (1) | 7 (12%) 51 (88%) |
| 5. Were materials characterised using other methods as well? | A—No (0) B—Yes (1) | 3 (5%) 55 (95%) |
| 6. Was the release of antimicrobial agents from the material investigated? | A—No (0) B—Yes, by disk diffusion (1) C—Yes, by a specialised method (2) | 27 (47%) 14 (24%) 17 (29%) |
| 7. Did the authors quantify the elemental composition of the antimicrobial product? | A—There is no measure of the final product composition (0) B—The final composition is calculated based on the starting materials (0) C—The presence of the antimicrobial ion is confirmed (e.g., by XPS) but not quantified (1) D—An appropriate method has been used to measure the elemental composition, e.g., ICP-MS (2) E—The composition is determined indirectly, e.g., by Rietveld refinement (2) | 6 (10%) 6 (10%) 10 (17%) 35 (60%) 1 (2%) |
| Reproducibility and validity of the antimicrobial tests | ||
| 8. Was the antimicrobial experiment performed on planktonic or surface-adherent bacteria? | A—Unclear if planktonic or adherent bacteria were measured (0) B—The antimicrobial assay was performed on planktonic bacteria (1) C—The antimicrobial assay was performed on surface-adherent bacteria (1) D—Both planktonic and surface-adherent bacteria were tested (2) | 6 (10%) 42 (72%) 5 (9%) 5 (9%) |
| 9. Were multiple species of microorganisms used for the antimicrobial studies? | A—Only 1 strain (0) B—Multiple strains with only 1 group (G−, G+ etc) (0) C—G+ and G− (1) D—G− or G+, and yeast (1) E—G+, and G−, and yeast (2) | 9 (16%) 2 (3%) 36 (62%) 1 (2%) 10 (17%) |
| Use of replicate experiments and statistics | ||
| 10. How many replicate experiments were performed? | A—No replicates were performed or replicates were not reported (0) B—N = 2 (0) C—N ≥ 3 (1) D—N ≥ 9 (2) | 16 (28%) 2 (3%) 40 (69%) 0 (0%) |
| Testing of material toxicity | ||
| 11. How was the (non-)toxicity of the materials tested? | A—No toxicity test was performed (0) B—Reference to a different study that rationalises why the current materials are or are not toxic (1) C—The study assessed a single measure of cell toxicity of the materials (1) D—The study assessed multiple of cellular/in vivo responses to the materials (2) | 22 (38%) 3 (5%) 15 (26%) 18 (31%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamphof, R.; Arts, J.; Cama, G.; Nelissen, R.; Pijls, B. Assessment of Quality in Antimicrobial Calcium Phosphate Research (AQUACAP): A Systematic Review. Materials 2025, 18, 1543. https://doi.org/10.3390/ma18071543
Kamphof R, Arts J, Cama G, Nelissen R, Pijls B. Assessment of Quality in Antimicrobial Calcium Phosphate Research (AQUACAP): A Systematic Review. Materials. 2025; 18(7):1543. https://doi.org/10.3390/ma18071543
Chicago/Turabian StyleKamphof, Robert, Jacobus Arts, Giuseppe Cama, Rob Nelissen, and Bart Pijls. 2025. "Assessment of Quality in Antimicrobial Calcium Phosphate Research (AQUACAP): A Systematic Review" Materials 18, no. 7: 1543. https://doi.org/10.3390/ma18071543
APA StyleKamphof, R., Arts, J., Cama, G., Nelissen, R., & Pijls, B. (2025). Assessment of Quality in Antimicrobial Calcium Phosphate Research (AQUACAP): A Systematic Review. Materials, 18(7), 1543. https://doi.org/10.3390/ma18071543






