PVP as an Oxygen Vacancy-Inducing Agent in the Development of Black 45S5 Bioactive Glass Fibrous Scaffolds Doped with Zn and Mg Using A-HSBS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterizations
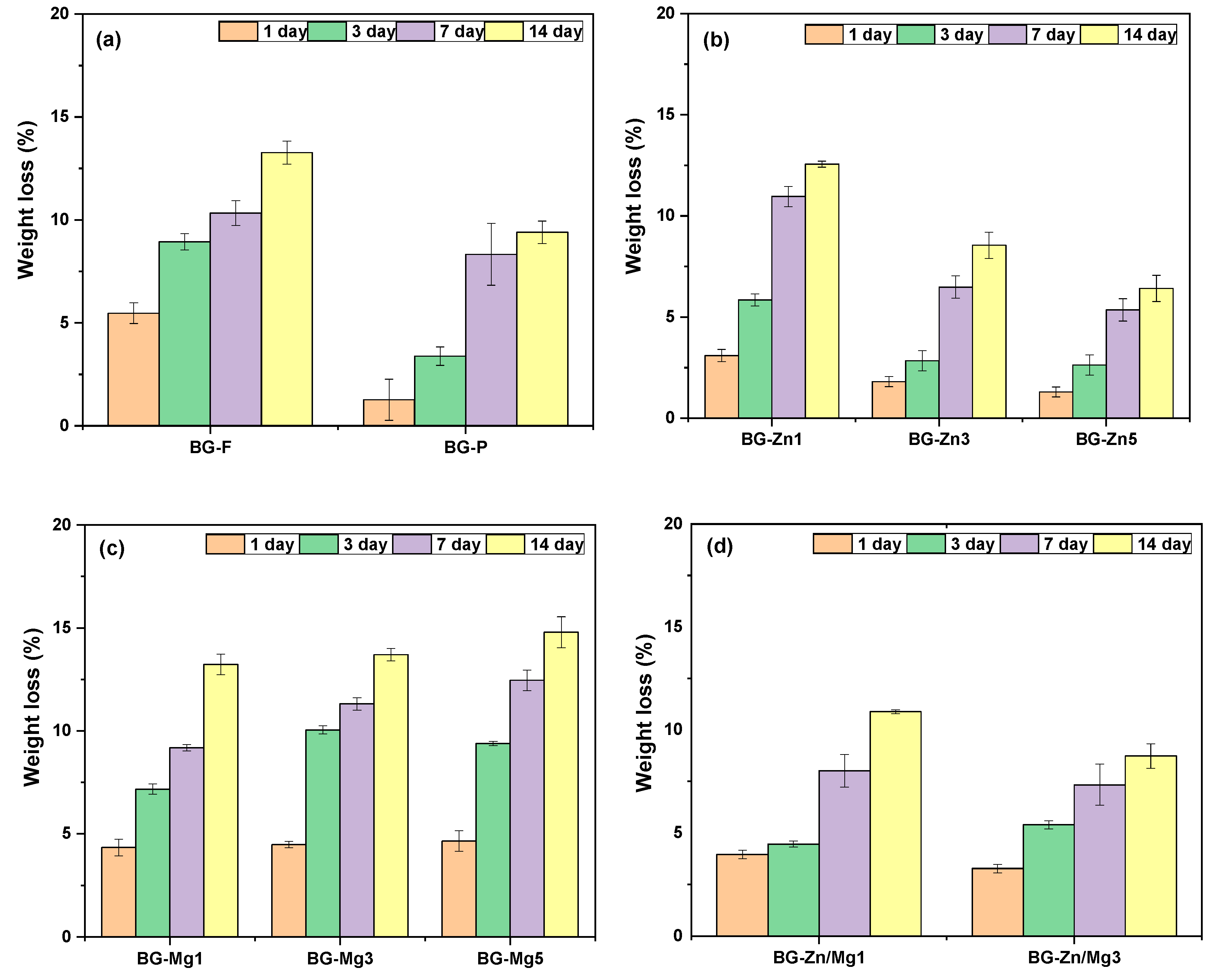
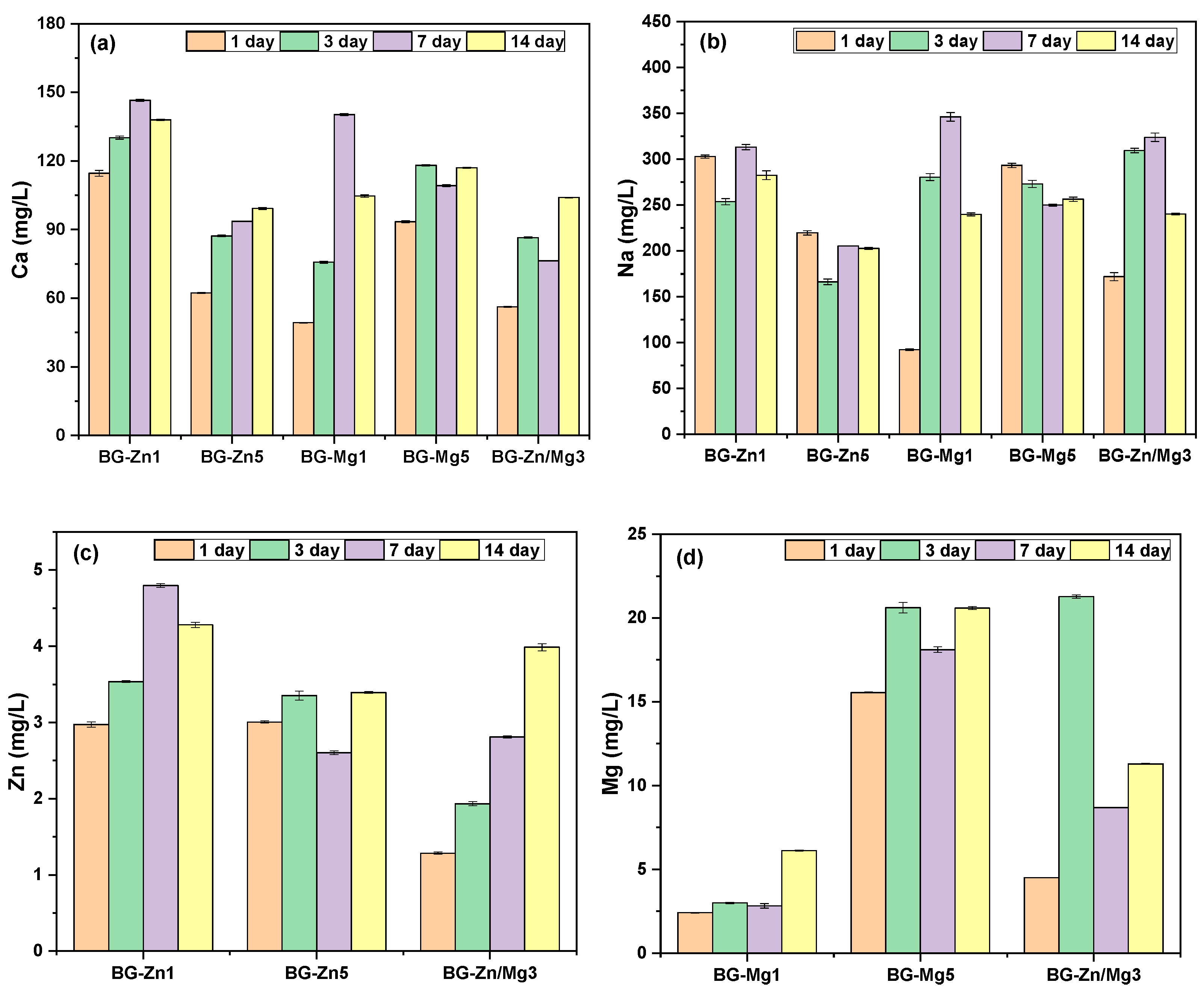
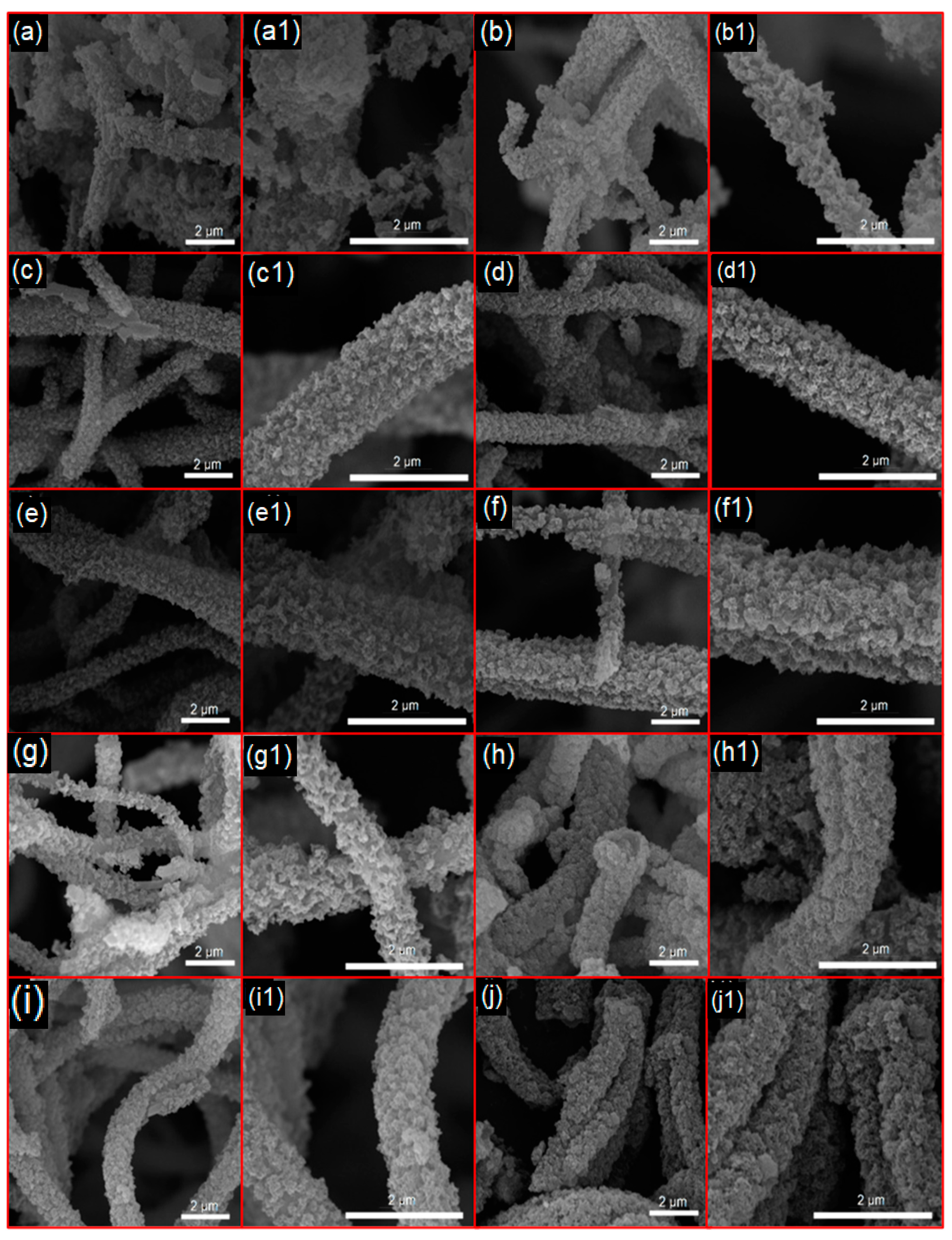
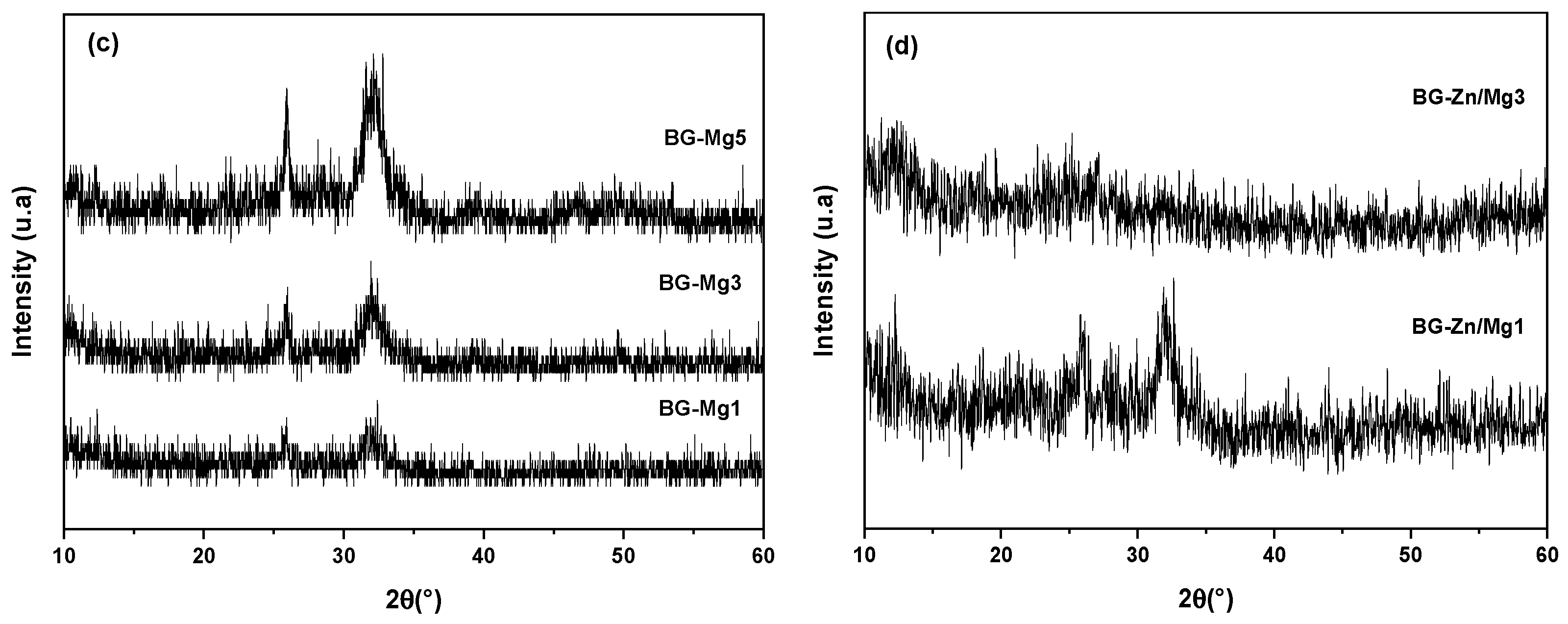
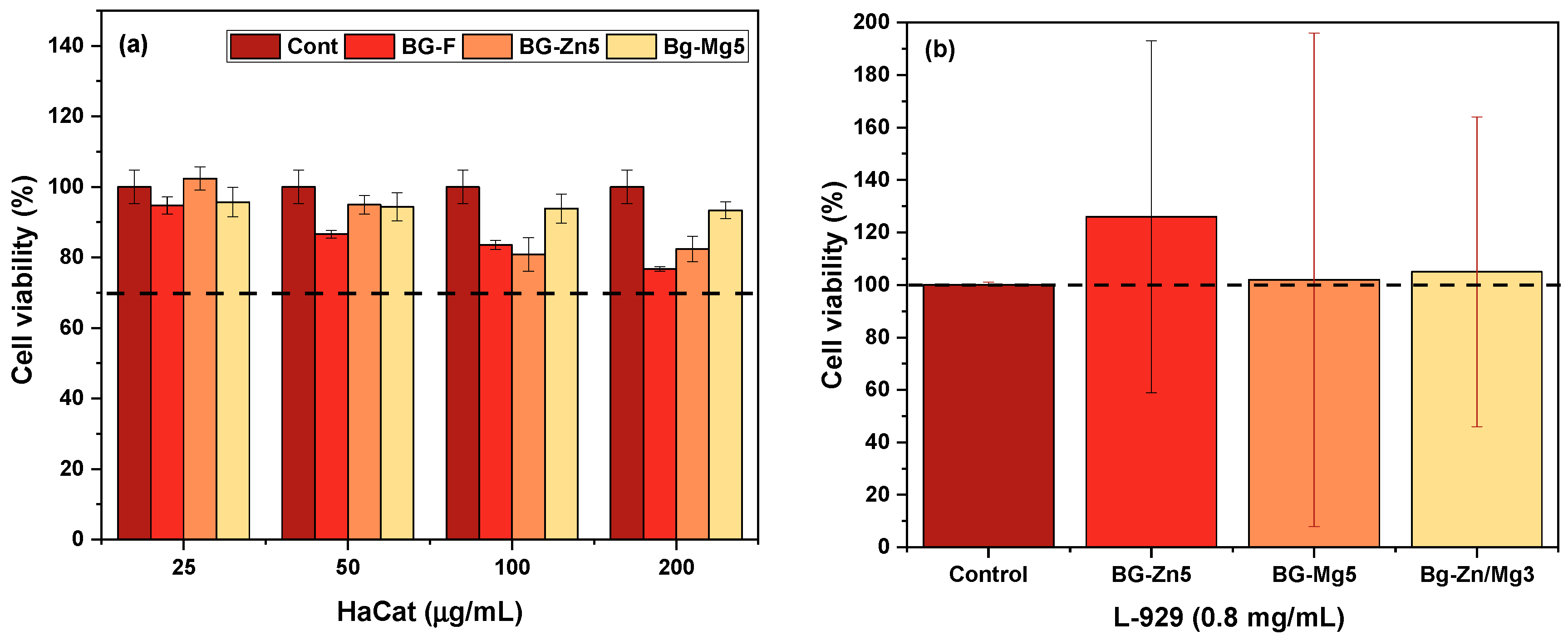
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Khater, G.A.; Safwat, E.M.; Farag, M.M.; Fathy, I.A.; Awad, H.M.; Abd-Elsatar, A.G.; Khater, A.G.A. Bioactive Glasses Based on Sodium Disilicate and Tetracalcium Phosphate Compositions for Bone Grafting Applications. Silicon 2025, 1–12. [Google Scholar] [CrossRef]
- Özel, C.; Çevlik, C.B.; Özarslan, A.C.; Emir, C.; Elalmis, Y.B.; Yücel, S. Evaluation of biocomposite putty with strontium and zinc co-doped 45S5 bioactive glass and sodium hyaluronate. Int. J. Biol. Macromol. 2023, 242, 124901. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, N.; Dini, G.; Poursamar, S.A.; Asadollahi, M.A. Sol-gel derived mesoporous 45S5 bioactive glass containing Mg and Zr ions: Synthesis, characterization, and in vitro biological investigation. Arab. J. Chem. 2024, 17, 105374. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Hammami, I.; Jakka, S.K.; Teixeira, S.S.; Silva, J.C.; Borges, J.P.; Graça, M.P.F. Influence of the Addition of Zinc, Strontium, or Magnesium Oxides to the Bioglass 45S5 Network on Electrical Behavior. Materials 2024, 17, 499. [Google Scholar] [CrossRef]
- Fellenberg, J.; Losch, S.; Arango-Ospina, M.; Hildenbrand, N.; Tripel, E.; Deng, L.; Renkawitz, T.; Westhauser, F.; Lehner, B.; Boccaccini, A.R. Targeting Bone Tumours with 45S5 Bioactive Glass. Int. J. Mol. Sci. 2024, 25, 10830. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Zhu, Y.; Gelinsky, M.; Chang, J.; Cuniberti, G.; Albrecht, V.; Friis, T.; Xiao, Y. Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater. 2011, 7, 3563–3572. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-containing bioactive glasses and glass-ceramics: From tissue regeneration to cancer therapeutic strategies. Mater. Sci. Eng. C 2021, 121, 111741. [Google Scholar] [CrossRef]
- Kermani, F.; Vojdani-Saghir, A.; Beidokhti, S.M.; Nazarnezhad, S.; Mollaei, Z.; Hamzehlou, S.; El-Fiqi, A.; Baino, F.; Kargozar, S. Iron (Fe)-doped mesoporous 45S5 bioactive glasses: Implications for cancer therapy. Transl. Oncol. 2022, 20, 101397. [Google Scholar] [CrossRef]
- Souza, L.; Ferreira, F.V.; Lopes, J.H.; Camilli, J.A.; Martin, R.A. Cancer inhibition and in vivo osteointegration and compatibility of gallium-doped bioactive glasses for osteosarcoma applications. ACS Appl. Mater. Interfaces 2022, 14, 45156–45166. [Google Scholar] [CrossRef]
- Moeini, A.; Hassanzadeh Chinijani, T.; Malek Khachatourian, A.; Vinicius Lia Fook, M.; Baino, F.; Montazerian, M. A critical review of bioactive glasses and glass–ceramics in cancer therapy. Int. J. Appl. Glass Sci. 2023, 14, 69–87. [Google Scholar] [CrossRef]
- Miola, M.; Pakzad, Y.; Banijamali, S.; Kargozar, S.; Vitale-Brovarone, C.; Yazdanpanah, A.; Bretcanu, O.; Ramedani, A.; Vernè, E.; Mozafari, M. Glass-ceramics for cancer treatment: So close, or yet so far? Acta Biomater. 2019, 83, 55–70. [Google Scholar] [CrossRef]
- Wang, L.; Long, N.J.; Li, L.; Lu, Y.; Li, M.; Cao, J.; Zhang, Y.; Zhang, Q.; Xu, S.; Yang, Z.; et al. Multi-functional bismuth-doped bioglasses: Combining bioactivity and photothermal response for bone tumor treatment and tissue repair. Light Sci. Appl. 2018, 7, 1. [Google Scholar] [CrossRef]
- Dang, W.; Li, T.; Li, B.; Ma, H.; Zhai, D.; Wang, X.; Chang, J.; Xiao, Y.; Wang, J.; Wu, C. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials 2018, 160, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dai, X.; Feng, W.; Chen, Y. Biomedical applications of MXenes: From nanomedicine to biomaterials. Acc. Mater. Res. 2022, 3, 785–798. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, C.; Zhai, D.; Luo, Y.; Chen, Y.; Lv, F.; Yi, Z.; Deng, Y.; Wang, J.; Chang, J.; et al. A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv. Funct. Mater. 2016, 26, 1197–1208. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses (MBGs) in cancer therapy: Full of hope and promise. Mater. Lett. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Ma, B.; Wu, J.; Chang, J.; Gelinsky, M.; Wu, C. Black bioceramics: Combining regeneration with therapy. Adv. Mater. 2020, 32, 2005140. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Yang, X.; Xie, Z. Fabrication of black-colored CuO–Al2O3–ZrO2 ceramics via heterogeneous nucleation method. Ceram. Int. 2012, 38, 2851–2856. [Google Scholar] [CrossRef]
