Evaluation of High-Temperature Sterilization Processes: Their Influence on the Mechanical Integrity of Additively Manufactured Polymeric Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for the Research
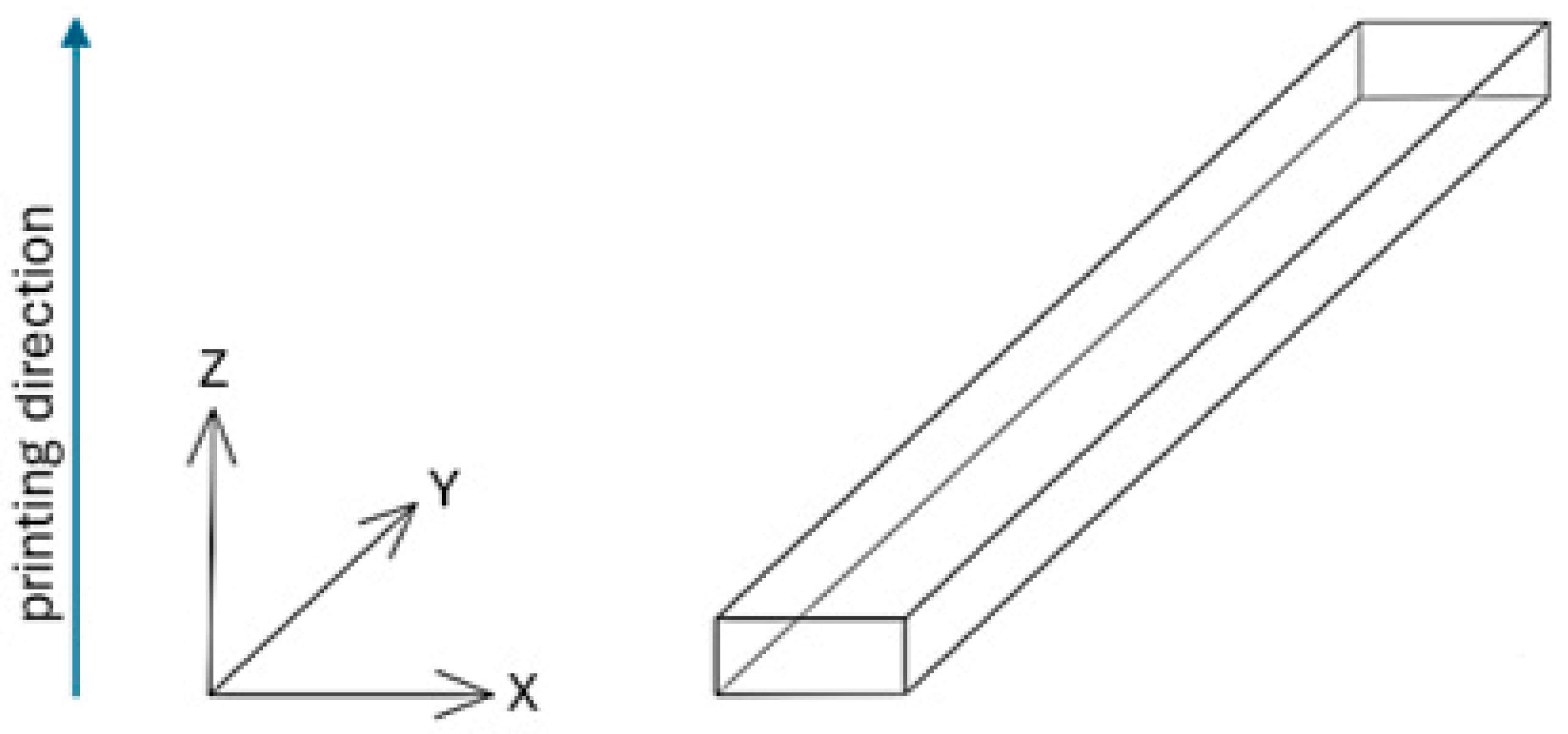
2.2. Manufacturing Process
2.3. Steam Sterilization
2.4. Testing Methods
3. Results and Discussion
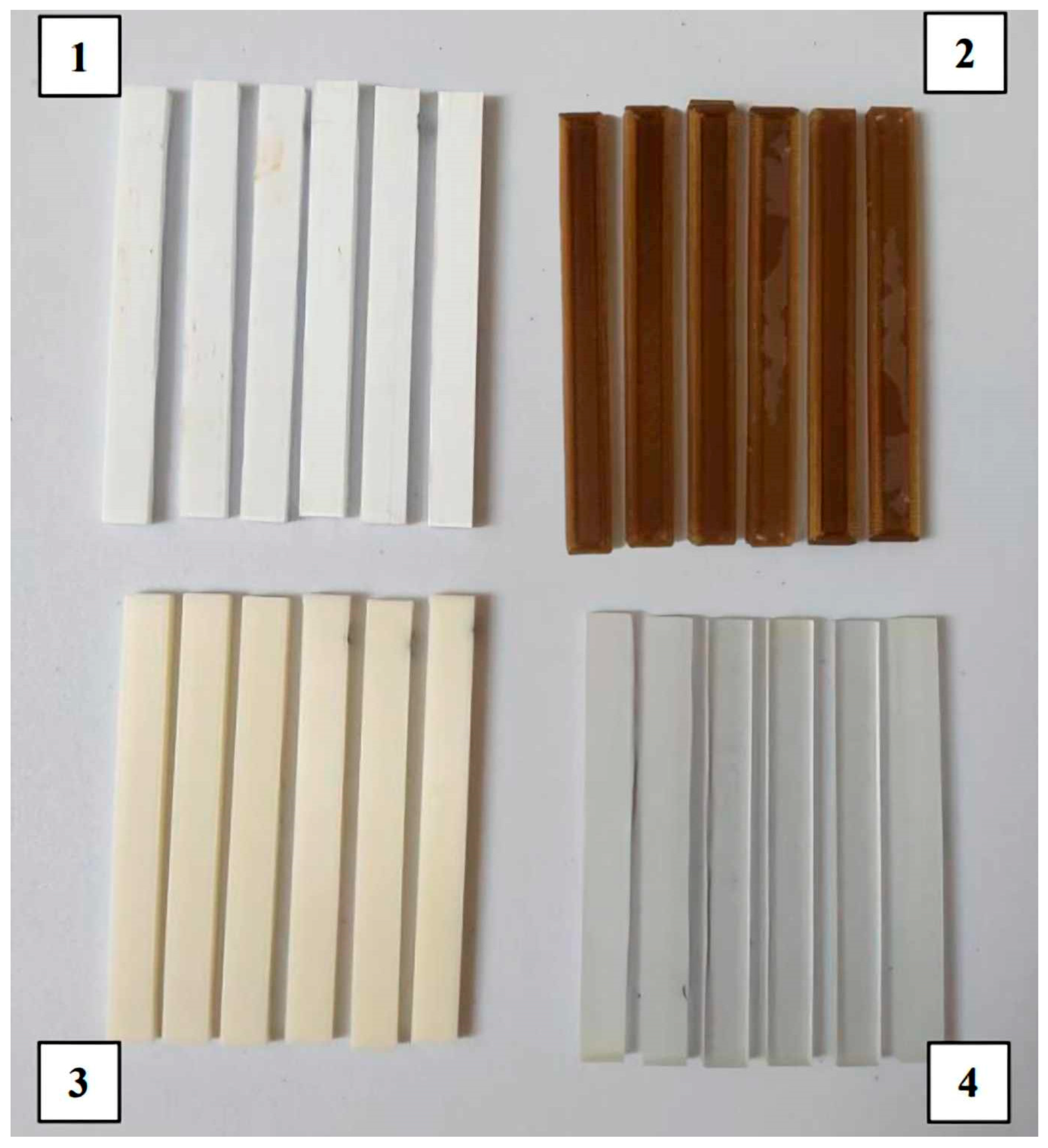
3.1. Visual Analysis of Samples After Bench Testing
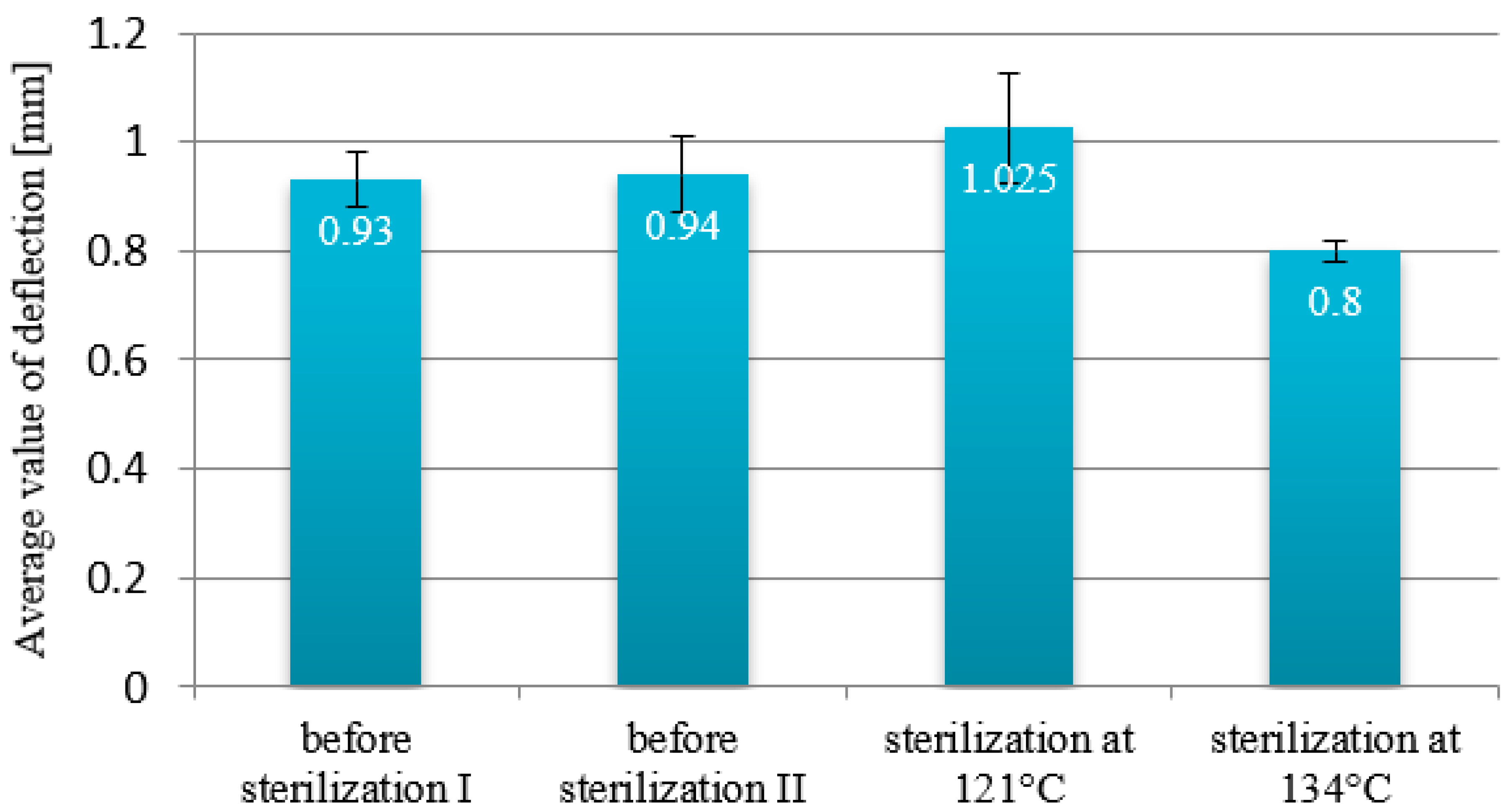
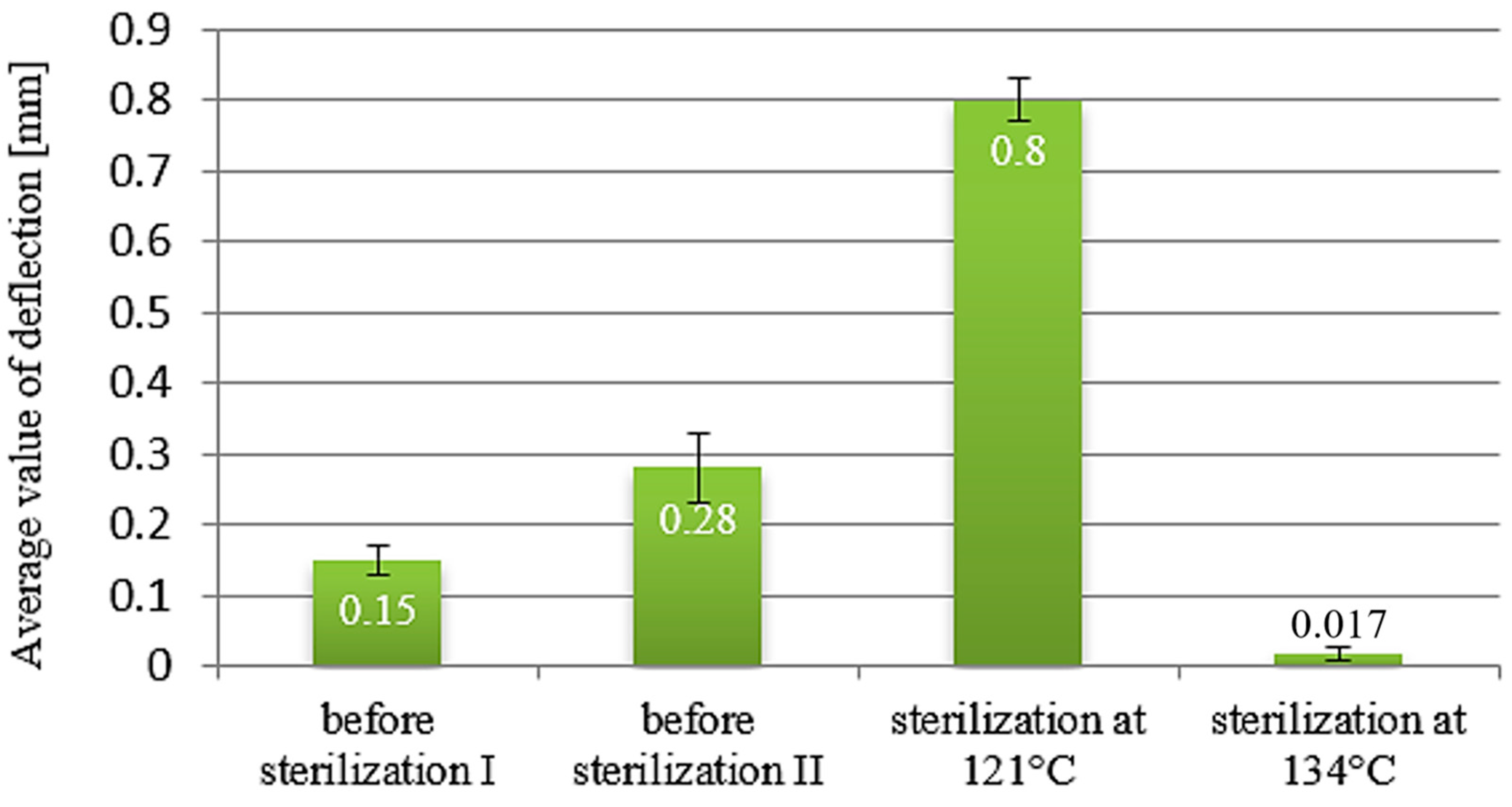
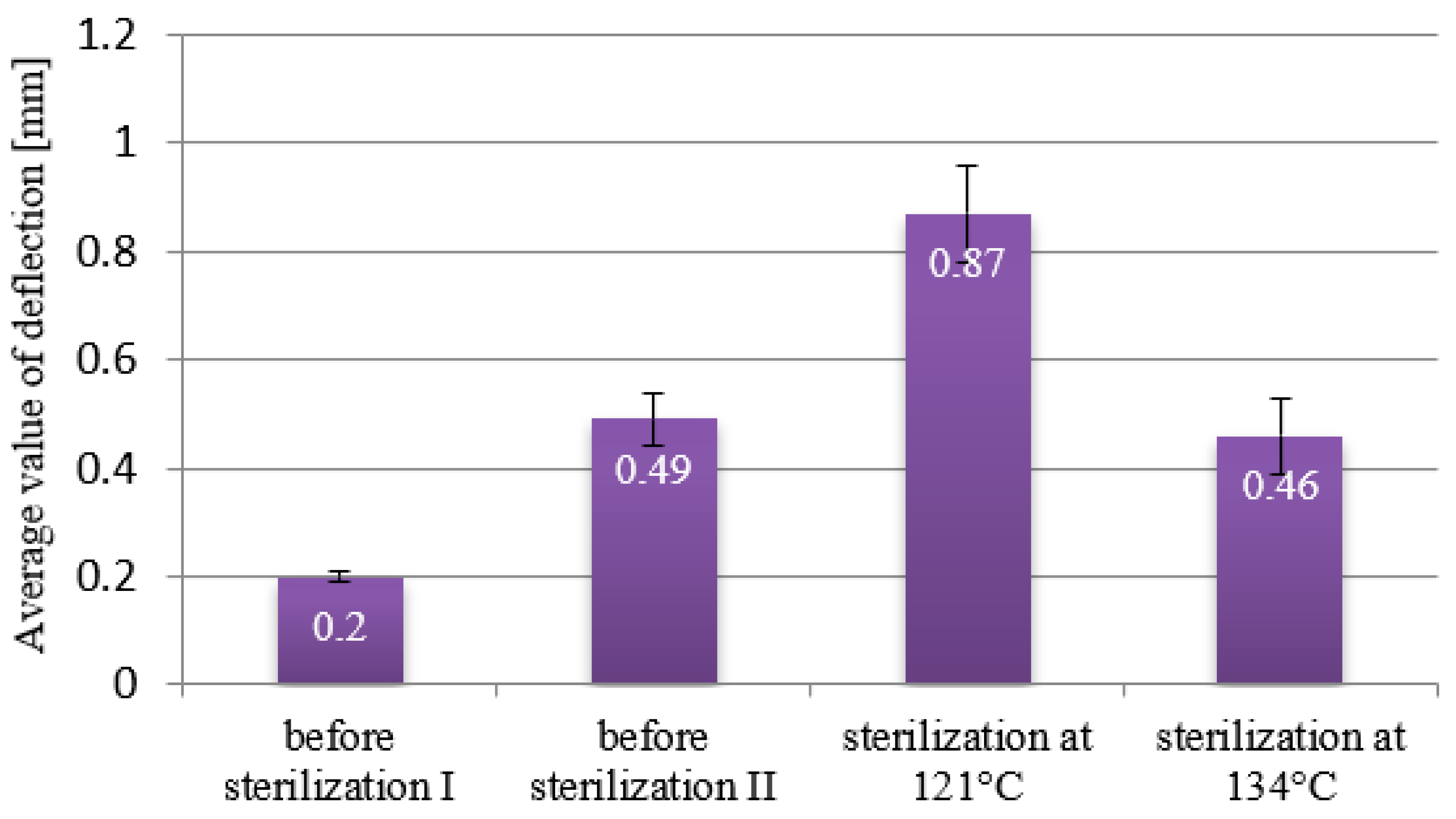
3.2. Deflection
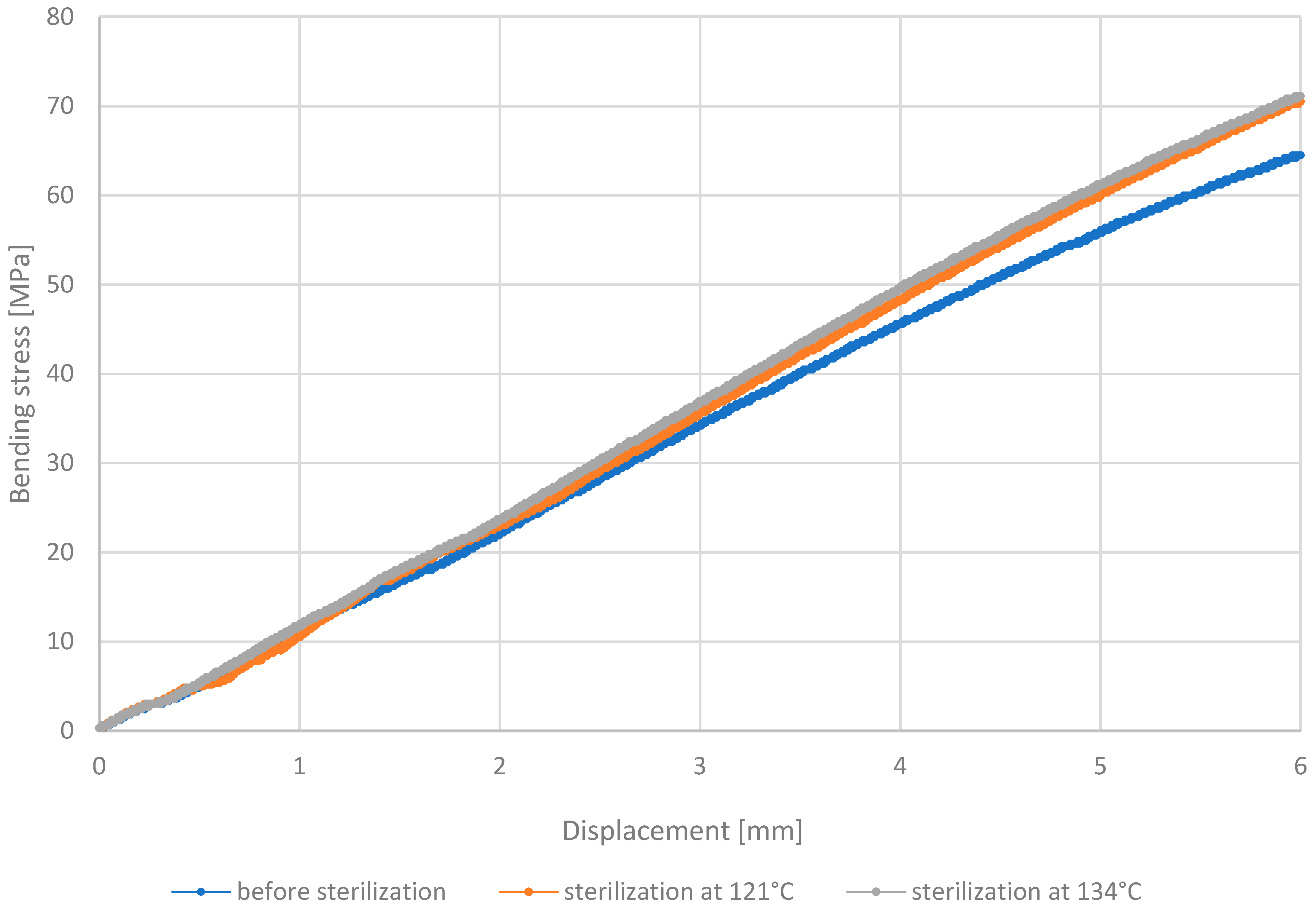
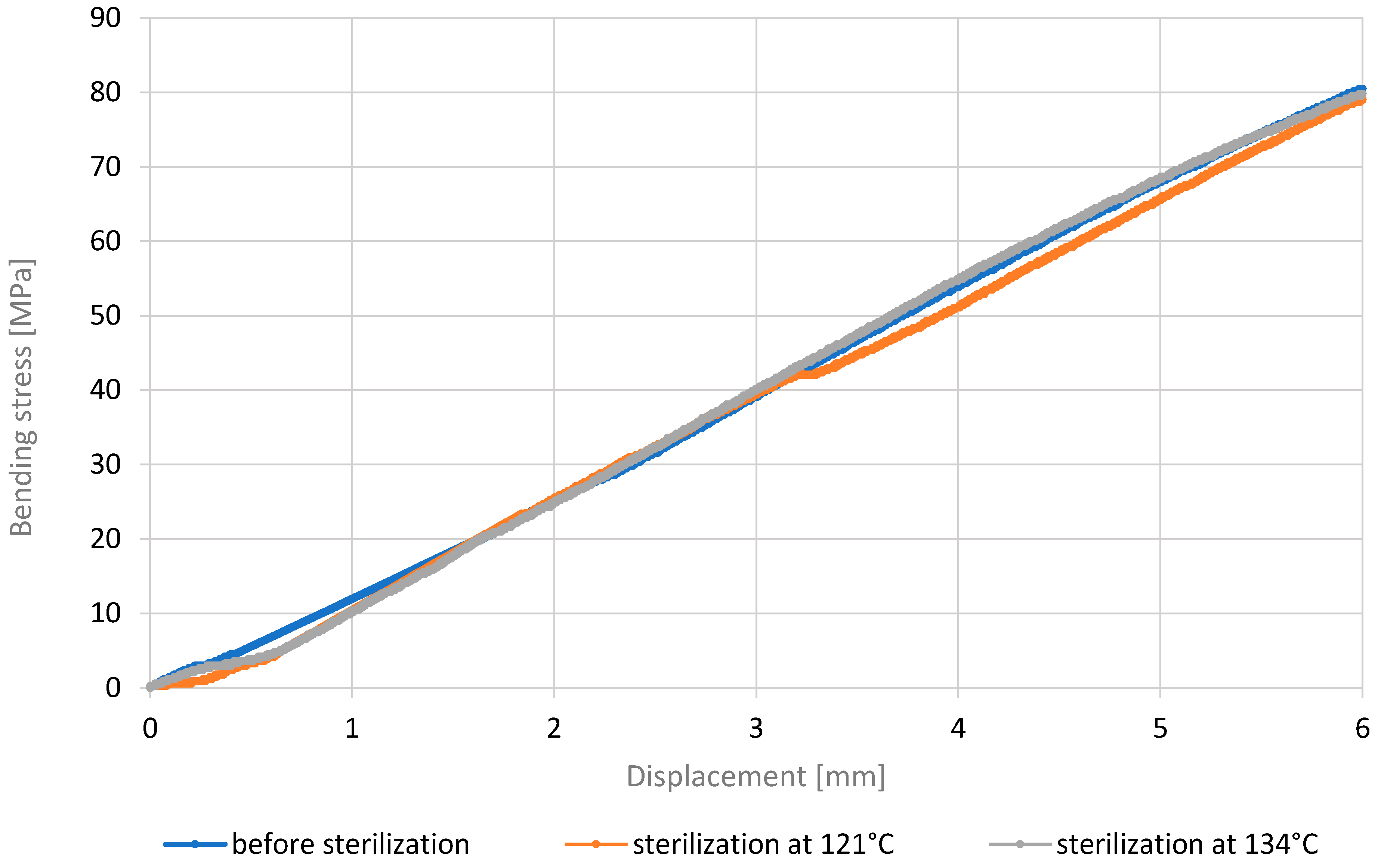
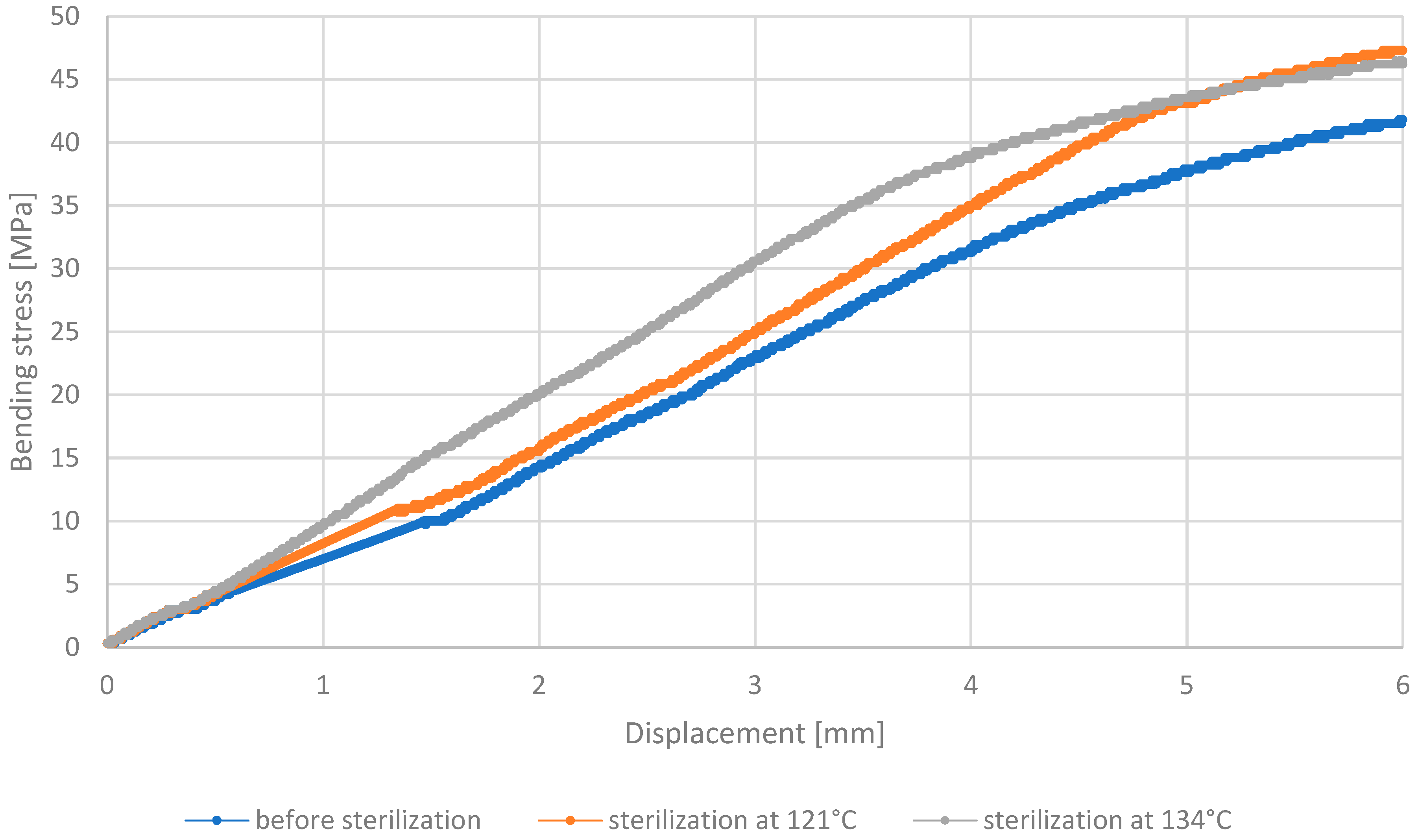
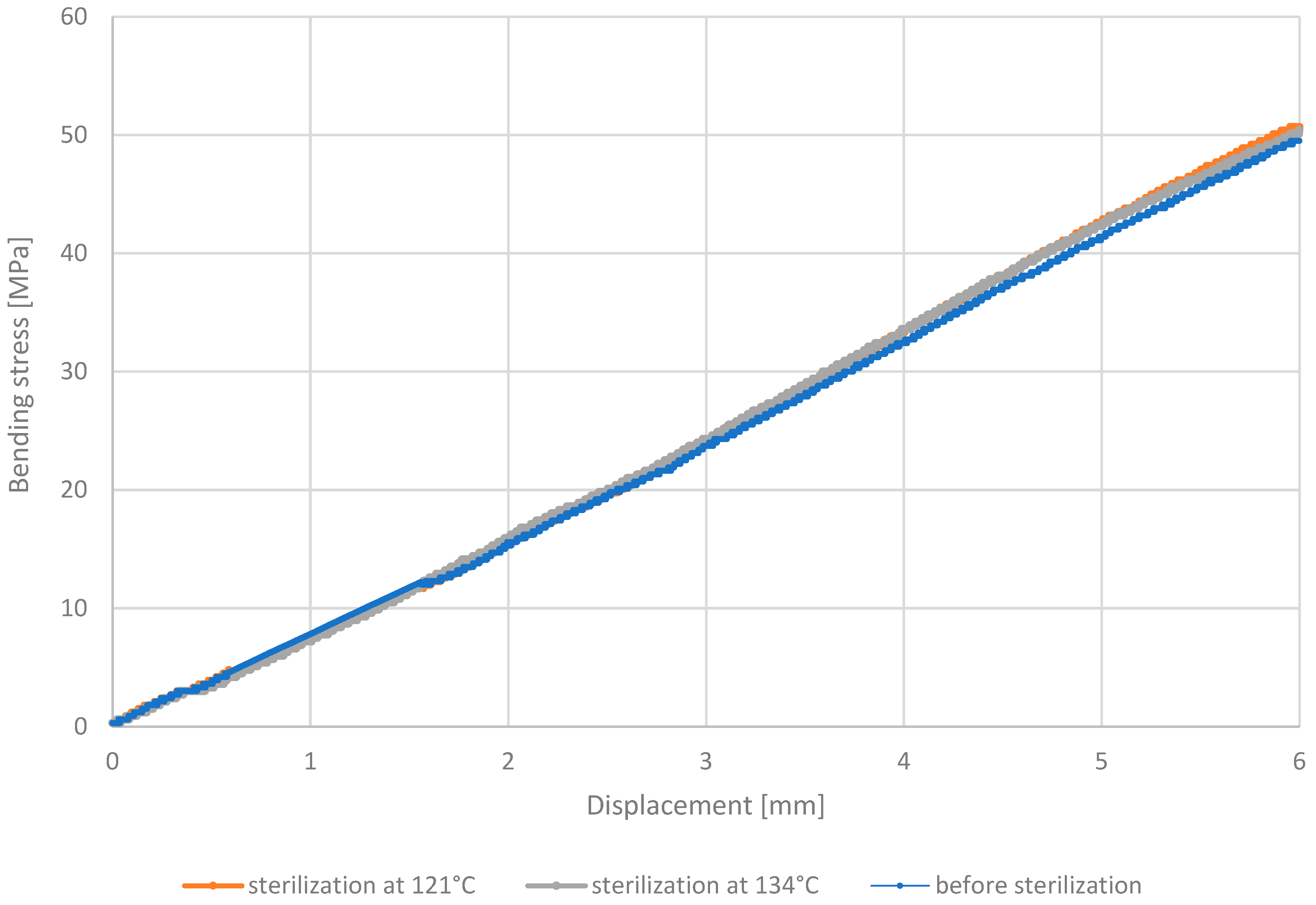
3.3. Three-Point Bending Test
3.4. Hardness
3.5. Test Results Obtained
4. Conclusions
- The steam sterilization process significantly affects the mechanical properties of polymer biomaterials such as MED610, PEEK, PETG HT100, and RGD720. Strength tests indicate varied reactions of these materials to the sterilization process, which is crucial for their further application in medicine.
- Creep tests and deflection measurements showed that steam sterilization leads to higher deflection values in sterilized beams. These results suggest that the temperature of the sterilization process is a significant factor that influences the mechanical properties of these materials.
- Analysis of the outcomes of the three-point bending test revealed that PETG HT100 and PEEK materials, characterized by high 3D printing temperatures, exhibit substantial resistance to the sterilization process, showing no significant differences in stress values after sterilization. On the contrary, the other materials examined showed increased bending stress values after sterilization, suggesting that this process may enhance their bending strength. The resistance of PETG HT100 and PEEK to sterilization suggests that materials with similar properties could be further developed to ensure high mechanical stability in medical applications. Furthermore, the observed increased bending strength in some materials after sterilization suggests that, in some cases, the sterilization process may enhance certain material properties, which could be leveraged in the design of materials for specific medical applications.
- The hardness test indicated that most materials, except PETG HT100, achieve higher hardness values after steam sterilization. The increased hardness of the material suggests a reduction in its susceptibility to permanent deformation under mechanical forces. PETG HT100 did not show significant differences in hardness values after sterilization, indicating its mechanical stability at elevated temperatures. The increase in hardness in many materials after sterilization suggests that engineers could design materials with improved resistance to permanent deformation, making them more durable for use in medical devices. The stability of PETG HT100 also highlights the potential for the development of materials that retain their mechanical properties under sterilization conditions, a critical factor for their use in the medical field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haerinia, M.; Shadid, R. Wireless Power Transfer Approaches for Medical Implants: A Review. Signals 2020, 1, 209–229. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Tejo-Otero, A.; Fenollosa-Artés, F. Development of Am Technologies for Metals in the Sector of Medical Implants. Metals 2020, 10, 686. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef]
- Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3d Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Picco, C.J.; Utomo, E.; McClean, A.; Domínguez-Robles, J.; Anjani, Q.K.; Volpe-Zanutto, F.; McKenna, P.E.; Acheson, J.G.; Malinova, D.; Donnelly, R.F.; et al. Development of 3D-Printed Subcutaneous Implants Using Concentrated Polymer/Drug Solutions. Int. J. Pharm. 2023, 631, 122477. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Diaz-Gomez, L.; Utomo, E.; Shen, T.; Picco, C.J.; Alvarez-Lorenzo, C.; Concheiro, A.; Donnelly, R.F.; Larrañeta, E. Use of 3d Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals 2021, 14, 921. [Google Scholar] [CrossRef]
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Braga, M.E.M.; et al. Porous Poly(ε-Caprolactone) Implants: A Novel Strategy for Efficient Intraocular Drug Delivery. J. Control. Release 2019, 316, 331–348. [Google Scholar] [CrossRef]
- Izdebska, A.; Lacheta, P.; Lis, A.; Nowak, J. Materiały Biodegradowalne i Bioresorbowalne Stosowane w Aplikacjach Medycznych, Zeszyty Studenckie Kół Naukowych, Politechnika Śląska, Gliwice, Poland. 2020, pp. 36–37. Available online: https://pimib.polsl.pl/pliki/imiib/11/Izdebska.pdf (accessed on 14 October 2024).
- Domsta, V.; Seidlitz, A. 3d-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Krivec, M.; Roshanghias, A.; Abram, A.; Binder, A. Exploiting the Combination of 3D Polymer Printing and Inkjet Ag-Nanoparticle Printing for Advanced Packaging. Microelectron. Eng. 2017, 176, 1–5. [Google Scholar] [CrossRef]
- Grzelak, K.; Łaszcz, J.; Polkowski, J.; Mastalski, P.; Kluczyński, J.; Łuszczek, J.; Torzewski, J.; Szachogłuchowicz, I.; Szymaniuk, R. Additive Manufacturing of Plastics Used for Protection against COVID19—The Influence of Chemical Disinfection by Alcohol on the Properties of Abs and Petg Polymers. Materials 2021, 14, 4823. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Foteinopoulos, P. Modelling of Additive Manufacturing Processes: A Review and Classification. Manuf. Rev. 2018, 5, 2. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J.; Kotta, S. 3D Printing in Medicine: Technology Overview and Drug Delivery Applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, L.; Wang, Y.; Gang, F.; Xu, N.; Li, T.; Sun, X. 3D Printing of Conductive Tissue Engineering Scaffolds Containing Polypyrrole Nanoparticles with Different Morphologies and Concentrations. Materials 2019, 12, 2491. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D Printing Metal Implants in Orthopedic Surgery: Methods, Applications and Future Prospects. J. Orthop. Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.A.; Hameed, P.; Whenish, R.; Elsen, R.S.; Aswin, G.; Jaiswal, A.K.; Prashanth, K.G.; Manivasagam, G. A Review on Development of Bio-Inspired Implants Using 3d Printing. Biomimetics 2021, 6, 65. [Google Scholar] [CrossRef]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA Implants for Controlled Drug Release: Impact of the Diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Shen, T.; Cornelius, V.A.; Corduas, F.; Mancuso, E.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; Larrañeta, E. Development of Drug Loaded Cardiovascular Prosthesis for Thrombosis Prevention Using 3D Printing. Mater. Sci. Eng. C 2021, 129, 112375. [Google Scholar] [CrossRef]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-Printed Reservoir-Type Implants Containing Poly(Lactic Acid)/Poly(Caprolactone) Porous Membranes for Sustained Drug Delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A New Era for Sterilization Based on Supercritical CO2 Technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Ao, T.; Mao, X.; Yan, X.; Javed, R.; Hou, W.; Wang, Y.; Sun, C.; Lin, S.; Yu, T.; et al. Sterilization and Disinfection Methods for Decellularized Matrix Materials: Review, Consideration and Proposal. Bioact. Mater. 2021, 6, 2927–2945. [Google Scholar] [CrossRef]
- Moradi, L.; Mohammadi Jobania, B.; Jafarnezhad-Ansariha, F.; Ghorbani, F.; Esmaeil-Pour, R.; Majidi Zolbina, M.; Kajbafzadeh, A.M. Evaluation of Different Sterilization Methods for Decellularized Kidney Tissue. Tissue Cell 2020, 66, 101396. [Google Scholar] [CrossRef]
- Jildeh, Z.B.; Wagner, P.H.; Schöning, M.J. Sterilization of Objects, Products, and Packaging Surfaces and Their Characterization in Different Fields of Industry: The Status in 2020. Phys. Status Solidi Appl. Mater. Sci. 2021, 218, 2000732. [Google Scholar] [CrossRef]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef]
- Pugliese, R.; Beltrami, B.; Regondi, S.; Lunetta, C. Polymeric Biomaterials for 3D Printing in Medicine: An Overview. Ann. 3D Print. Med. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Haryńska, A.; Carayon, I.; Kosmela, P.; Brillowska-Dąbrowska, A.; Łapiński, M.; Kucińska-Lipka, J.; Janik, H. Processing of Polyester-Urethane Filament and Characterization of FFF 3d Printed Elastic Porous Structures with Potential in Cancellous Bone Tissue Engineering. Materials 2020, 13, 4457. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether Ether Ketone (PEEK) and Its 3D Printed Implants Applications in Medical Field: An Overview. Clin. Epidemiol. Glob. Health 2019, 7, 571–577. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Li, W.; Yang, L. Emerging Biomaterials for Reproductive Medicine. Eng. Regen. 2021, 2, 230–245. [Google Scholar] [CrossRef]
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical Devices Biomaterials—A Review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2020, 234, 218–228. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. Extrusion-Based Additive Manufacturing of PEEK for Biomedical Applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material—A review. Colloids Surf. B Biointerfaces 2021, 203, 111726. [Google Scholar] [CrossRef] [PubMed]
- Trianziani, S. Dampak Pengurangan Ruang Terbuka Hijau (Rth) Perkotaan terhadap Peningkatan Suhu Udara dengan Metode Penginderaan Jauh. J. Agromet 2020, 4, 247–282. [Google Scholar]
- Zaupa, A.; Terraza, C.; Abarzúa-Illanes, P.N.; Byres, N.; Zavala, G.; Cuenca, J.; Hidalgo, C.; Viafara-Garcia, S.M.; Wolf, B.; Pino-Lagos, K.; et al. A Psychrophilic GelMA: Breaking Technical and Immunological Barriers for Multimaterial High-Resolution 3D Bioprinting. Biomacromolecules 2023, 24, 150–165. [Google Scholar] [CrossRef]
- Moharil, S.; Reche, A.; Durge, K. Polyetheretherketone (PEEK) as a Biomaterial: An Overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef]
- Available online: https://www.stratasys.com/En/Materials/Materials-Catalog/Polyjet-Materials/Biocompatible-Clear-Med6102/ (accessed on 14 October 2024).
- Available online: https://tech-labs.com/Stratasys/3d-Printing-Materials/Polyjet-Photopolymers/Rgd720 (accessed on 14 October 2024).
- Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4906721 (accessed on 14 October 2024).
- Available online: https://spectrumfilaments.com/wp-content/uploads/2022/05/en_tds_spectrum_petg_ht100.pdf (accessed on 14 October 2024).
- Available online: https://Www.Ensingerplastics.Com/Pl-Pl/Polwyroby/Selekcja-Tworzyw/Mechaniczne-Parametry (accessed on 14 October 2024).
- Available online: https://Cadxpert.Pl/Materialy-Do-Druku-3d/Biokompatybilne/ (accessed on 14 October 2024).
- ISO 178:2019; Plastics—Determination of Flexural Properties. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 17665:2024; Sterilization of Health Care Products—Moist Heat—Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2024.
- Mrówka, M.; Machoczek, T.; Jureczko, P.; Joszko, K.; Gzik, M.; Wolański, W.; Wilk, K. Mechanical, Chemical, and Processing Properties of Specimens Manufactured from Poly-Ether-Ether-Ketone (Peek) Using 3d Printing. Materials 2021, 14, 2717. [Google Scholar] [CrossRef]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of Layer Thickness and Raster Angle on the Mechanical Properties of 3D-Printed PEEK and a Comparative Mechanical Study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Polyether Ether Ketone (PEEK) and Its Manufacturing of Customised 3D Printed Dentistry Parts Using Additive Manufacturing. Clin. Epidemiol. Glob. Health 2019, 7, 654–660. [Google Scholar] [CrossRef]




| MED610 | PEEK | PET-G HT100 | RGD720 | |
|---|---|---|---|---|
| Tensile Strength [MPa] | 50–65 | 110 | 43 | 50–65 |
| Bending Strength [MPa] | 75–110 | 130 | 52 | 80–110 |
| Young’s Modulus [MPa] | 2000–3000 | 4100 | 1575 | 2300–2700 |
| Layer Thickness [mm] | Printing Speed [mm/s] | Nozzle Temperature [°C] | Bed Temperature [°C] | Chamber Temperature [°C] | Type of Infill | Number of Contours | Infill [%] | |
|---|---|---|---|---|---|---|---|---|
| MED610 | high quality | - | - | - | - | standard | - | 100 |
| PEEK | 0.15 | 50 | 420 | 100 | 110 | parallel lines | 5 | 100 |
| PET-G HT100 | 0.2 | 45 | 250 | 100 | - | parallel lines | 2 | 100 |
| RGD720 | high quality | - | - | - | - | standard | - | 100 |
|
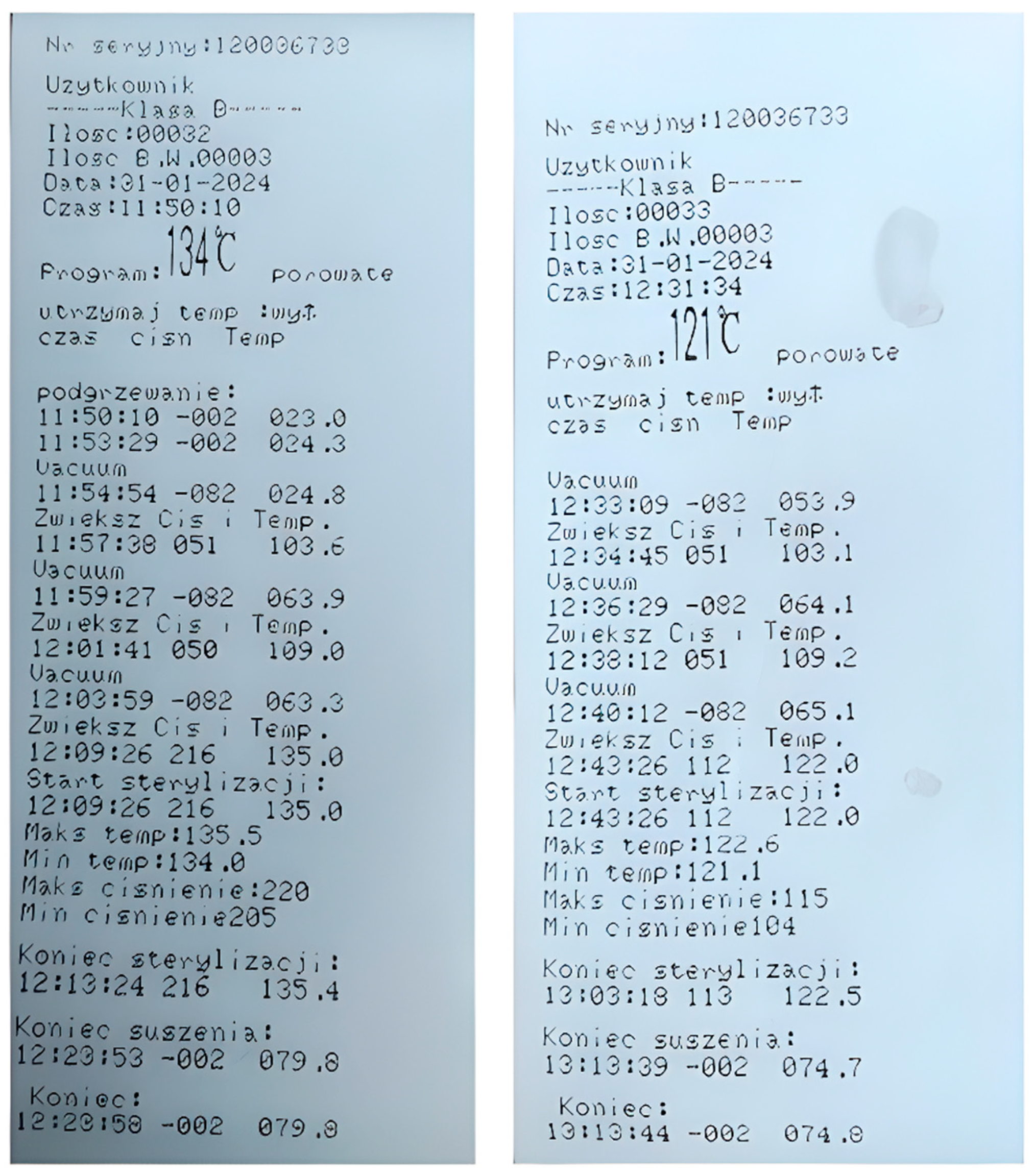
Max Temperature [°C] | Min Temperature [°C] | Max Pressure [MPa] | Min Pressure [MPa] | Class | Program | |
|---|---|---|---|---|---|---|
| Sterilization at 121 °C | 122.6 | 121.1 | 115 | 104 | B | 121 °C, porous |
| Sterilization w 134 °C | 135.5 | 124.0 | 220 | 205 | B | 134 °C, porous |
| Ref. |  |
| Sample after bending test |  |
| Sample after sterilization at temperature 121 °C and bending test |  |
| Sample after sterilization at temperature 134 °C and bending test |  |
| Ref. |  |
| Sample after bending test |  |
| Sample after sterilization at temperature 121 °C and bending test |  |
| Sample after sterilization at temperature 134 °C and bending test |  |
| Ref. |  |
| Sample after bending test |  |
| Sample after sterilization at temperature 121 °C and bending test |  |
| Sample after sterilization at temperature 134 °C and bending test |  |
| Ref. |  |
| Sample after bending test |  |
| Sample after sterilization at temperature. 121 °C and bending test |  |
| Sample after sterilization at temperature 134 °C and bending test |  |
| MED610 [N/mm2] | PEEK [N/mm2] | PET-G HT100 [N/mm2] | RGD720 [N/mm2] | |
|---|---|---|---|---|
| Test load [N] | 358 | 358 | 132 | 132 |
| Without Sterilization | 117.48 ± 8.56 | 79.02 ± 0.81 | 50.54 ± 3.31 | 35.44 ± 1.05 |
| After Sterilization at 121 °C | 134.02 ± 1.30 | 83.74 ± 12.59 | 49.98 ± 7.56 | 50.38 ± 9.05 |
| After Sterilization at 134 °C | 116.40 ± 10.97 | 84.86 ± 11.06 | 49.78 ± 6.69 | 45.44 ± 6.68 |
| Material | MED610 | PEEK | PET-G HT100 | RGD720 |
|---|---|---|---|---|
| Deflection Before Sterilization [mm] | 0.93–0.94 ± 0.07 | 0.25 ± 0.04 | 0.15–0.28 ± 0.02 | 0.2–0.49 ± 0.05 |
| Deflection After 121 °C [mm] | 1.025 ± 0.01 | 0.16 ± 0.05 | 0.8 ± 0.03 | 0.87 ± 0.09 |
| Deflection After 134 °C [mm] | 0.8 ± 0.02 | 0.35 ± 0.04 | 0.017 ± 0.01 | 0.46 ± 0.07 |
| Bending Strength Before Sterilization [MPa] | 70 | 81 | 49 | 44 |
| Bending Strength After 121 °C [MPa] | 64 | 79 | 51 | 47 |
| Bending Strength After 134 °C [MPa] | 71 | 80 | 50 | 46 |
| Hardness Before Sterilization [N/mm2] | 117.48 ± 8.56 | 79.02 ± 0.81 | 50.54 ± 3.31 | 35.44 ± 1.05 |
| Hardness After 121 °C [N/mm2] | 134.02 ± 1.30 | 83.74 ± 12.59 | 49.98 ± 7.56 | 50.38 ± 9.05 |
| Hardness After 134 °C [N/mm2] | 116.40 ± 10.97 | 84.86 ± 11.06 | 49.78 ± 6.69 | 45.44 ± 6.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbyrad, B.; Zaborniak, M.; Kochmański, Ł.; Jasik, K.; Kluczyński, J.; Budzik, G.; Turek, P. Evaluation of High-Temperature Sterilization Processes: Their Influence on the Mechanical Integrity of Additively Manufactured Polymeric Biomaterials. Materials 2025, 18, 1356. https://doi.org/10.3390/ma18061356
Zbyrad B, Zaborniak M, Kochmański Ł, Jasik K, Kluczyński J, Budzik G, Turek P. Evaluation of High-Temperature Sterilization Processes: Their Influence on the Mechanical Integrity of Additively Manufactured Polymeric Biomaterials. Materials. 2025; 18(6):1356. https://doi.org/10.3390/ma18061356
Chicago/Turabian StyleZbyrad, Barbara, Małgorzata Zaborniak, Łukasz Kochmański, Katarzyna Jasik, Janusz Kluczyński, Grzegorz Budzik, and Paweł Turek. 2025. "Evaluation of High-Temperature Sterilization Processes: Their Influence on the Mechanical Integrity of Additively Manufactured Polymeric Biomaterials" Materials 18, no. 6: 1356. https://doi.org/10.3390/ma18061356
APA StyleZbyrad, B., Zaborniak, M., Kochmański, Ł., Jasik, K., Kluczyński, J., Budzik, G., & Turek, P. (2025). Evaluation of High-Temperature Sterilization Processes: Their Influence on the Mechanical Integrity of Additively Manufactured Polymeric Biomaterials. Materials, 18(6), 1356. https://doi.org/10.3390/ma18061356










