Revealing the Mechanical Properties and Fracture Mechanism of Ag Paste Sintered Solder by Two Different Preparation Methods
Abstract
1. Introduction
2. Materials and Methods
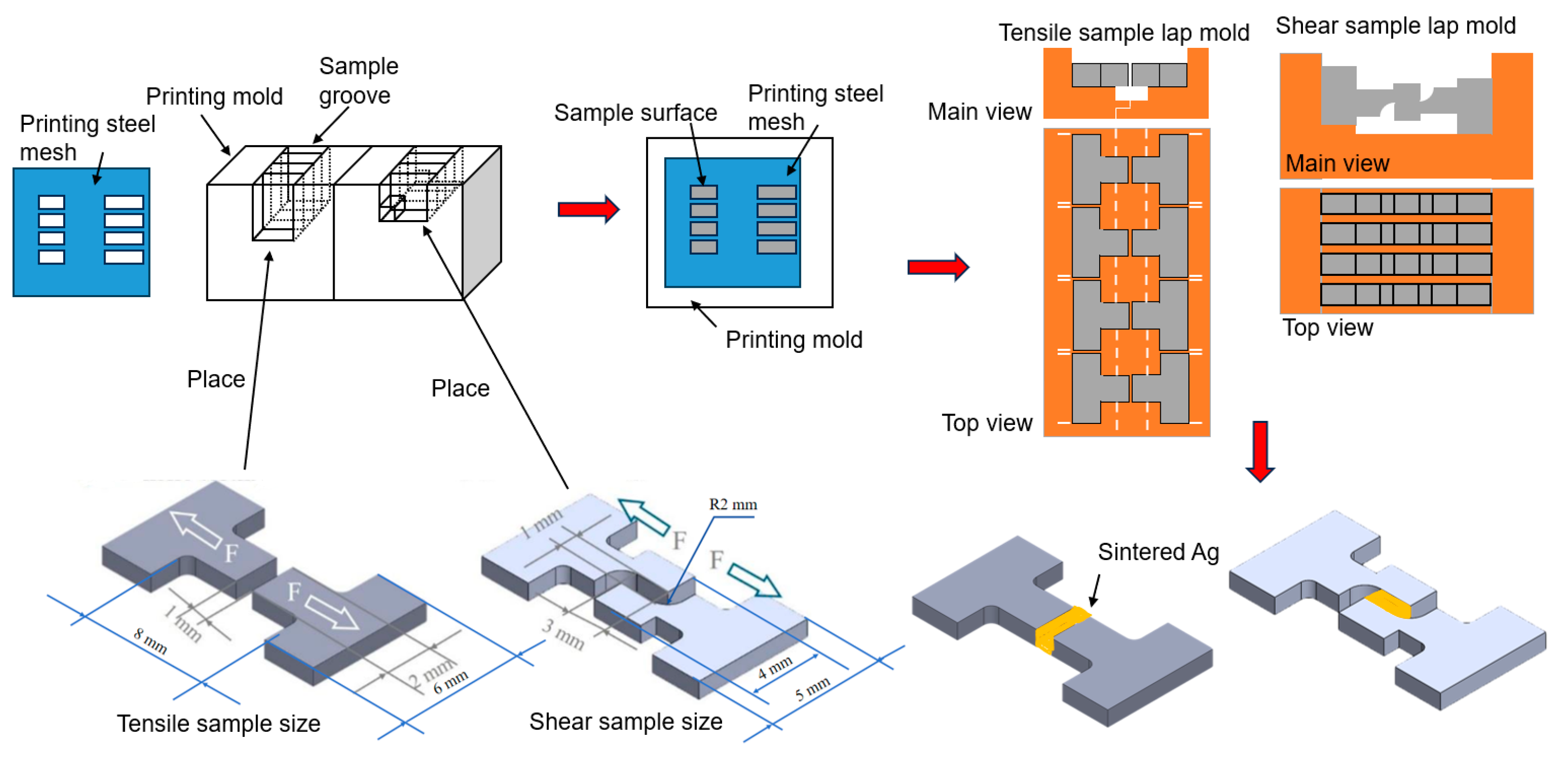
2.1. Sample Preparation
- (1)
- The in situ tensile/shear samples were machined from pure copper according to the designed dimensions by the electrical discharge machining (EDM) method, with the precision controlled within 10 μm. Subsequently, the samples were fixed on the fixture to ensure good contact between the workpiece and the electrodes. The workpiece was immersed in the electrolyte, and the power was turned on. Polishing was carried out at a voltage of 7 V for 1 min. Under the action of the electric field, the redox reaction of metal ions in the electrolyte occurs, and the microscopic protrusions on the metal surface are preferentially dissolved, thus achieving surface smoothing and removing oxides and contaminants on the sample surface. Immediately after polishing, the workpiece was taken out from the electrolyte and thoroughly washed with deionized water to remove the residual electrolyte.
- (2)
- The process of printing silver paste is shown in Figure 2. The samples were placed and fixed in the corresponding grooves of the mold. The printing steel mesh was placed on the surface of the printing mold and aligned with the printing area of the fixture, and then the silver paste was printed on the sample surface. Four samples could be printed and prepared at once. The thicknesses of the steel mesh were controlled at 0.1 mm and 1 mm, respectively. The openings in the printing area need to match the contact surfaces of the shear and tensile mold, and the dimensions of the samples are as shown in Figure 2. Subsequently, the printed tensile samples and the non-printed samples were fixed in the left and right molds, respectively. There are positioning lines on both sides of the mold to prevent misalignment after fitting. There are grooves in the middle part of the mold to prevent the sintered silver paste from contacting the mold. The two molds are assembled together through the shape matching of their bases to complete the lapping. For the shear samples, the printed samples with the concave side up were placed in the left-side groove of the mold, and then the other samples with the concave side down were placed in the right side groove and pressed tightly to achieve lapping with the left-side samples.
- (3)
- The lapped samples were placed in the furnace for sintering. The sintering temperature was 300 °C, the sintering time was 60 min, and the heating rate was 5 °C/min. The sintering process was carried out without pressure in N2 atmosphere. In the present study, pre-heating at 150 °C for 10 min was required to reduce the influence of the solvent on the silver paste.
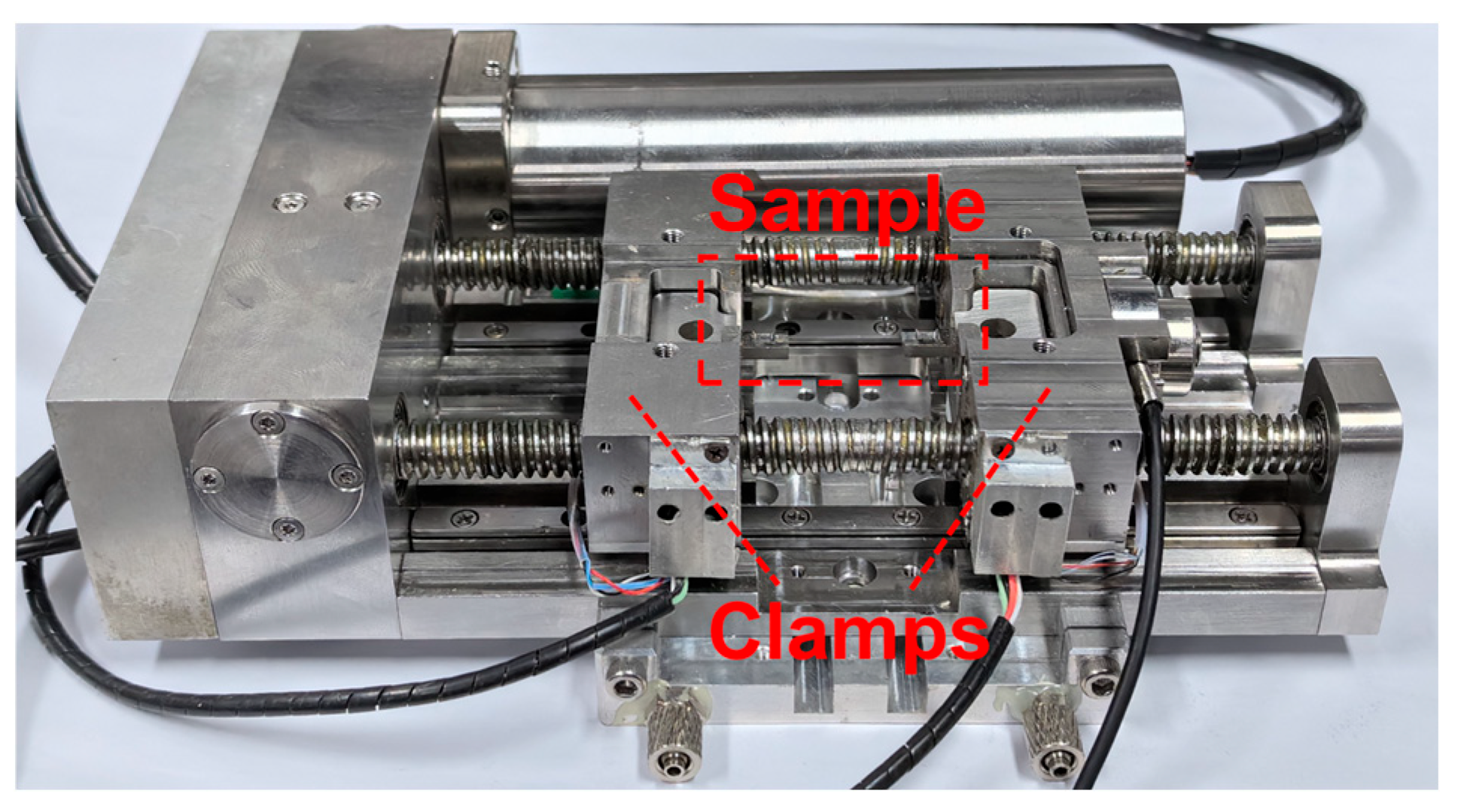
2.2. In Situ Tensile and Shear Tests
3. Results and Discussion
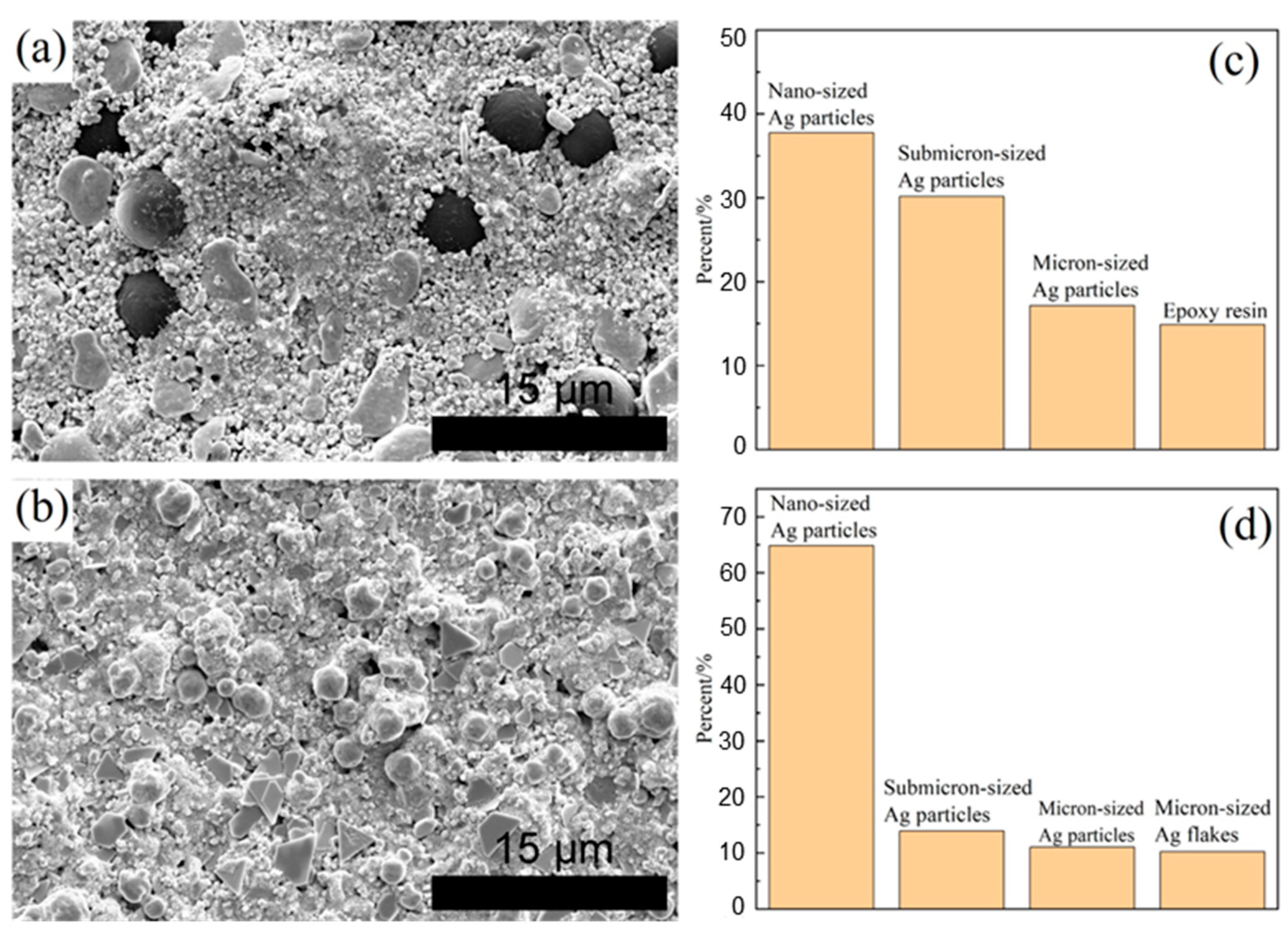
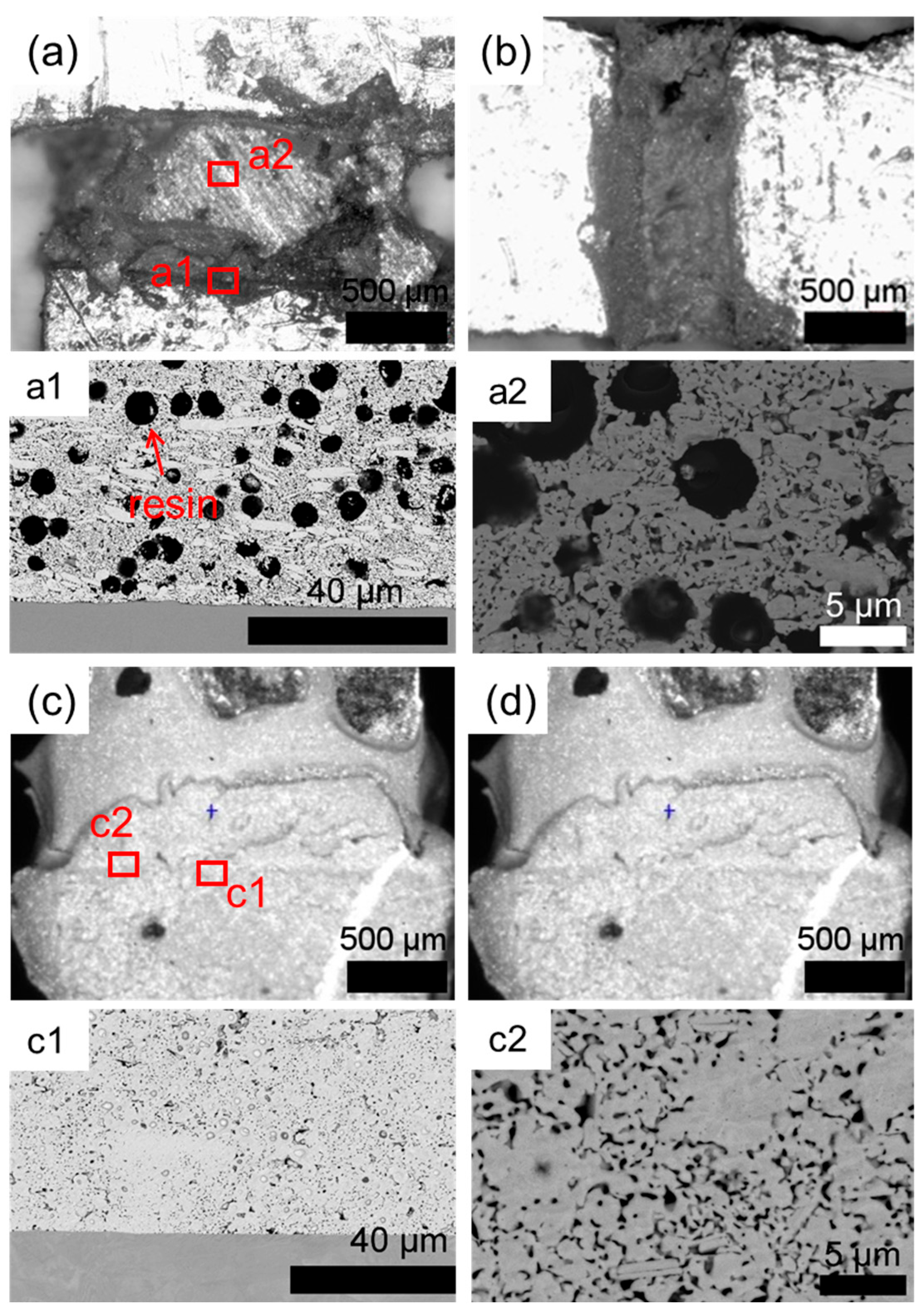