- Wang, X.; Tang, M. Bioceramic materials with ion-mediated multifunctionality for wound healing. Smart Med. 2022, 1, e20220032. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-black-phosphorus-reinforced 3D-printed scaffolds: A stepwise countermeasure for osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Niu, W.; Chen, M.; Guo, Y.; Wang, M.; Luo, M.; Cheng, W.; Wang, Y.; Lei, B. A multifunctional bioactive glass-ceramic nanodrug for post-surgical infection/cancer therapy-tissue regeneration. ACS Nano 2021, 15, 14323–14337. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Xue, J.; Wu, J.; Chang, J.; Wu, C. Defective black nano-titania thermogels for cutaneous tumor-induced therapy and healing. Nano Lett. 2019, 19, 2138–2147. [Google Scholar] [CrossRef]
- de Paula Neves, D.; dos Santos Santinoni, C.; Mori, G.G. Materiais Sintéticos e Impressão 3D na Regeneração Óssea Alveolar. Arch. Health Investig. 2022, 11, 304–317. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun biomimetic nanofibrous scaffolds: A promising prospect for bone tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef]
- Flores-Jacobo, A.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Effect of dopants on the physical, mechanical, and biological properties of porous scaffolds for bone tissue engineering. Biomed. Mater. Devices 2023, 1, 234–255. [Google Scholar] [CrossRef]
- Araújo, M.E.B.; Farias, R.M.C.; Araujo, R.N.; Maciel, P.P.; Bonan, P.R.F.; Barboza, C.A.G.; Melo, J.C.; Menezes, R.R.; Neves, G.A. Cu-doped 70S bioactive glass fibrous membranes produced using solution blow spinning (SBS). Ceram. Int. 2024, 50, 41257–41267. [Google Scholar] [CrossRef]
- Silva, V.C.; Farias, R.M.C.; Bonan, R.F.; Cartaxo, J.M.; Medeiros, E.S.; Figueiredo, L.R.F.; Neves, G.A.; Menezes, R.R. Novel synthesis of BCP cotton-wool-like nanofibrous scaffolds by air-heated solution blow spinning (A-HSBS) technique. Ceram. Int. 2023, 49, 24084–24092. [Google Scholar] [CrossRef]
- Barros, L.N.L.C.; Silva, V.C.; de Araujo, R.N.; Silva, D.B.; Neves, G.d.A.; Menezes, R.R. Production of 3D fibrous structure of ICIE16 bioactive glass by air-heated solution blow spinning (A-HSBS). Mater. Lett. 2024, 365, 136440. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Preparation and in vitro characterization of electrospun 45S5 bioactive glass nanofibers. Ceram. Int. 2015, 41, 417–425. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Investigation of in vitro mineralization of silicate-based 45S5 and 13-93 bioactive glasses in artificial saliva for dental applications. Ceram. Int. 2017, 43, 3531–3539. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Balakumar, S. Phase separation induced shell thickness variations in electrospun hollow Bioglass 45S5 fiber mats for drug delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 15316–15323. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Milan, P.B.; Amoupour, M.; Kermani, F.; Gorgani, S.; Nazarnezhad, S.; Hooshmand, S.; Baino, F. Osteogenic potential of magnesium (Mg)-doped multicomponent bioactive glass: In vitro and in vivo animal studies. Materials 2022, 15, 318. [Google Scholar] [CrossRef]
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv. Sci. 2019, 6, 1900209. [Google Scholar] [CrossRef] [PubMed]
- Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Pereira, R.M.; Rodrigues, K.F.; Ribas, R.G.; da Silva, D.M.; Thim, G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021, 47, 2999–3012. [Google Scholar] [CrossRef]
- Liu, W.; Guo, S.; Tang, Z.; Wei, X.; Gao, P.; Wang, N.; Li, X.; Guo, Z. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 2020, 528, 664–670. [Google Scholar] [CrossRef]
- Hohenbild, F.; Arango Ospina, M.; Schmitz, S.I.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. An in vitro evaluation of the biological and osteogenic properties of magnesium-doped bioactive glasses for application in bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 12703. [Google Scholar] [CrossRef]
- Wetzel, R.; Blochberger, M.; Scheffler, F.; Hupa, L.; Brauer, D.S. Mg or Zn for Ca substitution improves the sintering of bioglass 45S5. Sci. Rep. 2020, 10, 15964. [Google Scholar] [CrossRef]
- Wetzel, R.; Bartzok, O.; Brauer, D.S. Influence of low amounts of zinc or magnesium substitution on ion release and apatite formation of Bioglass 45S5. J. Mater. Sci. Mater. Med. 2020, 31, 86. [Google Scholar] [CrossRef]
- Karimi, M.; Asadi-Eydivand, M.; Abolfathi, N.; Chehrehsaz, Y.; Solati-Hashjin, M. The effect of pore size and layout on mechanical and biological properties of 3D-printed bone scaffolds with gradient porosity. Polym. Compos. 2023, 44, 1343–1359. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.J.R.; Silva, J.C.; Soares, P.I.P.; Borges, J.P. Polyvinylpyrrolidone nanofibers incorporating mesoporous bioactive glass for bone tissue engineering. Biomimetics 2023, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Standard, I. Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. Int. Organ. Stand. 2009, 10, 978157020355. [Google Scholar]
- Associação Brasileira de Normas, T. ABNT NBR ISO/IEC 17025: 2005-Requisitos Gerais Para a Competência de Laboratórios de Ensaio e Calibração. General Requirements for the Competence of Testing and Calibration Laboratories, 2nd ed.; ABNT: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Medeiros, E.L.G.; Gomes, D.S.; Santos, A.M.C.; Vieira, R.H.; de Lima, I.L.; Rocha, F.S.; Castro-Filice, L.d.S.; Medeiros, E.S.; Neves, G.A.; Menezes, R.R. 3D nanofibrous bioactive glass scaffolds produced by one-step spinning process. Ceram. Int. 2021, 47, 102–110. [Google Scholar] [CrossRef]
- Bhol, P.; Mohanty, M.; Mohanty, P.S. Polymer-matrix stabilized metal nanoparticles: Synthesis, characterizations and insight into molecular interactions between metal ions, atoms and polymer moieties. J. Mol. Liq. 2021, 325, 115135. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Immobilized materials for removal of toxic metal ions from surface/groundwaters and aqueous waste streams. Environ. Sci. Process. Impacts 2016, 18, 429–444. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge-and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Alibe, I.M.; Matori, K.A.; Sidek, H.A.A.; Yaakob, Y.; Rashid, U.; Alibe, A.M.; Zaid, M.H.M.; Nasir, S.; Nasir, M.M. Effects of polyvinylpyrrolidone on structural and optical properties of willemite semiconductor nanoparticles by polymer thermal treatment method. J. Therm. Anal. Calorim. 2019, 136, 2249–2268. [Google Scholar] [CrossRef]
- Qu, J.; Liu, W.; Liu, R.; He, J.; Liu, D.; Feng, Z.; Feng, Z.; Li, R.; Li, C. Evolution of oxygen vacancies in cerium dioxide at atomic scale under CO2 reduction. Chem Catal. 2023, 3, 100759. [Google Scholar] [CrossRef]
- Li, G.; Blake, G.R.; Palstra, T.T.M. Vacancies in functional materials for clean energy storage and harvesting: The perfect imperfection. Chem. Soc. Rev. 2017, 46, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Ji, X.; Kang, Y.; Ouyang, J.; Chen, Y.; Artzi, D.; Zeng, X.; Xiao, Y.; Feng, C.; Qi, B.; Kim, N.Y.; et al. Synthesis of ultrathin biotite nanosheets as an intelligent theranostic platform for combination cancer therapy. Adv. Sci. 2019, 6, 1901211. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Z.; Strand, J.; Munde, M.S.; Shluger, A.L. Mechanisms of oxygen vacancy aggregation in SiO2 and HfO2. Front. Phys. 2019, 7, 43. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect chemistry in heterogeneous catalysis: Recognition, understanding, and utilization. Acs Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef]
- Anjali, K.K.; Sivakumar, M. Optical absorption enhancement of PVP capped TiO2 nanostructures in the visible region. Solid State Ion. 2019, 337, 33–41. [Google Scholar] [CrossRef]
- Srilakshmi, P.; Sivakumar, M.; Kathirvel, A.; Maheswari, A.U. Influence of annealing atmosphere for controlling oxygen vacancies of PVP-capped TiO2 nanoparticles. J. Nanopart. Res. 2021, 23, 1–14. [Google Scholar] [CrossRef]
- Orellana, W.; Chacham, H. Energetics of carbon and oxygen impurities and their interaction with vacancies in cubic boron nitride. Phys. Rev. B 2000, 62, 10135. [Google Scholar] [CrossRef]
- Londos, C.A.; Sgourou, E.N.; Chroneos, A. Oxygen-vacancy defects in electron-irradiated Si: The role of carbon in their behavior. J. Mater. Sci. Mater. Electron. 2014, 25, 914–921. [Google Scholar] [CrossRef]
- Ni, D.; Zhang, J.; Wang, J.; Hu, P.; Jin, Y.; Tang, Z.; Yao, Z.; Bu, W.; Shi, J. Oxygen vacancy enables markedly enhanced magnetic resonance imaging-guided photothermal therapy of a Gd3+-doped contrast agent. ACS Nano 2017, 11, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Hu, Y.; Wang, X.; Liu, G.; Wang, Z.; Liu, Z.; Tian, Q.; Zhu, M.; Shi, X.; Chen, Z. Dynamically tuning near-infrared-induced photothermal performances of TiO2 nanocrystals by Nb doping for imaging-guided photothermal therapy of tumors. Nanoscale 2017, 9, 9148–9159. [Google Scholar] [CrossRef]
- Yu, N.; Peng, C.; Wang, Z.; Liu, Z.; Zhu, B.; Yi, Z.; Zhu, M.; Liu, X.; Chen, Z. Dopant-dependent crystallization and photothermal effect of Sb-doped SnO2 nanoparticles as stable theranostic nanoagents for tumor ablation. Nanoscale 2018, 10, 2542–2554. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Raucci, M.G.; Zheng, K.; Boccaccini, A.R.; Ambrosio, L. Oxygen-Deficient Bioceramics: Combination of Diagnosis, Therapy, and Regeneration. Adv. Mater. 2023, 35, 2302858. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Huang, T.; Sun, C.; Chen, W.; Zou, H.; He, X.; Shen, J.; Xiao, Y. Oxygen vacancy-rich nickel oxide nanoplatforms for enhanced photothermal and chemodynamic therapy combat methicillin-resistant Staphylococcus aureus. Acta Biomater. 2024, 182, 275–287. [Google Scholar] [CrossRef]
- Fang, Q.; Hong, C.; Liu, Z.; Pan, Y.; Lin, J.; Zheng, J.; Zhang, J.; Chen, T.; Ma, X.; Wu, A. Oxygen Vacancy Defect Enhanced NIR-II Photothermal Performance of BiOxCl Nanosheets for Combined Phototherapy of Cancer Guided by Multimodal Imaging. Adv. Healthc. Mater. 2024, 13, 2303200. [Google Scholar] [CrossRef] [PubMed]
- Moghanian, A.; Ghorbanoghli, A.; Kazem-Rostami, M.; Pazhouheshgar, A.; Salari, E.; Saghafi Yazdi, M.; Alimardani, T.; Jahani, H.; Sharifian Jazi, F.; Tahriri, M. Novel antibacterial Cu/Mg-substituted 58S-bioglass: Synthesis, characterization and investigation of in vitro bioactivity. Int. J. Appl. Glass Sci. 2020, 11, 685–698. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Variation of the properties of sol–gel synthesized bioactive glass 45S5 in organic and inorganic acid catalysts. Mater. Adv. 2021, 2, 413–425. [Google Scholar] [CrossRef]
- Peniche, C.; Zaldívar, D.; Pazos, M.; Páz, S.; Bulay, A.; Román, J.S. Study of the thermal degradation of poly (N-vinyl-2-pyrrolidone) by thermogravimetry–FTIR. J. Appl. Polym. Sci. 1993, 50, 485–493. [Google Scholar] [CrossRef]
- Xu, L.; Che, L.; Zheng, J.; Huang, G.; Wu, X.; Chen, P.; Zhang, L.; Hu, Q. Synthesis and thermal degradation property study of N-vinylpyrrolidone and acrylamide copolymer. RSC Adv. 2014, 4, 33269–33278. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison between bioactive sol-gel and melt-derived glasses/glass-ceramics based on the multicomponent SiO2–P2O5–CaO–MgO–Na2O–K2O system. Materials 2020, 13, 540. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardi, M.; Bianco, A.; Ravaglioli, A.; Montanaro, L. Sol–gel derived 45S5 bioglass: Synthesis, microstructural evolution and thermal behaviour. J. Mater. Sci. Mater. Med. 2012, 23, 1849–1866. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkali-mediated sol–gel method. Mater. Lett. 2007, 61, 3251–3253. [Google Scholar] [CrossRef]
- Ramsheh, M.R.; Behnamghader, A.; Khanlarkhani, A. Sol-gel synthesis, in vitro behavior, and human bone marrow-derived mesenchymal stem cell differentiation and proliferation of bioactive glass 58S. Iran. Biomed. J. 2021, 25, 180. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal decomposition of metal nitrates in air and hydrogen environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Stoia, M.; Barvinschi, P.; Barbu-Tudoran, L. Thermal decomposition of metal nitrates: PVA–TEOS gels for obtaining M (II) ferrite/silica nanocomposites. J. Therm. Anal. Calorim. 2013, 113, 21–30. [Google Scholar] [CrossRef]
- Xiong, Y.; Washio, I.; Chen, J.; Cai, H.; Li, Z.-Y.; Xia, Y. Poly(vinyl pyrrolidone): A dual functional reductant and stabilizer for the facile synthesis of noble metal nanoplates in aqueous solutions. Langmuir 2006, 22, 8563–8570. [Google Scholar] [CrossRef]
- Pirayesh, H.; Nychka, J.A. Sol–gel synthesis of bioactive glass-ceramic 45S5 and its in vitro dissolution and mineralization behavior. J. Am. Ceram. Soc. 2013, 96, 1643–1650. [Google Scholar] [CrossRef]
- Elakkiya, K.; Raja, C.A.; Balakumar, S. Devitrite (Na2Ca3Si6O16) phase dominated nanostructured 45S5 bioactive glass: Exploring its structural and biological properties. Biomed. Mater. 2024, 19, 025039. [Google Scholar] [CrossRef]
- Thomas, A.; Bera, J. Crystallization and sintering behavior of glass-ceramic powder synthesized by sol-gel process. J. Aust. Ceram. Soc. Vol. 2016, 52, 87–91. [Google Scholar]
- Dave, H.K.; Tank, Y.N.; Prajapati, A.R. Synthesis and fabrication of bioactive glass for bone implant using 3D printing setup. Prog. Addit. Manuf. 2023, 9, 1825–1834. [Google Scholar] [CrossRef]
- Nawaz, Q.; de Pablos-Martín, A.; Martins de Souza e Silva, J.; Hurle, K.; Jaimes, A.T.C.; Brauer, D.S.; Boccaccini, A.R. New insights into the crystallization process of sol-gel–derived 45S5 bioactive glass. J. Am. Ceram. Soc. 2020, 103, 4234–4247. [Google Scholar] [CrossRef]
- Adhikari, J.; Dasgupta, S.; Barui, A.; Ghosh, M.; Saha, P. Functionalized mesoporous SiO2-CaO-Na2O-P2O5 based nanometric glass-ceramic particles with enhanced dispersibility and bioactivity. J. Sol-Gel Sci. Technol. 2023, 106, 757–774. [Google Scholar] [CrossRef]
- Novak, S.; Orives, J.R.; Nalin, M.; Unalan, I.; Boccaccini, A.R.; de Camargo, E.R. Quaternary bioactive glass-derived powders presenting submicrometric particles and antimicrobial activity. Ceram. Int. 2022, 48, 29982–29990. [Google Scholar] [CrossRef]
- Veláquez-González, C.S.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Effect of Ta2O5 content on the microstructural properties of 45S5 bioglass glass-ceramic scaffolds. Boletín de la Sociedad Española de Cerámica y Vidrio 2024, 63, 304–315. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.Z.; Rezabeigi, E.; Drew, R.A.L. Crystallization behavior of combeite in 45S5 Bioglass® via controlled heat treatment. J. Non-Cryst. Solids 2018, 502, 176–183. [Google Scholar] [CrossRef]
- Montazerian, M.; Shearer, A.; Mauro, J.C. Perspectives on the impact of crystallization in bioactive glasses and glass-ceramics. Int. J. Ceram. Eng. Sci. 2024, 6, e10194. [Google Scholar] [CrossRef]
- Topalović, V.S.; Grujić, S.R.; Živanović, V.D.; Matijašević, S.D.; Nikolić, J.D.; Stojanović, J.N.; Smiljanić, S.V. Bioactive glass-ceramics prepared by powder sintering and crystallization of polyphosphate glass containing strontium. Ceram. Int. 2017, 43, 12061–12069. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Graça, M.P.F.; Prezas, P.R.; Kumar, J.S.; Melo, B.M.G.; Sales, A.J.M.; Almeida, A.F.; Valente, M.A. Structural, thermal, morphological and dielectric investigations on 45S5 glass and glass-ceramics. J. Non-Cryst. Solids 2021, 562, 120780. [Google Scholar] [CrossRef]
- Zarifah, N.A.; Lim, W.F.; Matori, K.A.; Sidek, H.A.A.; Wahab, Z.A.; Zainuddin, N.; Salleh, M.A.; Fadilah, B.N.; Fauzana, A.N. An elucidating study on physical and structural properties of 45S5 glass at different sintering temperatures. J. Non-Cryst. Solids 2015, 412, 24–29. [Google Scholar] [CrossRef]
- Xin, R.; Zhang, Q.; Gao, J. Identification of the wollastonite phase in sintered 45S5 bioglass and its effect on in vitro bioactivity. J. Non-Cryst. Solids 2010, 356, 1180–1184. [Google Scholar] [CrossRef]
- Fagerlund, S.; Massera, J.; Hupa, M.; Hupa, L. T–T–T behaviour of bioactive glasses 1–98 and 13–93. J. Eur. Ceram. Soc. 2012, 32, 2731–2738. [Google Scholar] [CrossRef]
- Spirandeli, B.R.; Campos, T.M.B.; Ribas, R.G.; Thim, G.P.; de Sousa Trichês, E. Evaluation of colloidal and polymeric routes in sol-gel synthesis of a bioactive glass-ceramic derived from 45S5 bioglass. Ceram. Int. 2020, 46, 20264–20271. [Google Scholar] [CrossRef]
- Sugumaran, V.; Subramanian, B. Influence of Ageing Time on Crystallization Kinetics and Hydroxyapatite Mimicking Silicorhenanite (β-Na2Ca4(PO4 2SiO4) Phase Evolution in 45S5® Bioactive Glass for Advanced Biomedical Applications. Mater. Chem. Phys. 2024, 327, 129893. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Sola, A. An overview of the effects of thermal processing on bioactive glasses. Sci. Sinter. 2010, 42, 307–320. [Google Scholar] [CrossRef]
- Ranga, N.; Gahlyan, S.; Duhan, S. Antibacterial efficiency of Zn, Mg and Sr doped bioactive glass for bone tissue engineering. J. Nanosci. Nanotechnol. 2020, 20, 2465–2472. [Google Scholar] [CrossRef]
- Wetzel, R.; Bartzok, O.; Hupa, L.; Brauer, D.S. Low Mg or Zn substitution for improved thermal properties of Bioglass 45S5. Mater. Lett. 2019, 256, 126599. [Google Scholar] [CrossRef]
- Norouzi, A.; Banijamali, S.; Keshavarzi, A. Sinter-crystallization, phase development and microstructural evaluations of ZnO containing 45S5® glass-ceramics. Mater. Today Proc. 2018, 5, 15696–15701. [Google Scholar] [CrossRef]
- Verné, E.; Bretcanu, O.; Balagna, C.; Bianchi, C.L.; Cannas, M.; Gatti, S.; Vitale-Brovarone, C. Early stage reactivity and in vitro behavior of silica-based bioactive glasses and glass-ceramics. J. Mater. Sci. Mater. Med. 2009, 20, 75–87. [Google Scholar] [CrossRef]
- Lefebvre, L.; Chevalier, J.; Gremillard, L.; Zenati, R.; Thollet, G.; Bernache-Assolant, D.; Govin, A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007, 55, 3305–3313. [Google Scholar] [CrossRef]
- Santos, K.W.; Costa, K.; Gonçalves, I.S.; Alves, M.; Lauda, D.P.; Vasconcellos, L.M.R.; Campos, T.M.B.; Oliveira, I.R. Understanding the structural complexities of bioactive glass 45S5: An analysis of manufacturing methods and their outcomes in orthopedic applications. Ceram. Int. 2024, 50, 51043–51054. [Google Scholar] [CrossRef]
- de Siqueira, L.; Campos, T.M.B.; Camargo, S.E.A.; Thim, G.P.; Triches, E.S. Structural, crystallization and cytocompatibility evaluation of the 45S5 bioglass-derived glass-ceramic containing niobium. J. Non-Cryst. Solids 2021, 555, 120629. [Google Scholar] [CrossRef]
- Saberi, A.; Behnamghader, A.; Aghabarari, B.; Yousefi, A.; Majda, D.; Huerta, M.V.M.; Mozafari, M. 3D direct printing of composite bone scaffolds containing polylactic acid and spray dried mesoporous bioactive glass-ceramic microparticles. Int. J. Biol. Macromol. 2022, 207, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.; Morterra, C. Carbonate Formation on Bioactive Glasses. Langmuir 2004, 20, 6382–6388. [Google Scholar] [CrossRef] [PubMed]
- Perardi, A.; Cerrruti, M.; Morterra, C. Carbonate formation on sol-gel bioactive glass 58S and on Bioglass® 45S5. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2005; Volume 155, pp. 461–469. [Google Scholar] [CrossRef]
- Hao, C.; Zhao, Y.; Zhou, Y.; Zhou, L.; Xu, Y.; Wang, D.; Xu, D. Interactions between metal chlorides and poly(vinyl pyrrolidone) in concentrated solutionsand solid-state films. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1589–1598. [Google Scholar] [CrossRef]
- Mohamed Kamari, H.; Goodarz Naseri, M.; Saion, E.B. A novel research on behavior of zinc ferrite nanoparticles in different concentration of poly(vinyl pyrrolidone) (PVP). Metals 2014, 4, 118–129. [Google Scholar] [CrossRef]
- Dadol, G.C.; Kilic, A.; Tijing, L.D.; Lim, K.J.A.; Cabatingan, L.K.; Tan, N.P.B.; Stojanovska, E.; Polat, Y. Solution blow spinning (SBS) and SBS-spun nanofibers: Materials, methods, and applications. Mater. Today Commun. 2020, 25, 101656. [Google Scholar] [CrossRef]
- Weng, L.; Boda, S.K.; Teusink, M.J.; Shuler, F.D.; Li, X.; Xie, J. Binary doping of strontium and copper enhancing osteogenesis and angiogenesis of bioactive glass nanofibers while suppressing osteoclast activity. ACS Appl. Mater. Interfaces 2017, 9, 24484–24496. [Google Scholar] [CrossRef]
- Misra, S.K.; Watts, P.C.P.; Valappil, S.P.; Silva, S.R.P.; Roy, I.; Boccaccini, A.R. Poly(3-hydroxybutyrate)/Bioglass® composite films containing carbon nanotubes. Nanotechnology 2007, 18, 075701. [Google Scholar] [CrossRef]
- Desogus, L.; Cuccu, A.; Montinaro, S.; Orrù, R.; Cao, G.; Bellucci, D.; Sola, A.; Cannillo, V. Classical Bioglass® and innovative CaO-rich bioglass powders processed by Spark Plasma Sintering: A comparative study. J. Eur. Ceram. Soc. 2015, 35, 4277–4285. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008–02029. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Wei, Y.; Gu, Y.; Tang, N.; Liu, Z.; Li, T.; Xiao, Z.; Yu, J.; Han, L. A novel Na2Ca2Si3O9: Tb3+ glass ceramic with high luminescence efficiency and robust thermal stability for white LED lighting application. Ceram. Int. 2024, 50, 45508–45519. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sepantafar, M.; Muhamad, N.; Bakar Sulong, A. How does scaffold porosity conduct bone tissue regeneration? Adv. Eng. Mater. 2021, 23, 2100463. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Fathi, A.; bin Abd Razak, N.A.; Kadri, N.A.; Sheikhi, A.; Baino, F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021, 136, 1–36. [Google Scholar] [CrossRef]
- Ferraris, S.; Vernè, E. Surface Functionalization of Bioactive Glasses: Reactive Groups, Biomolecules and Drugs on Bioactive Surfaces for Smart and Functional Biomaterials. R. Soc. Chem. 2016, 9, 221–235. [Google Scholar] [CrossRef]
- Ma, J.; Huang, B.X.; Zhao, X.C.; Ban, C.L.; Hao, X.H.; Wang, C.Z. Substitutions of zinc in mesoporous silicate-based glasses and their physicochemical and biological properties. J. Non-Cryst. Solids 2018, 491, 98–105. [Google Scholar] [CrossRef]
- Courthéoux, L.; Lao, J.; Nedelec, J.M.; Jallot, E. Controlled bioactivity in zinc-doped sol−gel-derived binary bioactive glasses. J. Phys. Chem. C 2008, 112, 13663–13667. [Google Scholar] [CrossRef]
- Lusvardi, G.; Zaffe, D.; Menabue, L.; Bertoldi, C.; Malavasi, G.; Consolo, U. In vitro and in vivo behaviour of zinc-doped phosphosilicate glasses. Acta Biomater. 2009, 5, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Strobel, L.A.; Kneser, U.; Boccaccini, A.R. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed. Glas. 2015, 1, 51–69. [Google Scholar] [CrossRef]
- Aina, V.; Bonino, F.; Morterra, C.; Miola, M.; Bianchi, C.L.; Malavasi, G.; Marchetti, M.; Bolis, V. Influence of the Chemical Composition on Nature and Activity of the Surface Layer of Zn-Substituted Sol−Gel (Bioactive) Glasses. J. Phys. Chem. C 2011, 115, 2196–2210. [Google Scholar] [CrossRef]
- Prabhu, M.; Kavitha, K.; Manivasakan, P.; Rajendran, V.; Kulandaivelu, P. Synthesis, characterization and biological response of magnesium-substituted nanobioactive glass particles for biomedical applications. Ceram. Int. 2013, 39, 1683–1694. [Google Scholar] [CrossRef]
- Matic, T.; Daou, F.; Cochis, A.; Barac, N.; Ugrinovic, V.; Rimondini, L.; Veljovic, D. Multifunctional Sr, Mg-Doped Mesoporous Bioactive Glass Nanoparticles for Simultaneous Bone Regeneration and Drug Delivery. Int. J. Mol. Sci. 2024, 25, 8066. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chattopadhyay, N.; Kifor, O.; Butters, R.R., Jr.; Sugimoto, T.; Brown, E.M. Mouse Osteoblastic Cell Line (MC3T3-E1) Expresses Extracellular Calcium (Ca2+o)–Sensing Receptor and Its Agonists Stimulate Chemotaxis and Proliferation of MC3T3-E1 Cells. J. Bone Miner. Res. 1998, 13, 1530–1538. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Lin, Z.; Zhou, P.; Zhang, X.; Zhang, W.; Chen, Q.; Kou, D.; Ying, X.; Shen, Y.; et al. Effects of extracellular calcium on viability and osteogenic differentiation of bone marrow stromal cells in vitro. Hum. Cell 2013, 26, 114–120. [Google Scholar] [CrossRef]
- Tsitlakidis, S.; Hohenbild, F.; Saur, M.; Moghaddam, A.; Kunisch, E.; Renkawitz, T.; Gonzalo de Juan, I.; Westhauser, F. Reduced Sodium Portions Favor Osteogenic Properties and Cytocompatibility of 45S5-Based Bioactive Glass Particles. Biomimetics 2023, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Boyd, D.; Moane, S.; Bennett, M. The effect of composition on ion release from Ca–Sr–Na–Zn–Si glass bone grafts. J. Mater. Sci. Mater. Med. 2009, 20, 2207–2214. [Google Scholar] [CrossRef]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K.; Onuma, K.; Kanzaki, N.; Sogo, Y.; Ichinose, N. Zinc-releasing calcium phosphate for stimulating bone formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, A.; Uchiyama, S. Bioavailability of zinc yeast in rats: Stimulatory effect on bone calcification in vivo. J. Health Sci. 2004, 50, 75–81. [Google Scholar] [CrossRef]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2006, 17, 489–494. [Google Scholar] [CrossRef]
- Aina, V.; Perardi, A.; Bergandi, L.; Malavasi, G.; Menabue, L.; Morterra, C.; Ghigo, D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem. -Biol. Interact. 2007, 167, 207–218. [Google Scholar] [CrossRef]
- Juliano, S.A.; Serafim, L.F.; Duay, S.S.; Heredia Chavez, M.; Sharma, G.; Rooney, M.; Comert, F.; Pierce, S.; Radulescu, A.; Cotten, M.L.; et al. A potent host defense peptide triggers DNA damage and is active against multidrug-resistant gram-negative pathogens. ACS Infect. Dis. 2020, 6, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.d.; Haberbeck, L.U.; Riella, H.G.; Ribeiro, D.H.B.; Carciofi, B.A.M. Antibacterial activity of zinc oxide nanoparticles synthesized by solochemical process. Braz. J. Chem. Eng. 2019, 36, 885–893. [Google Scholar] [CrossRef]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W.; et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L. Calcium and magnesium ions are membrane-active against stationary-phase Staphylococcus aureus with high specificity. Sci. Rep. 2016, 6, 20628. [Google Scholar] [CrossRef]
- Wetzel, R.; Brauer, D.S. Apatite formation of substituted Bioglass 45S5: SBF vs. Tris. Mater. Lett. 2019, 257, 126760. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Dietzel, A. Die Kationenfeldstärken und ihre Beziehungen zu Entglasungsvorgängen, zur Verbindungsbildung und zu den Schmelzpunkten von Silicaten. Z. Für Elektrochem. Angew. Phys. Chem. 1942, 48, 9–23. [Google Scholar] [CrossRef]
- Chen, X.; Brauer, D.S.; Karpukhina, N.; Waite, R.D.; Barry, M.; McKay, I.J.; Hill, R.G. ‘Smart’acid-degradable zinc-releasing silicate glasses. Mater. Lett. 2014, 126, 278–280. [Google Scholar] [CrossRef]
- Elgayar, I.; Hill, R.; Chen, X.; Bubb, N.; Wood, D. Dielectric spectroscopy and dissolution studies of bioactive glasses. Int. J. Appl. Glass Sci. 2017, 8, 418–427. [Google Scholar] [CrossRef]
- Zhao, R.; Shi, L.; Gu, L.; Qin, X.; Song, Z.; Fan, X.; Zhao, P.; Li, C.; Zheng, H.; Li, Z.; et al. Evaluation of bioactive glass scaffolds incorporating SrO or ZnO for bone repair: In vitro bioactivity and antibacterial activity. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040910. [Google Scholar] [CrossRef]
- Du, R.L.; Chang, J.; Ni, S.Y.; Zhai, W.Y.; Wang, J.Y. Characterization and in vitro bioactivity of zinc-containing bioactive glass and glass-ceramics. J. Biomater. Appl. 2006, 20, 341–360. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Correia, R.N.; Fernandes, M.H. Effects of Si speciation on the in vitro bioactivity of glasses. Biomaterials 2002, 23, 371–379. [Google Scholar] [CrossRef]
- Abushahba, F.; Söderling, E.; Aalto-Setälä, L.; Sangder, J.; Hupa, L.; Närhi, T.O. Antibacterial properties of bioactive glass particle abraded titanium against Streptococcus mutans. Biomed. Phys. Eng. Express 2018, 4, 045002. [Google Scholar] [CrossRef]
- Elalmış, Y. Effect of Al2O3 doping on antibacterial activity of 45S5 bioactive glass. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 419–428. [Google Scholar] [CrossRef]
- Hu, S.; Chang, J.; Liu, M.; Ning, C. Study on antibacterial effect of 45S5 Bioglass®. J. Mater. Sci. Mater. Med. 2009, 20, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Kaysinger, K.K.; Ramp, W.K. Extracellular pH modulates the activity of cultured human osteoblasts. J. Cell. Biochem. 1998, 68, 83–89. [Google Scholar] [CrossRef]
- Fliefel, R.; Popov, C.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Mesenchymal stem cell proliferation and mineralization but not osteogenic differentiation are strongly affected by extracellular pH. J. Cranio-Maxillofac. Surg. 2016, 44, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Galow, A.-M.; Rebl, A.; Koczan, D.; Bonk, S.M.; Baumann, W.; Gimsa, J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem. Biophys. Rep. 2017, 10, 17–25. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive glasses—Structure and properties. Angew. Chem. Int. Ed. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Zhang, D.; Hupa, M.; Hupa, L. In situ pH within particle beds of bioactive glasses. Acta Biomater. 2008, 4, 1498–1505. [Google Scholar] [CrossRef]
- Fopase, R.; Pandey, L.M.; Srinivasan, A. Effect of systematic substitution of Na2O for SiO2 on devitrification and bioactivity of sol-gel derived 69.5 SiO2-24.5 CaO-6P2O5 ceramics. Mater. Chem. Phys. 2024, 313, 128731. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, P.; Pyare, R. Synthesis, characterization, mechanical and biological properties of biocomposite based on zirconia containing 1393 bioactive glass with hydroxyapatite. Ceram. Int. 2020, 46, 10442–10451. [Google Scholar] [CrossRef]
- Lu, X.; Kolzow, J.; Chen, R.R.; Du, J. Effect of solution condition on hydroxyapatite formation in evaluating bioactivity of B2O3 containing 45S5 bioactive glasses. Bioact. Mater. 2019, 4, 207–214. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.-F.; Tariq, U.; Butt, F.K.; Abdolahi, A.; Hussain, R. Synthesis, characterization, in vitro bioactivity and antimicrobial activity of magnesium and nickel doped silicate hydroxyapatite. Ceram. Int. 2015, 41, 11886–11898. [Google Scholar] [CrossRef]
- Stanciu, G.A.; Sandulescu, I.; Savu, B.; Stanciu, S.G.; Paraskevopoulos, K.M.; Chatzistavrou, X.; Kontonasaki, E.; Koidis, P. Investigation of the hydroxyapatite growth on bioactive glass surface. J. Biomed. Pharm. Eng. 2007, 1, 34–39. [Google Scholar]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Faia-Torres, A.B.; Guimond-Lischer, S.; Rottmar, M.; Charnley, M.; Goren, T.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials 2014, 35, 9023–9032. [Google Scholar] [CrossRef]
- Gabbai-Armelin, P.R.; Souza, M.T.; Kido, H.W.; Tim, C.R.; Bossini, P.S.; Fernandes, K.R.; Magri, A.M.P.; Parizotto, N.A.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A. Characterization and biocompatibility of a fibrous glassy scaffold. J. Tissue Eng. Regen. Med. 2017, 11, 1141–1151. [Google Scholar] [CrossRef]
- Thomas, N.G.; Manoharan, A.; Anbarasu, A. Preclinical evaluation of sol-gel synthesized modulated 45s5-bioglass based biodegradable bone graft intended for alveolar bone regeneration. J. Hard Tissue Biol. 2021, 30, 303–308. [Google Scholar] [CrossRef]
- Majumdar, S.; Hira, S.K.; Tripathi, H.; Kumar, A.S.; Manna, P.P.; Singh, S.P.; Krishnamurthy, S. Synthesis and characterization of barium-doped bioactive glass with potential anti-inflammatory activity. Ceram. Int. 2021, 47, 7143–7158. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Zorba, T.; Papadopoulou, L.; Pavlidou, E.; Chatzistavrou, X.; Paraskevopoulos, K.; Koidis, P. Hydroxy carbonate apatite formation on particulate bioglass in vitro as a function of time. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2002, 37, 1165–1171. [Google Scholar] [CrossRef]
- Macon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef]
- Mahato, A.; De, M.; Bhattacharjee, P.; Kumar, V.; Mukherjee, P.; Singh, G.; Kundu, B.; Balla, V.K.; Nandi, S.K. Role of calcium phosphate and bioactive glass coating on in vivo bone healing of new Mg–Zn–Ca implant. J. Mater. Sci. Mater. Med. 2021, 32, 55. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Camy, S.; Combes, C.; Marsan, O.; Canceill, T.; Cazalbou, S. Bioceramic powders for bone regeneration modified by high-pressure CO2 process. J. Mater. Sci. 2021, 56, 3387–3403. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pyare, R. Characterization of ZnO substituted 45S5 bioactive glasses and glass-ceramics. J. Mater. Sci. Res. 2012, 1, 207. [Google Scholar] [CrossRef]
- Miola, M.; Verné, E.; Ciraldo, F.E.; Cordero-Arias, L.; Boccaccini, A.R. Electrophoretic deposition of chitosan/45S5 bioactive glass composite coatings doped with Zn and Sr. Front. Bioeng. Biotechnol. 2015, 3, 159. [Google Scholar] [CrossRef]
- Souza, M.T.; Crovace, M.C.; Schröder, C.; Eckert, H.; Peitl, O.; Zanotto, E.D. Effect of magnesium ion incorporation on the thermal stability, dissolution behavior and bioactivity in Bioglass-derived glasses. J. Non-Cryst. Solids 2013, 382, 57–65. [Google Scholar] [CrossRef]
- Salim, S.A.S.; Mohamad, H.; Noor, S. Influence of MgO on Sol-Gel Derived SiO2-CaO-Na2O-P2O5 Bioglass System. J. Phys. Conf. Ser. 2018, 1082, 012034. [Google Scholar] [CrossRef]
- Unalan, I.; Rimoli, I.H.; Mutlu, N.; Michálek, M.; Abraham, G.A.; Liverani, L.; Boccaccini, A.R. Cotton wool-like ion-doped bioactive glass nanofibers: Investigation of Zn and Cu combined effect. Biomed. Mater. 2024, 19, 065001. [Google Scholar] [CrossRef] [PubMed]
- Salesa, B.; Sabater i Serra, R.; Serrano-Aroca, Á. Zinc chloride: Time-dependent cytotoxicity, proliferation and promotion of glycoprotein synthesis and antioxidant gene expression in human keratinocytes. Biology 2021, 10, 1072. [Google Scholar] [CrossRef]
- Holmes, A.M.; Mackenzie, L.; Roberts, M.S. Disposition and measured toxicity of zinc oxide nanoparticles and zinc ions against keratinocytes in cell culture and viable human epidermis. Nanotoxicology 2020, 14, 263–274. [Google Scholar] [CrossRef]
- Michaelsson, G.; Ljunghall, K.; Danielson, B.G. Zinc in epidermis and dermis in healthy subjects. Acta Derm. -Venereol. 1980, 60, 295–299. [Google Scholar] [CrossRef]
- Lange, T.S.; Kirchberg, K.; Bielinsky, A.K.; Leuker, A.; Bank, I.; Ruzicka, T.; Scharffetter-Kochanek, K. Divalent cations (Mg2+, Ca2+) differentially influence the β1 integrin-mediated migration of human fibroblasts and keratinocytes to different extracellular matrix proteins. Exp. Dermatol. 1995, 4, 130–137. [Google Scholar] [CrossRef]
- Grzesiak, J.J.; Pierschbacher, M.D. Changes in the concentrations of extracellular Mg++ and Ca++ down-regulate E-cadherin and up-regulate α2β1 integrin function, activating keratinocyte migration on type I collagen. J. Investig. Dermatol. 1995, 104, 768–774. [Google Scholar] [CrossRef]
- