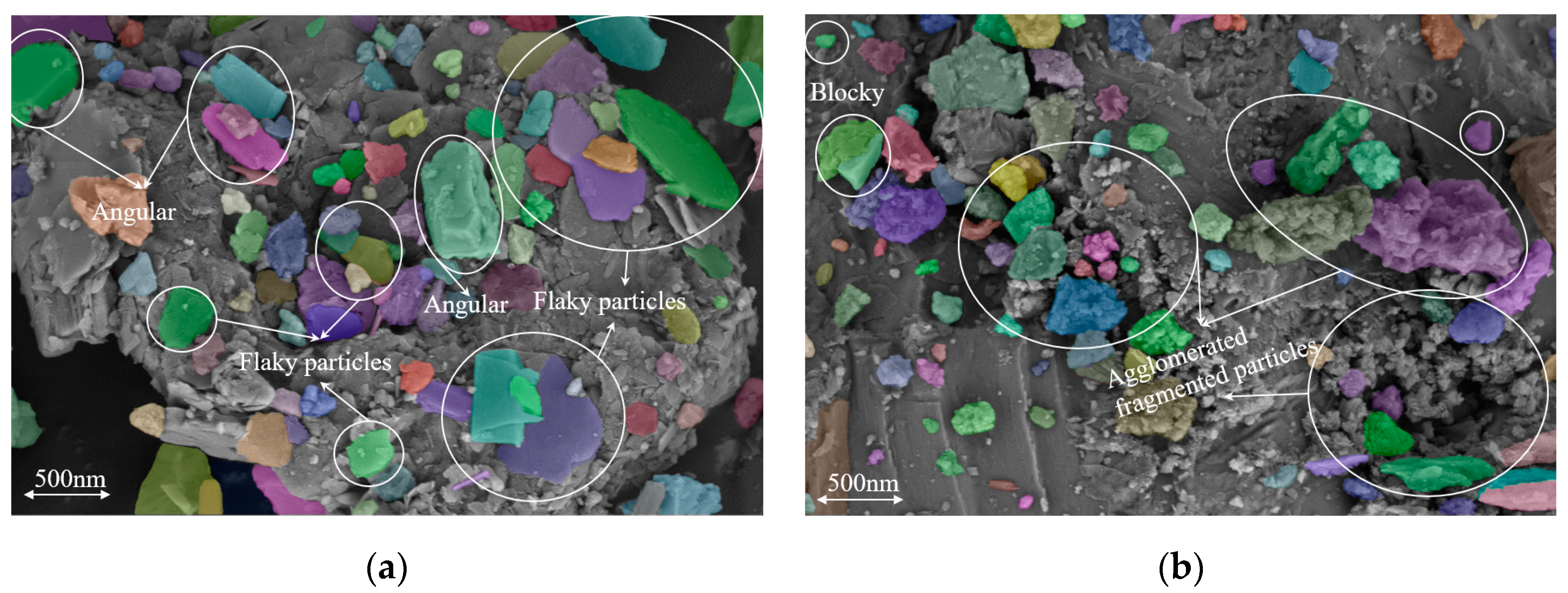
3.3. Results of FTIR
The FTIR spectra of the two asphalt mastics are shown in
Figure 13. Both the base asphalt and the two asphalt mastics show two strong absorption peaks at 2920 cm
−1 and 2850 cm
−1, corresponding to the asymmetric and symmetric stretching vibrations of -CH
2- groups in orderly arranged long-chain alkyls [
33]. The area of these peaks remains largely unchanged, suggesting that neither LP nor SSP influences the unsaturated hydrocarbons in the asphalt. Additionally, strong absorption peaks at 1456 cm
−1 and 1375 cm
−1—resulting from the scissoring vibration of methylene (-CH
2-) and the symmetric bending vibration of methyl (-CH
3-) groups—represent the relative content of free aliphatic compounds. Around 719 cm
−1, an in-plane rocking vibration absorption peak of -CH
2- groups is observed [
34], reflecting the relative content of free long-chain aliphatic compounds. Comparing the ratios of peak areas at 1455, 1375, and 719 cm
−1 to the total peak area in the 400–4000 cm
−1 range for the two asphalt mastics reveals a gradual weakening of these peaks with increasing filler content. This trend indicates a reduction in the relative content of aliphatic compounds within the asphalt mastic as the filler content increases, which results in a higher proportion of heavy components, thereby explaining the enhanced high-temperature performance of asphalt mastic with LP and SSP.
As shown in
Figure 13, the peak areas of SSP asphalt mastic at 1455 cm
−1, 1375 cm
−1, 719 cm
−1, and 872 cm
−1 are smaller than those of LP asphalt mastic. This observation indicates that SSP has adsorbed a greater amount of free aliphatic compounds, resulting in an increased relative content of heavy components in SSP asphalt mastic, which enhances its high-temperature performance. These findings align with the results of previous experiments. In summary, after mixing with asphalt, both LP and SSP fillers adsorb more polar molecules from the asphalt without disrupting the fillers’ crystalline structure or chemical bonds and without generating new functional groups. The interaction between the fillers and asphalt is limited to physical and chemical adsorption, with no evidence of a chemical reaction [
35]. Although no chemical reactions occurred between LP or SSP and the asphalt, both fillers absorbed the polar components of the asphalt, indicating that the asphalt wrapped the crystalline structures of the two fillers, leading to a reduction in the FTIR absorption peaks. Furthermore, as shown in
Figure 13, the peak intensity of SSP is lower than that of LP. This can be attributed to the fact that SSP has a specific surface area 7.06% higher than that of LP, resulting in a stronger adsorption capacity compared to LP.
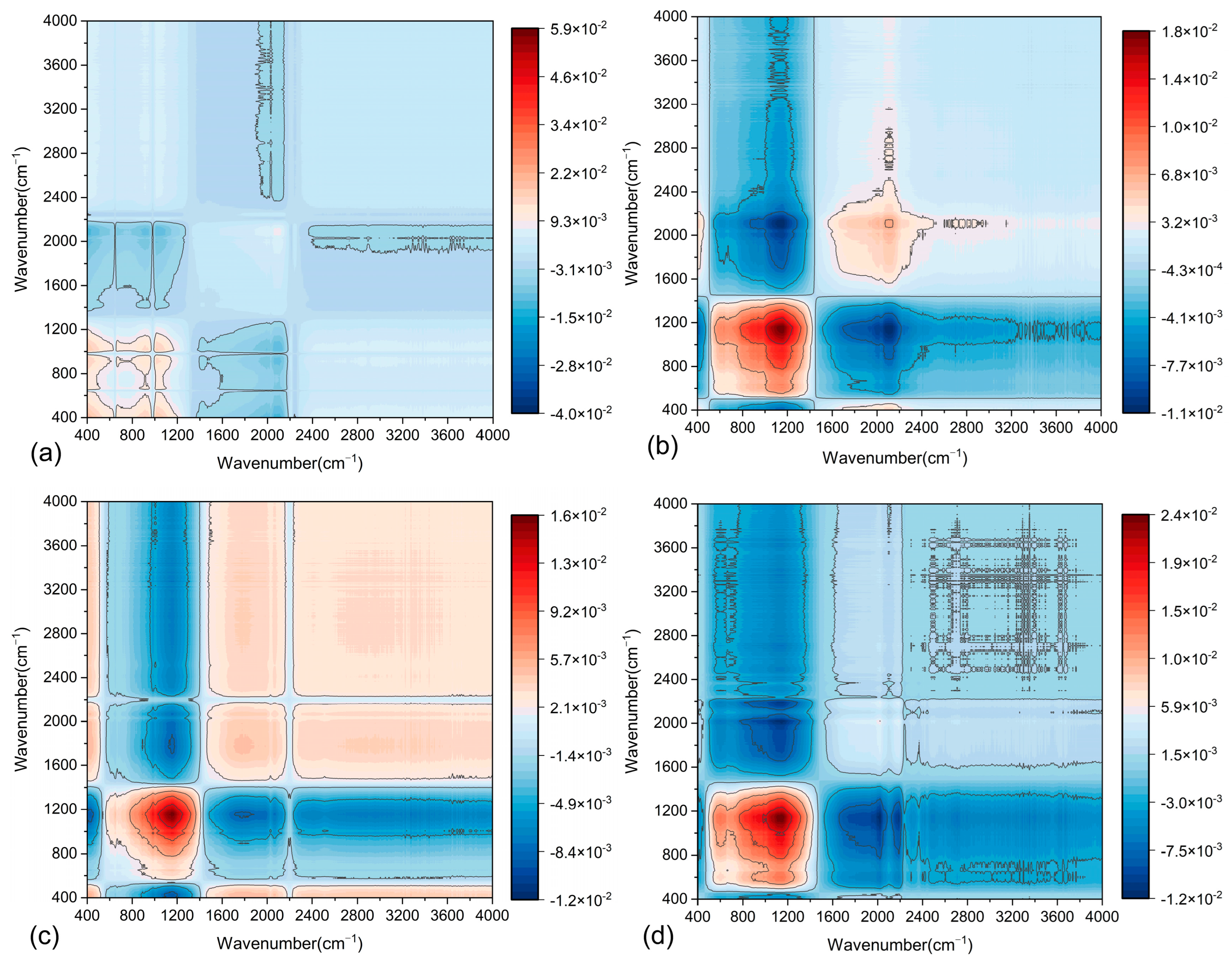
As an advanced spectroscopic technique, two-dimensional infrared correlation spectroscopy (2D-COS) surpasses conventional one-dimensional FTIR in exploring structural and interactive changes within complex polymer systems under external perturbations at the molecular level. By extending the original FTIR into a second dimension, 2D-COS enhances the spectral resolution through correlations between distinct wavenumbers, thereby providing comprehensive insights into dynamic molecular interactions. Specifically, 2D-COS accurately resolves intermolecular interactions within polymer chains, such as van der Waals forces and hydrogen bonding, by identifying synchronized or asynchronous spectral responses under controlled perturbations.
The application of 2D-COS to asphalt mastic enables the precise determination of functional group content variations and interaction heterogeneities under diverse conditions, providing critical insights into the physicochemical compatibility between asphalt and fillers. This approach effectively resolves dynamic molecular-level responses to external stimuli, thereby clarifying the evolution of interfacial interactions driven by hydrogen bonding or van der Waals forces. By using 2D-COS, the differences in functional group content and their interactions under various F/A ratios can be compared. This aids in understanding the physicochemical properties of the two asphalt mastics. In 2D-COS, the intensity of each pixel represents the interaction strength between different wavenumbers. In the synchronous spectrum, pixel intensities reflect the positive and negative correlations of vibrations associated with different chemical bonds. The asynchronous spectrum, on the other hand, reveals sequential relationships by analyzing dynamic changes occurring at different time points. As shown in
Figure 14 and
Figure 15, the synchronous 2D-COS spectra of the two asphalt mastics display auto-correlation peaks and cross-peaks along the diagonal and off-diagonal lines, respectively. These peaks indicate the positive and negative correlations in the spectral intensity variations in filler-related variables. In contrast, the asynchronous 2D-COS spectra exhibit cross-peaks along both the diagonal and off-diagonal lines. The appearance of these cross-peaks may be associated with correlations in functional group variations, which are attributed to intermolecular or intramolecular interactions.
From
Figure 14a, it is evident that in the synchronous 2D-COS spectrum of LP-modified asphalt mastics, strong positive correlation peaks appear in the 800–1200 cm
−1 range, with the peak intensity at 1093 cm
−1 being the most prominent. This indicates that the functional groups in this region respond strongly to changes in the F/A ratio. The analysis suggests that significant interactions occur between LP and asphalt, particularly in the Si-O and Al-O bond regions. In contrast, distinct negative correlation peaks are observed at 2000 cm
−1 and within the 2800–4000 cm
−1 range, indicating that the functional groups in these regions are less affected by variations in LP content. Furthermore, the peak at 2920 cm
−1 shows minimal variation, which is attributed to the antisymmetric stretching vibration absorption band of -CH
2-. This suggests that LP primarily influences unsaturated hydrocarbons.
Figure 13b shows the asynchronous 2D-COS spectrum of LMA. The results reveal sequential changes within the 800–1200 cm
−1 region, suggesting that these changes are primarily driven by the initial distribution and diffusion of the filler. Additionally, several cross-peaks are observed, with the following four peak pairs showing the highest intensities: (1096, 1485 cm
−1), (1120, 2000 cm
−1), (1120, 2115 cm
−1), and (1100, 2300 cm
−1).
Figure 15 presents the 2D-COS spectrum of SMA. Compared to LMA, the positions of the auto-correlation peaks have shifted. In the synchronous 2D-COS spectrum, all auto-correlation peaks of SMA exhibit negative correlations, with the lowest intensities observed in the 400–1400 cm
−1 range. Higher signal intensities are observed in the 1200–1600 cm
−1 and 2800–3600 cm
−1 ranges. These stronger signals are likely attributable to the effects of oxides (e.g., Fe-O, Ca-O) in the steel slag powder on the asphalt matrix. Distinct asynchronous signals are observed in the 2800–3600 cm
−1 range, which are associated with the dynamic responses of C-H bond vibrations within the asphalt matrix. This observation suggests that the steel slag powder may induce a minor molecular rearrangement process within the asphalt matrix.
Figure 14 and
Figure 15 indicate that the structure of LMA is predominantly enhanced through physical adsorption, while the SSP-modified asphalt mastic may involve trace chemical reactions (primarily physical adsorption) that lead to molecular rearrangement within the matrix. This process requires a longer duration to reach a stable state. Consequently, the 2DIR spectra of the two asphalt mastics exhibit distinct morphological characteristics. The synchronous spectrum of LMA demonstrates stronger physical interactions, with minimal dynamic changes observed in the asynchronous spectrum. In contrast, the synchronous spectrum of SMA reveals more pronounced chemical adsorption, while its asynchronous spectrum displays significant dynamic changes. This suggests a stronger molecular reorganization capability within the SMA.
In the original spectrum, numerous overlapping peaks are observed in the fingerprint region. Significant differences in the auto-correlation peaks of the spectra for the two asphalt mastics are identified within the 400–1300 cm
−1, 1200–2400 cm
−1, and 2500–3000 cm
−1 intervals. To improve the resolution and obtain more detailed spectral information, characteristic extraction was performed for these three feature bands. This approach produced clearer synchronous and asynchronous 2D-COS spectra, as illustrated in
Figure 16 and
Figure 17.
To compare the effects of LP and SSP on the functional groups of asphalt mastics at the same F/A ratios, the synchronous 2D-COS spectra of the two fillers under four F/A ratios (0.6, 0.8, 1.0, and 1.2) were analyzed, as shown in
Figure 18. The results reveal that as the F/A ratio increases, both the positions and intensities of the auto-peaks undergo significant changes. Specifically, the number of positive auto-peaks initially increases and then decreases, while the intensity of positive auto-peaks within the 400–1300 cm
−1 range gradually strengthens. This indicates that the influence of LP and SSP on functional groups becomes more pronounced as the F/A ratio increases.
From
Figure 18a–d, changes in the color intensity illustrate significant variations in specific chemical bonds or molecular groups within the samples. These variations are particularly evident in the 800–1200 cm
−1 range (associated with mineral components) and the 2800–3600 cm
−1 range (corresponding to the stretching vibrations of C-H bonds in the asphalt matrix). Additionally, the gradual enhancement in the intensity of positive auto-correlation peaks suggests that the addition of LP or SSP promotes the strengthening of specific intermolecular interactions. This may be related to particle-filling effects and their reinforcement of the asphalt matrix structure.
In the 800–1200 cm
−1 range, the increase in peak intensity suggests that the Si-O and Al-O bonds in the mineral components of LP or SSP enhance the interactions between the particles and the asphalt matrix, leading to a significant filling effect. As the F/A ratio increases (see
Figure 18d), the intensity of red peaks in this region becomes more pronounced, reflecting a higher proportion of particles relative to the matrix. In the 2800–3600 cm
−1 range—which corresponds to the stretching vibrations of C-H bonds—the band may be influenced by interactions between the asphalt components and the surface substances of LP or SSP. Although the wavenumber changes remain relatively stable, the intensity increases at higher F/A ratios (F/A = 1.0 and F/A = 1.2). This suggests that the physical adsorption of the fillers may influence the saturated hydrocarbons and aromatic hydrocarbons within the asphalt matrix.
At low F/A ratios (0.6 and 0.8), the spectral signals are relatively dispersed, indicating weak interactions between LP or SSP and the matrix in the modified asphalt mastic. As the F/A ratio increased, the spectral signals became more concentrated with significantly enhanced intensity, indicating that the incorporation of filler markedly altered the intermolecular interaction patterns within the mastic, potentially inducing the formation of a more stable structure.
In both asphalt mastics, the positive auto-correlation peaks in the synchronous spectra exhibited enhanced intensity with increasing F/A ratio, indicating a denser microstructure and strengthened interactions between the filler and asphalt, which concomitantly promoted the development of a three-dimensional molecular network. Concurrently, the asynchronous spectra demonstrated a reduced variation in peak distribution, suggesting a progressive stabilization of structural homogeneity within the asphalt mastic at higher F/A ratios.
To further evaluate the potential of SSP as a novel filler, this study systematically summarizes the comprehensive performance of various new fillers based on recent research findings from other scholars, as shown in
Table 8. By comparing the advantages and disadvantages of SSP, LP, fly ash (FA), and direct coal liquefaction residue (DCLR) in terms of high-temperature performance, adhesion capability, temperature sensitivity, environmental impact, and economic cost of the asphalt mastic, it was found that each type of filler possesses unique performance characteristics. For instance, LP exhibits the lowest cost, while FA and DCLR significantly enhance the high-temperature performance of asphalt mastic. However, when considering all evaluation metrics comprehensively, SSP not only effectively improves the performance of asphalt mastic but also demonstrates lower economic costs and excellent environmental sustainability, thereby enhancing the performance of asphalt mastic while reducing its environmental impact.
In asphalt mastic, although SSP demonstrates superior performance and better process compatibility compared to LP or other fillers, its regional supply limitations may hinder large-scale applications. To address this issue, future research plans will focus on exploring alternative supply strategies or developing hybrid filler systems to mitigate supply constraints and further optimize its application potential.