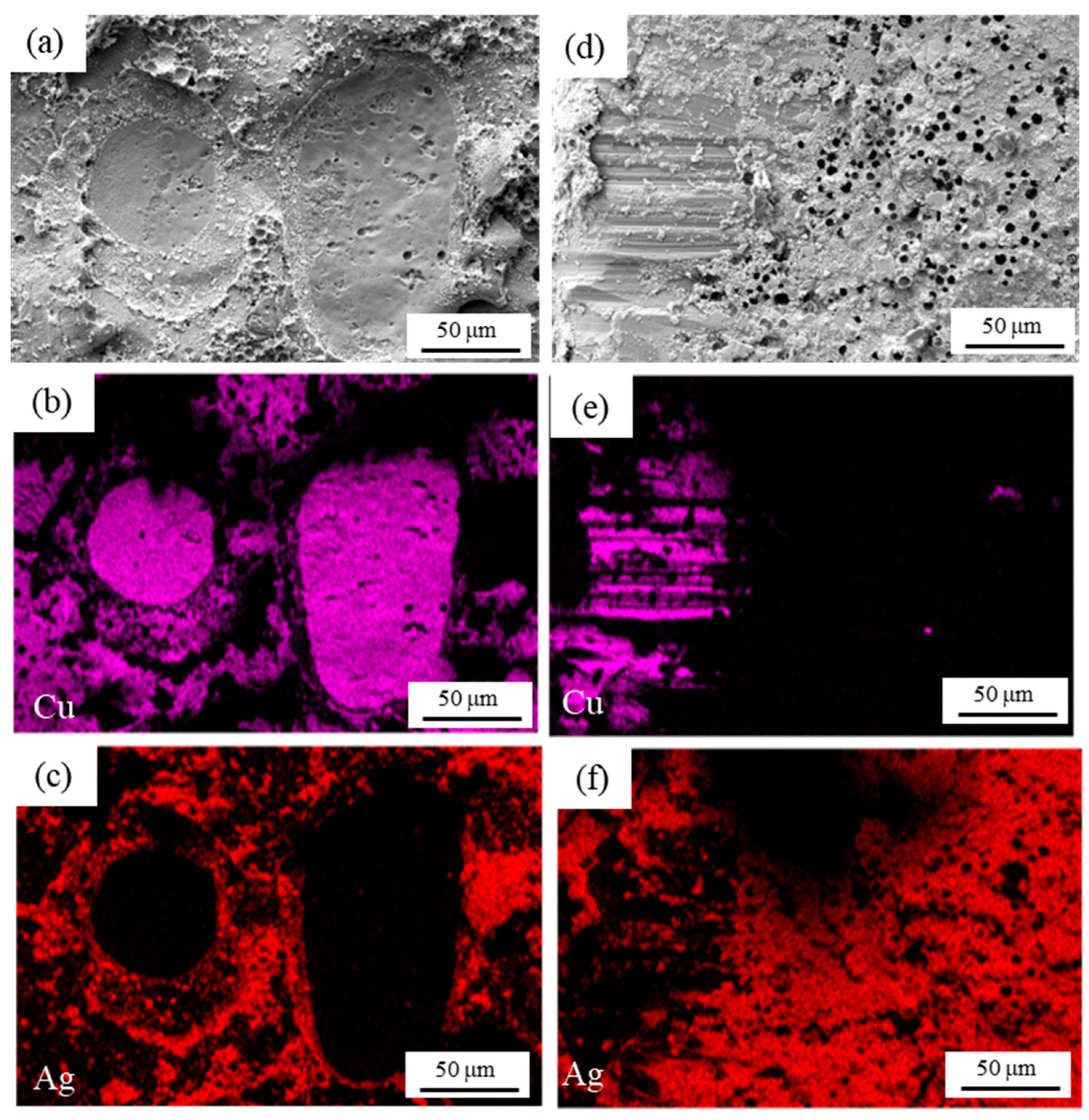
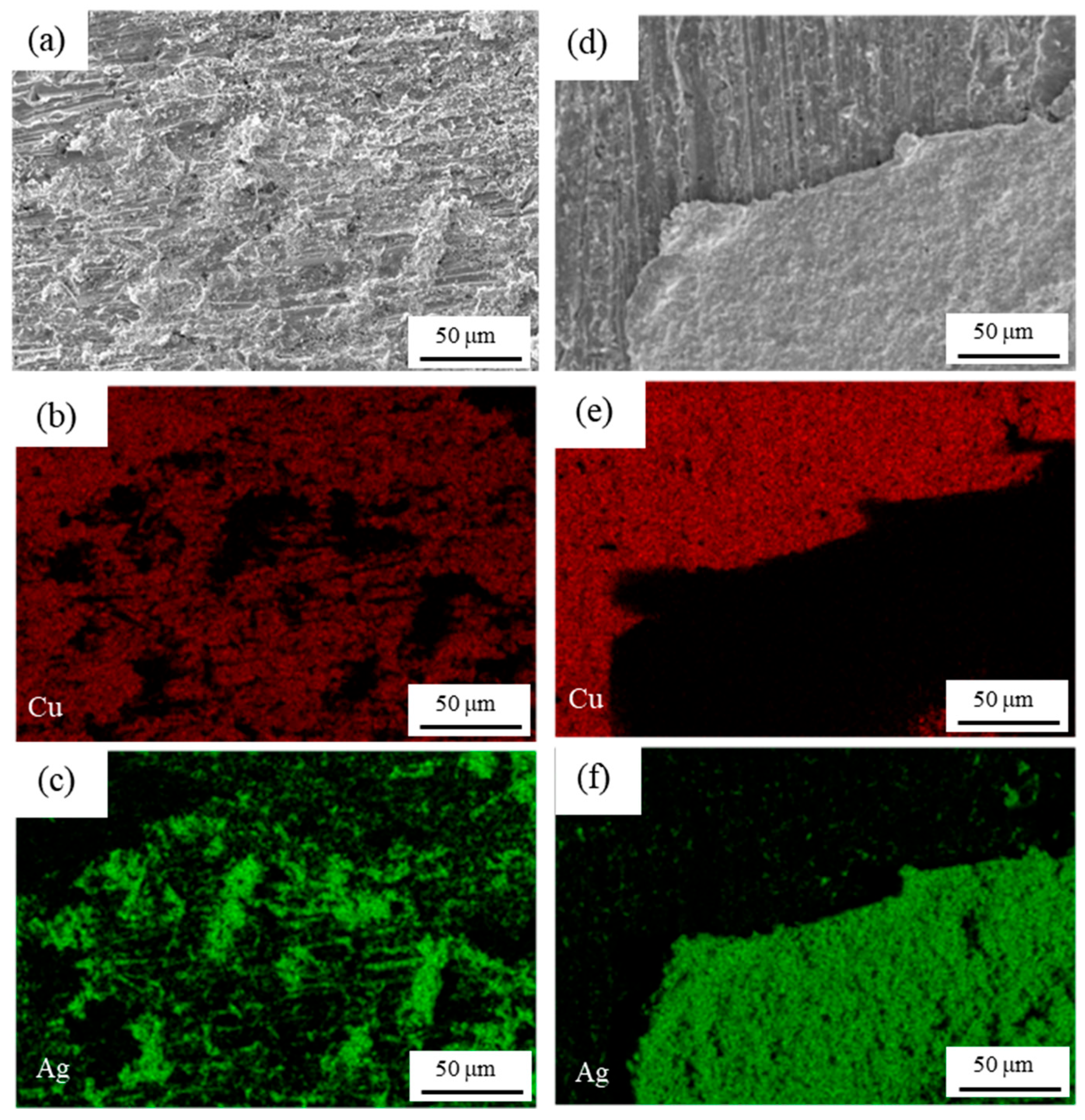
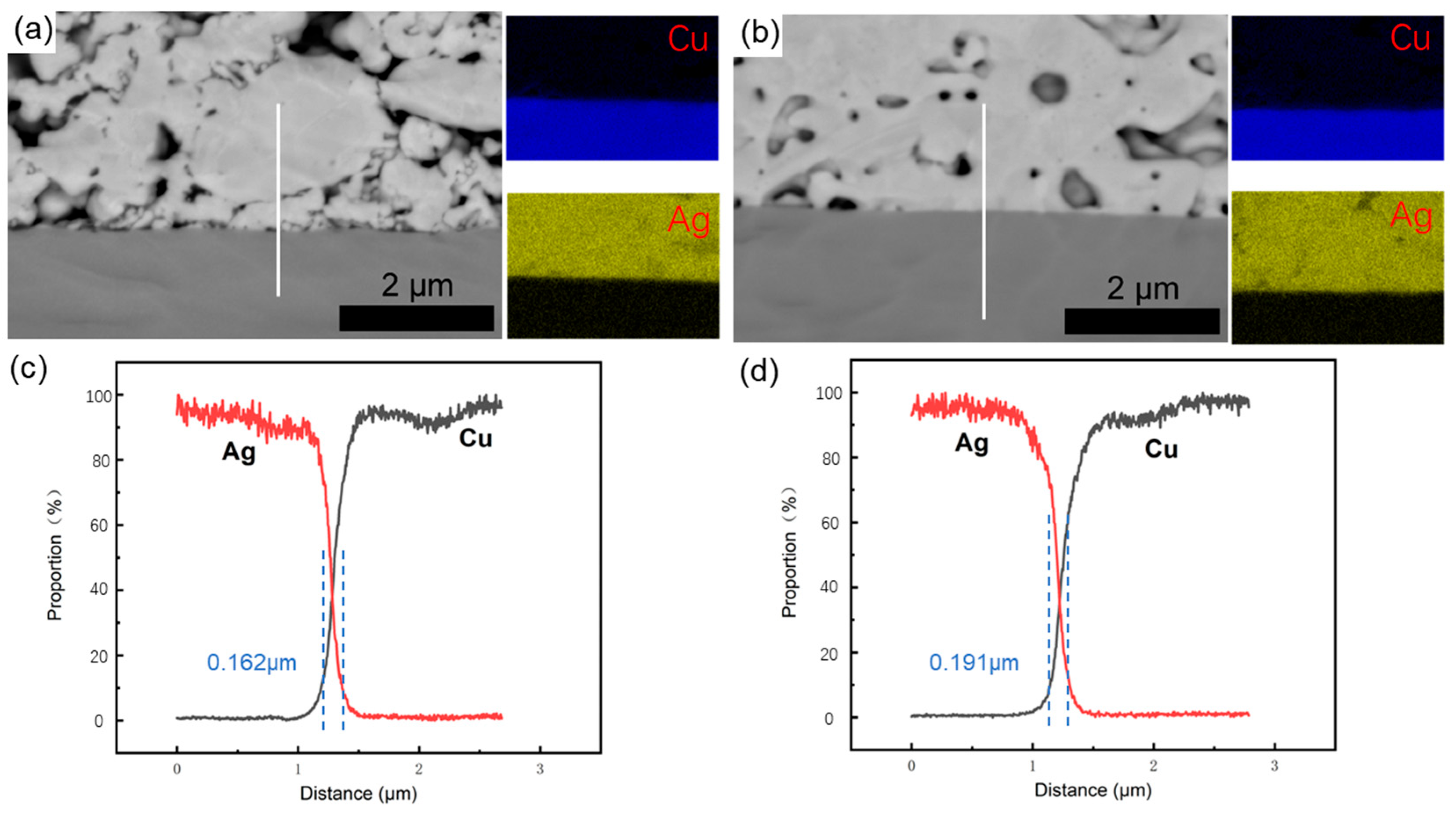
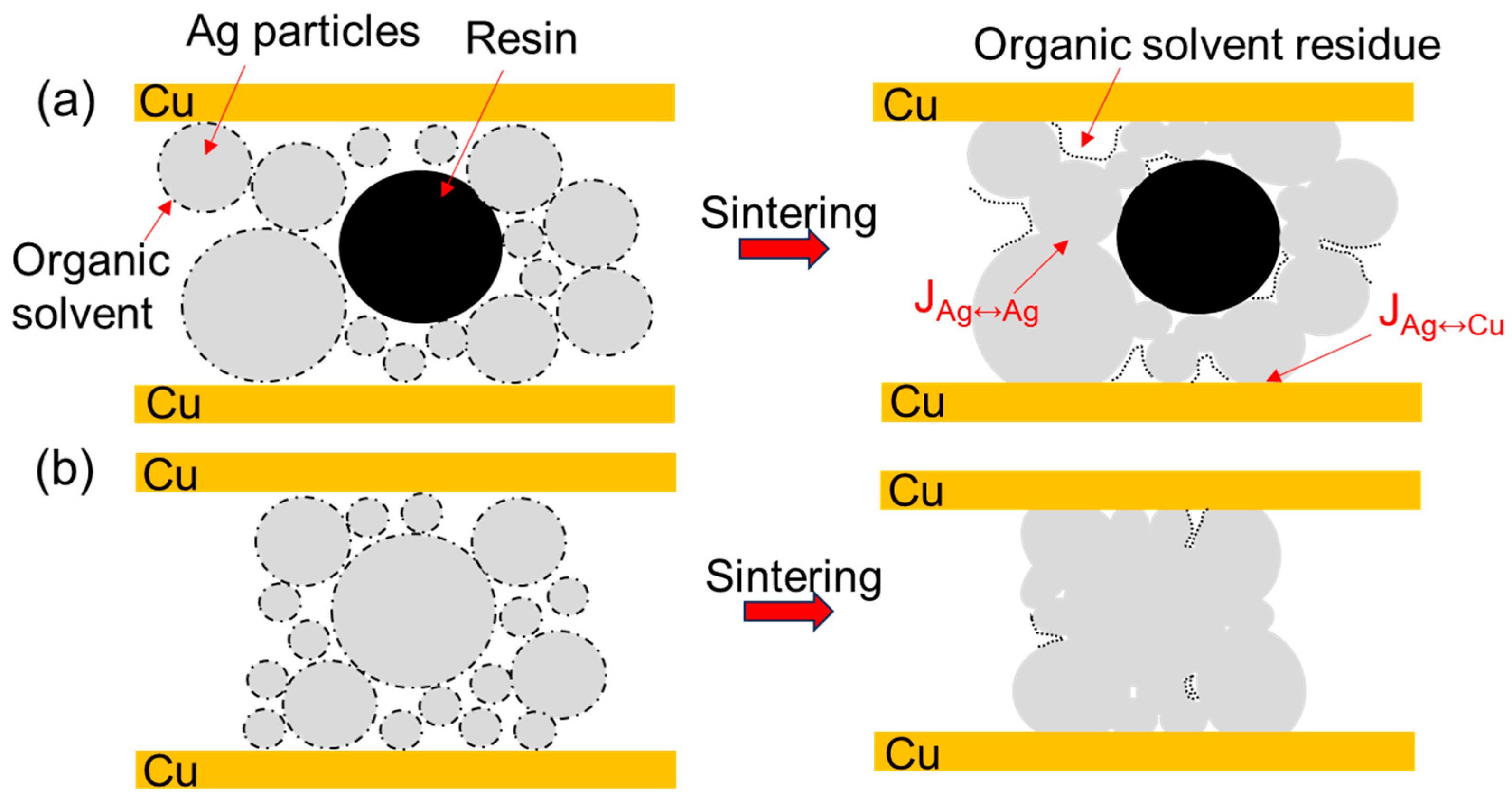
3.1. Original Microstructure of Ag-Cu Sintering Joints
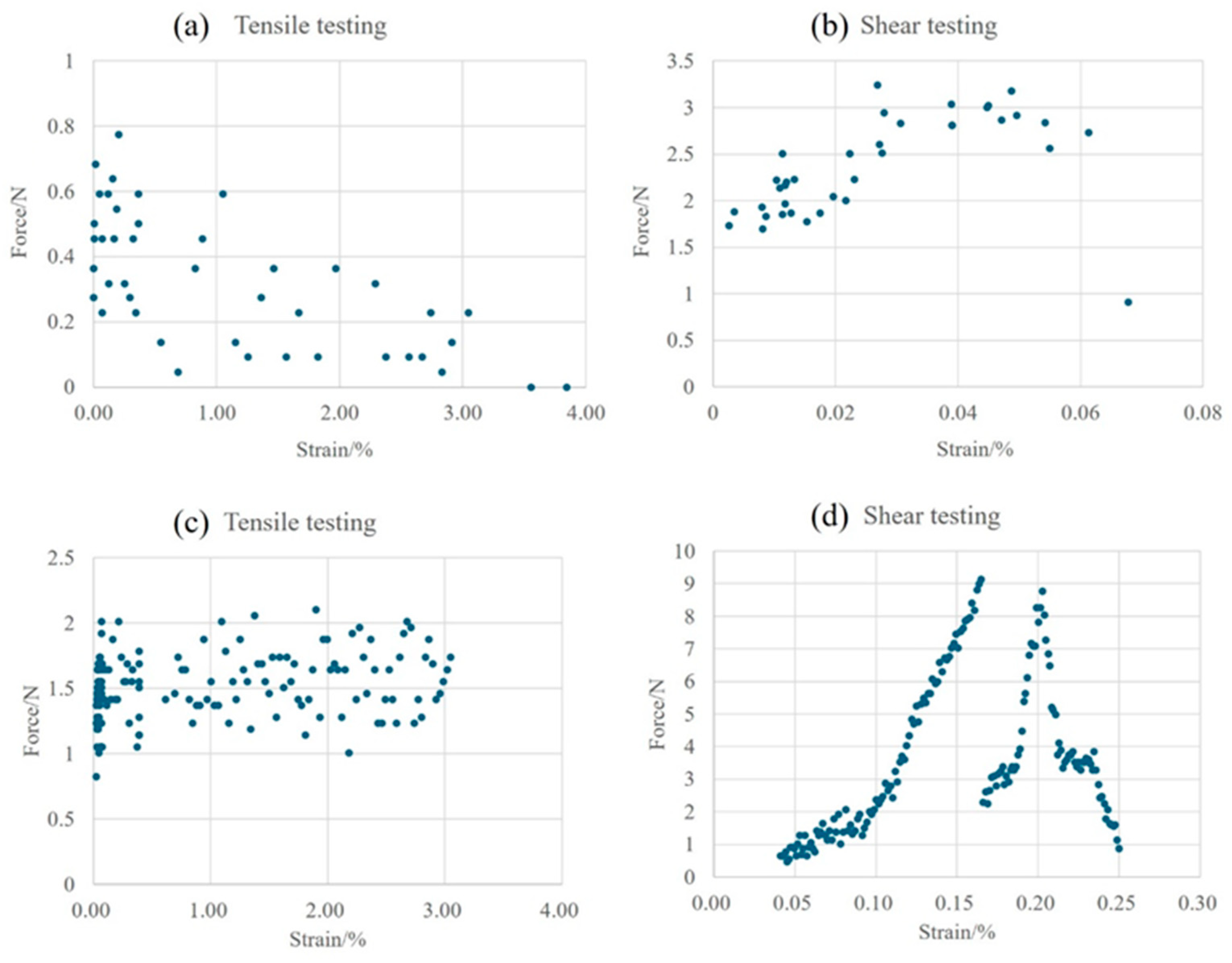
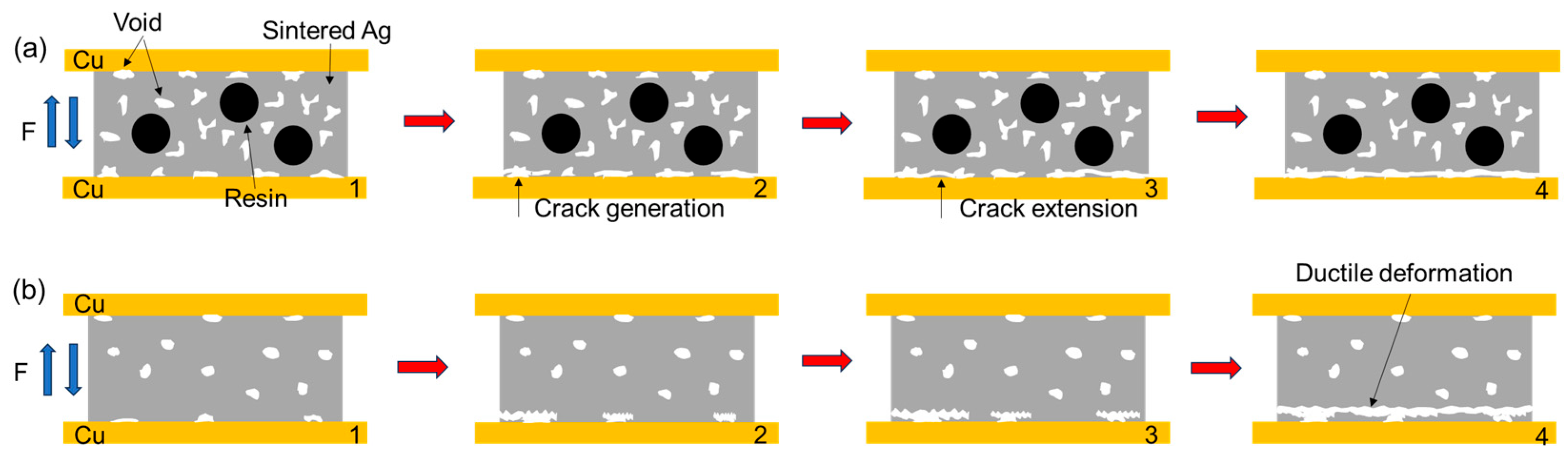
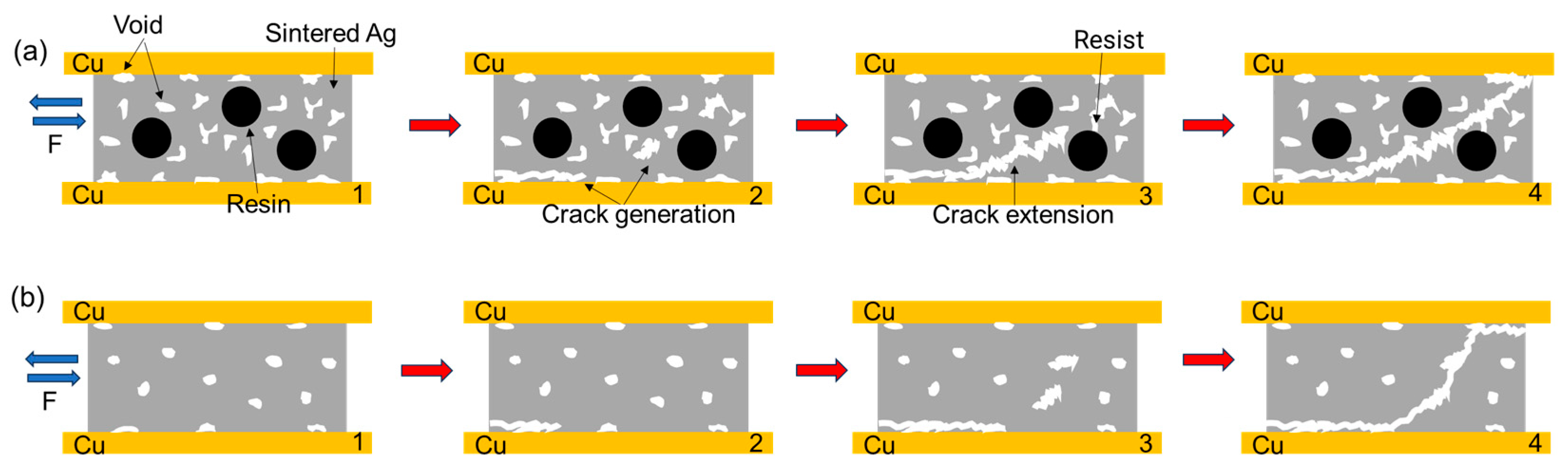
3.2. In Situ Tensile and Shear Testing
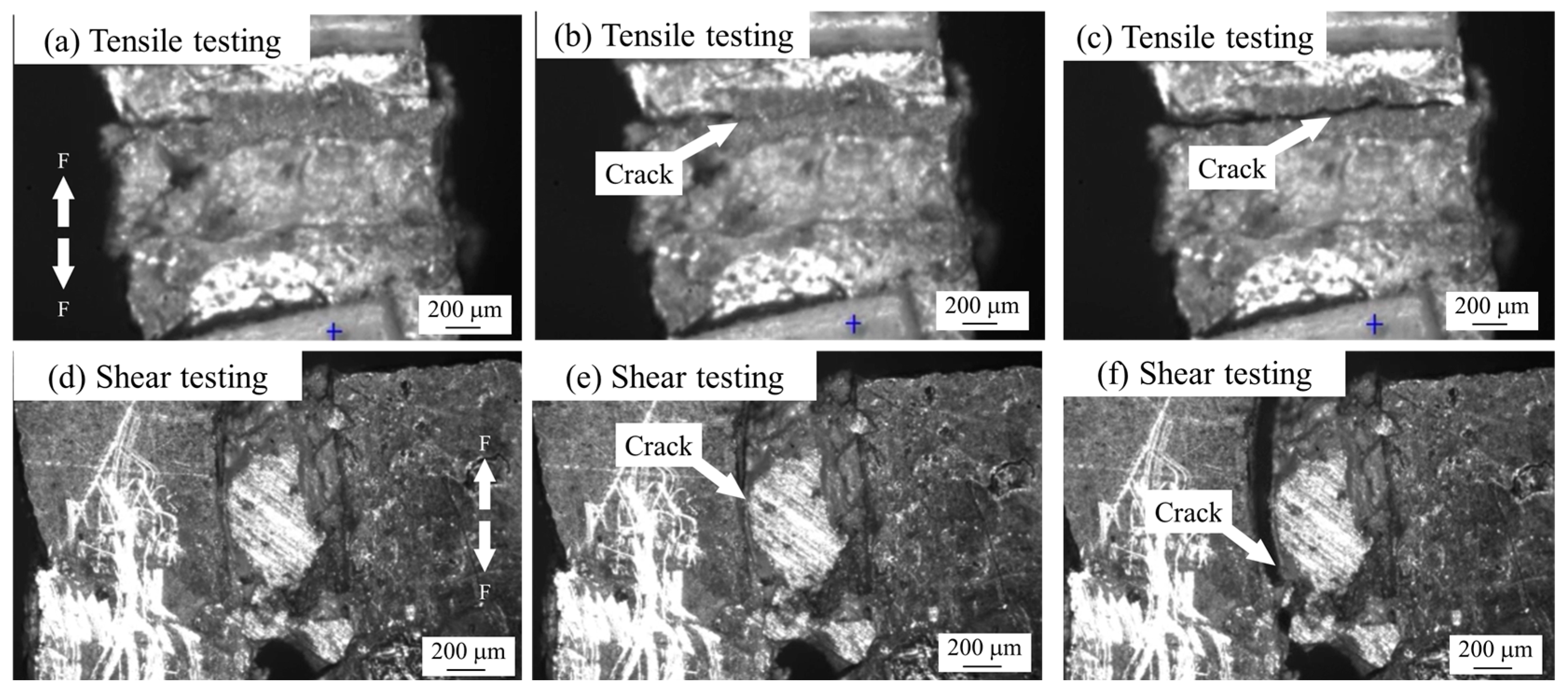
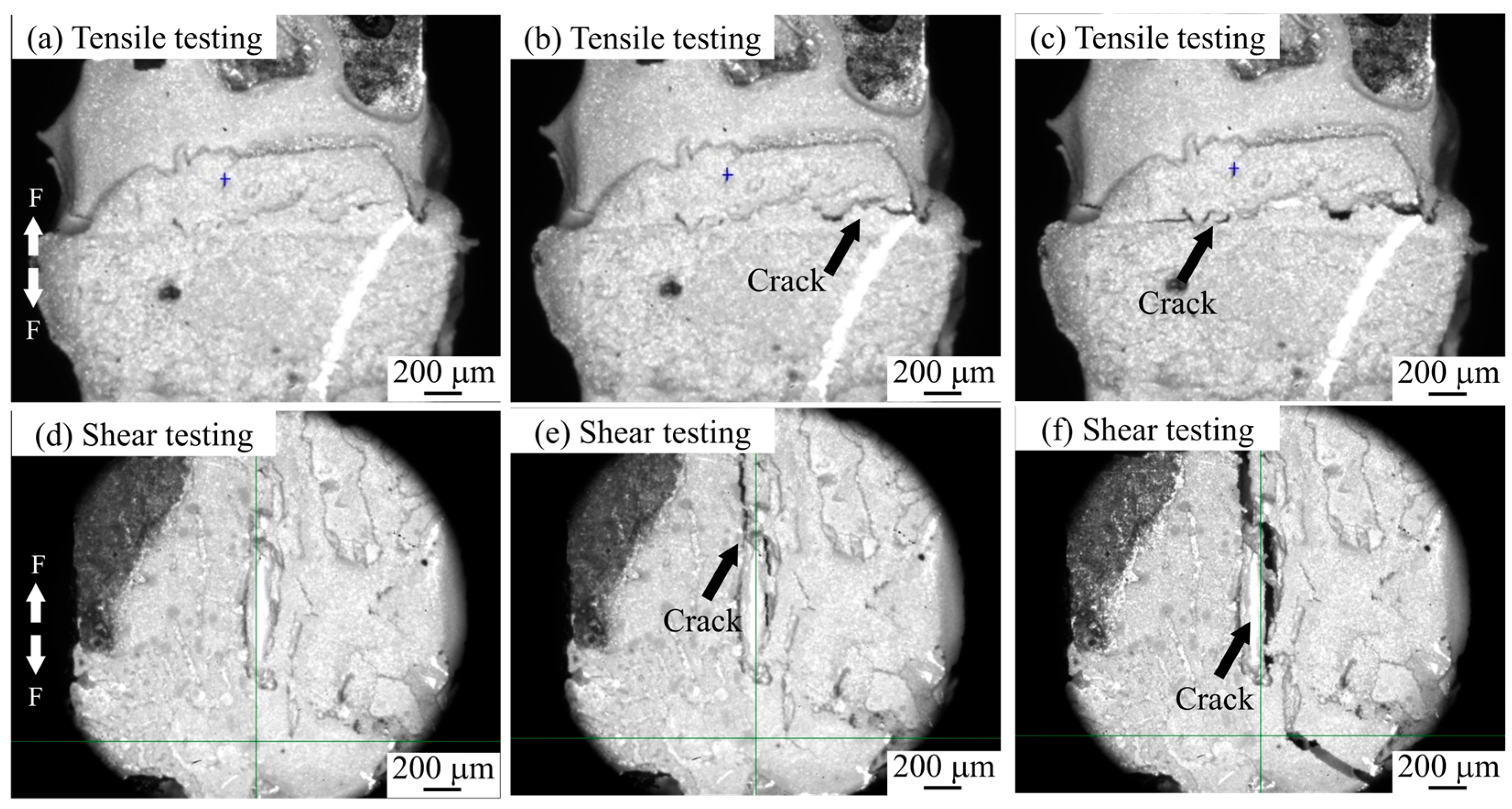
3.3. Fracture Mechanism Analysis
4. Conclusions
- (1)
- In the tensile test, both joints are in Ag-Cu interface fracture mode, and in the shear test, composite fracture mode occurs.
- (2)
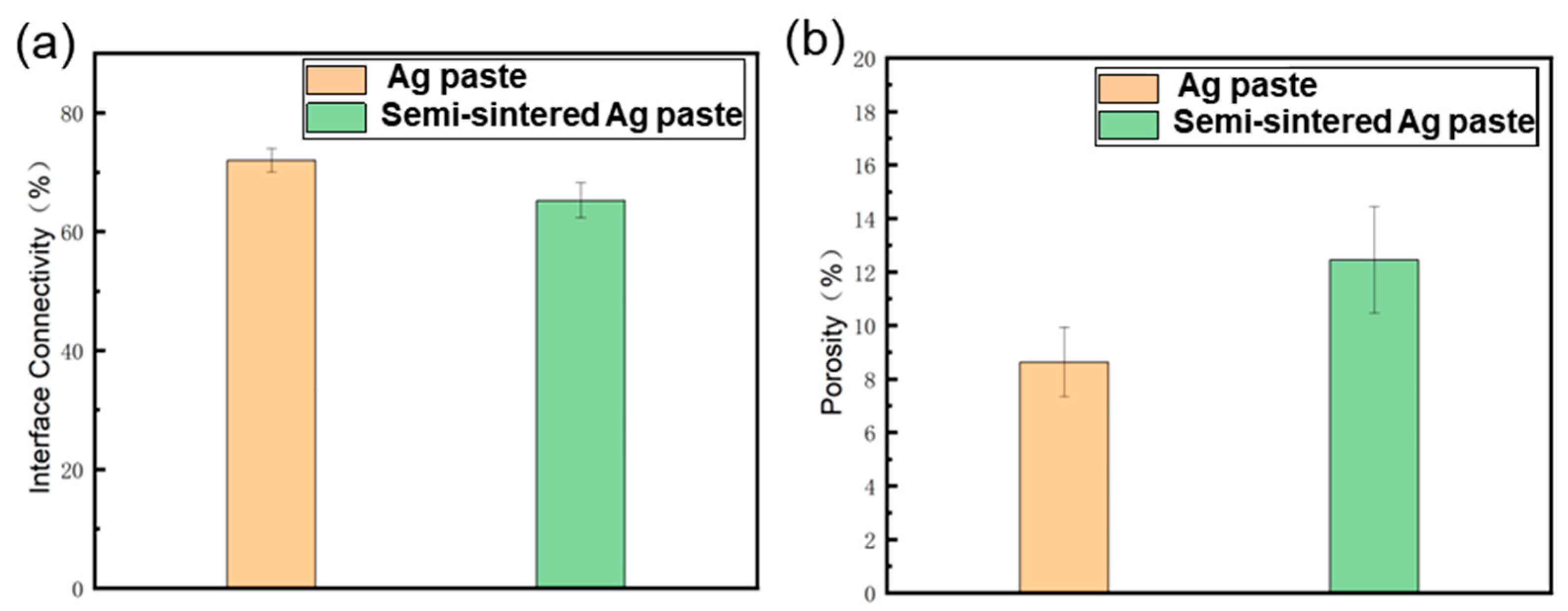
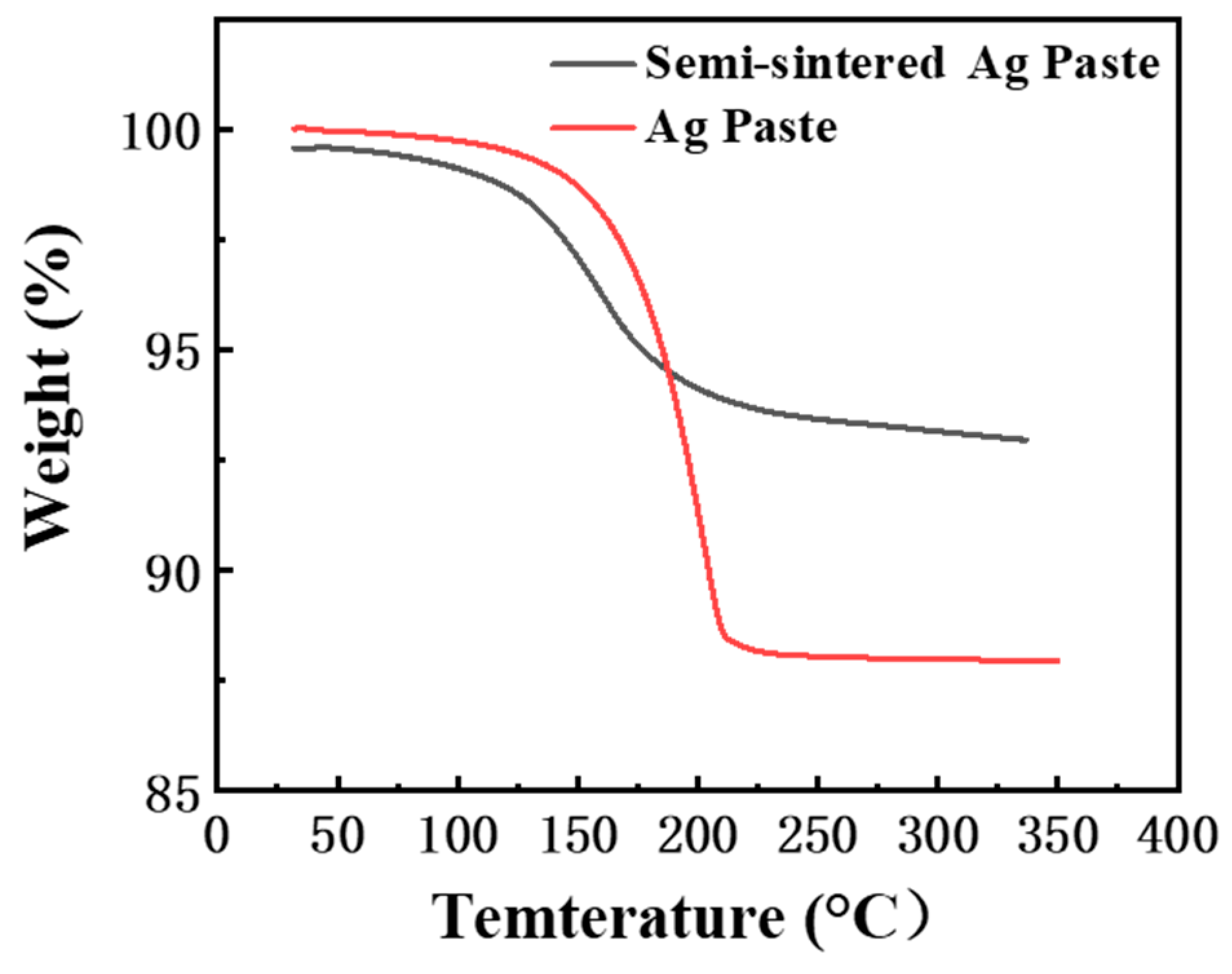
- Due to the resins and organic substances blocking the diffusion of Ag atoms, the semi-sintered Ag joint is mainly surface diffusion-dominated during the sintering densification process, while the sintered Ag joint is the result of the combined action of surface diffusion and grain-boundary diffusion, resulting in a higher porosity and a lower interface connection rate in the semi-sintered Ag joint.
- (3)
- During the tensile process, cracks are preferentially generated and propagated in the pores at the interface. The sintered Ag joint has a higher interface connection rate and better plasticity in the silver layer, so it has a high tensile strength.
- (4)
- During the shear process, there are more pores in the sintered body and at the Ag-Cu interface of the semi-sintered Ag joint, causing stress concentration and rapid failure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.Y.; Mei, Y.H.; Li, X.; Lu, G.Q. Correlation between interfacial microstructure and bonding strength of sintered nanosilver on ENIG and electroplated Ni/Au direct-bond-copper (DBC) substrates. J. Alloys Compd. 2016, 675, 317–324. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.T.; Liu, G.M.; Li, C.F.; Kurosaka, S.; Nagao, S.; Suganuma, K. Enhancement of bonding strength in Ag sinter joining on Au surface finished substrate by increasing Au grain-size. Appl. Surf. Sci. 2019, 485, 468–475. [Google Scholar] [CrossRef]
- Gao, L.Y.; Yang, H.K.; Chen, X.; Tang, W.D.; Huang, X.M.; Liu, Z.Q. The development of porous metallic materials: A short review of fabrication, characteristics, and applications. Phys. Scr. 2023, 98, 122001. [Google Scholar] [CrossRef]
- Zhao, S.; Dai, Y.W.; Qin, F.; Li, Y.N.; An, T.; Gong, Y.P. Effect of surface finish metallization layer on shearing fracture toughness of sintered silver bonded joints. Eng. Fract. Mech. 2022, 264, 108355. [Google Scholar] [CrossRef]
- Bai, J.G.F.; Lu, G.Q. Thermomechanical reliability of low-temperature sintered silver die attached SiC power device assembly. IEEE Trans. Device Mater. Reliab. 2006, 6, 436–441. [Google Scholar] [CrossRef]
- Knoerr, M.; Schletz, A. Power semiconductor joining through sintering of silver nanoparticles: Evaluation of influence of parameters time, temperature and pressure on density, strength and reliability. In Proceedings of the 2010 6th International Conference on Integrated Power Electronics Systems, Nuremberg, Germany, 16–18 March 2010; pp. 1–6. [Google Scholar]
- Mohd Zubir, N.S.; Zhang, H.; Zou, G.; Bai, H.; Deng, Z.; Feng, B.; Wu, A.; Liu, L.; Zhou, Y.N. Large-Area Die-Attachment Sintered by Organic-Free Ag Sintering Material at Low Temperature. J. Electron. Mater. 2019, 48, 7562–7572. [Google Scholar] [CrossRef]
- Stuckner, J.A.; Lu, G.Q.; Mitsuhara, M.; Reynolds, W.T.; Murayama, M. The Influence of Processing Conditions on the 3-D Interconnected Structure of Nanosilver Paste. IEEE Trans. Electron. Devices 2017, 64, 494–499. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Wang, L.G.; Fan, X.J.; Zhang, G.Q.; Sun, F.L. Effect of Sintering Pressure on the Porosity and the Shear Strength of the Pressure-Assisted Silver Sintering Bonding. IEEE Trans. Device Mater. Reliab. 2018, 18, 240–246. [Google Scholar] [CrossRef]
- Fan, T.K.; Zhang, H.; Shang, P.J.; Li, C.F.; Chen, C.T.; Wang, J.X.; Liu, Z.Q.; Zhang, H.; Suganuma, K. Effect of electroplated Au layer on bonding performance of Ag pastes. J. Alloys Compd. 2018, 731, 1280–1287. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.T.; Jiu, J.T.; Nagao, S.; Suganuma, K. High-temperature reliability of low-temperature and pressureless micron Ag sintered joints for die attachment in high-power device. J. Mater. Sci. Mater. Electron. 2018, 29, 8854–8862. [Google Scholar] [CrossRef]
- de Wit, R. Silver Sintering Die Attach Developments for RF, Power and Automotive Applications. In Proceedings of the 2021 23rd European Microelectronics and Packaging Conference & Exhibition (EMPC), Gothenburg, Sweden, 13–16 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Jung, K.H.; Min, K.D.; Lee, C.J.; Park, B.G.; Jeong, H.; Koo, J.M.; Lee, B.; Jung, S.B. Effect of epoxy content in Ag nanoparticle paste on the bonding strength of MLCC packages. Appl. Surf. Sci. 2019, 495, 143487. [Google Scholar] [CrossRef]
- Sasaki, K.; Mizumura, N. Development of low-temperature sintering nano-ag pastes using lowering modulus technologies. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 897–902. [Google Scholar] [CrossRef]
- Sasaki, K.; Mizumura, N.; Tsuno, A.; Yagci, S.; Kopp, G. Development of low-temperature sintering nano-silver die attach materials for bare Cu application. In Proceedings of the 2017 21st European Microelectronics and Packaging Conference (EMPC) & Exhibition, Warsaw, Poland, 10–13 September 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Jin, H.H.; Kanagavel, S.; Chin, W.F. Novel conductive paste using hybrid silver sintering technology for high reliability power semiconductor packaging. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 27–30 May 2014; pp. 1790–1795. [Google Scholar] [CrossRef]
- Liang, Z.T.; Wang, H.Y.; Zhou, B.; Li, G.Y.; Chen, Z.H.; Fu, Z.W.; Chen, S.; Yang, X.F.; Peng, C.; Huang, C.M. Thermo-Mechanical Stability of Resin-Added Sintered Silver Joints Between Si Die and Ni-/Au-Electroplated Substrate. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 1516–1525. [Google Scholar] [CrossRef]
- An, L.H.; Wu, X.D.; Wang, K.; Li, R.Z.; Li, Z.Q.; Li, G.Q. Crack modes and toughening strategies of bioinspired 3D printed double-helicoidal architectures. Int. J. Mech. Sci. 2023, 253, 108388. [Google Scholar] [CrossRef]
- Fu, S.C.; Zhao, M.; Shan, H.Y.; Li, Y. Fabrication of large-area interconnects by sintering of micron Ag paste. Mater. Lett. 2018, 226, 26–29. [Google Scholar] [CrossRef]
- Ma, L.M.; Wang, Y.C.; Jia, Q.; Zhang, H.Q.; Wang, Y.S.; Li, D.; Zou, G.S.; Guo, F. Low-Temperature-Sintered Nano-Ag Film for Power Electronics Packaging. J. Electron. Mater. 2024, 53, 228–237. [Google Scholar] [CrossRef]
- Shen, X.W.; Li, J.J.; Xi, S. High Strength Die-Attach Joint Formation by Pressureless Sintering of Organic Amine Modified Ag Nanoparticle Paste. Nanomaterials 2022, 12, 3351. [Google Scholar] [CrossRef]
- Morita, T.; Ide, E.; Yasuda, Y.; Hirose, A.; Kobayashi, K. Study of Bonding Technology Using Silver Nanoparticles. Jpn. J. Appl. Phys. 2008, 47, 6615. [Google Scholar] [CrossRef]
- Asoro, M.A.; Damiano, J.; Ferreira, P.J. Size Effects on the Melting Temperature of Silver Nanoparticles: In-situ TEM Observations. Microsc. Microanal. 2009, 15, 706–707. [Google Scholar] [CrossRef]
- Wang, W.G.; Zou, G.S.; Deng, Z.Y.; Jia, Q.; Feng, B.; Liu, L. Effects of Oxygen Content on Low-Temperature Bonding Using Organic-Free Silver Nanostructured Film with Different Types of Substrate Metallization. J. Electron. Mater. 2024, 53, 3870–3886. [Google Scholar] [CrossRef]
- Yang, F.; Hu, B.; Peng, Y.; Hang, C.J.; Chen, H.T.; Lee, C.W.; Wei, J.; Li, M.Y. Ag microflake-reinforced nano-Ag paste with high mechanical reliability for high-temperature applications. J. Mater. Sci. Mater. Electron. 2019, 30, 5526–5535. [Google Scholar] [CrossRef]
- Wakamoto, K.; Mochizuki, Y.; Otsuka, T.; Nakahara, K.; Namazu, T. Temperature Dependence on Tensile Mechanical Properties of Sintered Silver Film. Materials 2020, 13, 4061. [Google Scholar] [CrossRef] [PubMed]
- Wakamoto, K.; Yasugi, D.; Otsuka, T.; Nakahara, K.; Namazu, T. Fracture Mechanism of Sintered Silver Film Revealed by In Situ SEM Uniaxial Tensile Loading. IEEE Trans. Compon. Packag. Manuf. Technol. 2024, 14, 240–250. [Google Scholar] [CrossRef]
- Yao, Y.; Gong, H. Damage and viscoplastic behavior of sintered nano-silver joints under shear loading. Eng. Fract. Mech. 2019, 222, 106741. [Google Scholar] [CrossRef]
- Ma, K.; Liu, X.; Sun, Y.M.; Song, Y.F.; Feng, Z.; Zhou, Y.; Liu, S. A Micromechanical Analysis to the Viscoplastic Behavior of Sintered Silver Joints under Shear Loading. Materials 2023, 16, 4472. [Google Scholar] [CrossRef]
- Zabihzadeh, S.; Cugnoni, J.; Duarte, L.I.; Van Petegem, S.; Van Swygenhoven, H. Deformation behavior of nano-porous polycrystalline silver. Part II: Simulations. Acta Mater. 2017, 131, 564–573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Yang, H.-K.; Huang, X.; Gao, L.-Y.; Liu, Z.-Q. Revealing the Mechanical Properties and Fracture Mechanism of Ag Paste Sintered Solder by Two Different Preparation Methods. Materials 2025, 18, 1435. https://doi.org/10.3390/ma18071435
Liang J, Yang H-K, Huang X, Gao L-Y, Liu Z-Q. Revealing the Mechanical Properties and Fracture Mechanism of Ag Paste Sintered Solder by Two Different Preparation Methods. Materials. 2025; 18(7):1435. https://doi.org/10.3390/ma18071435
Chicago/Turabian StyleLiang, Jialong, Hao-Kun Yang, Xingming Huang, Li-Yin Gao, and Zhi-Quan Liu. 2025. "Revealing the Mechanical Properties and Fracture Mechanism of Ag Paste Sintered Solder by Two Different Preparation Methods" Materials 18, no. 7: 1435. https://doi.org/10.3390/ma18071435
APA StyleLiang, J., Yang, H.-K., Huang, X., Gao, L.-Y., & Liu, Z.-Q. (2025). Revealing the Mechanical Properties and Fracture Mechanism of Ag Paste Sintered Solder by Two Different Preparation Methods. Materials, 18(7), 1435. https://doi.org/10.3390/ma18071435







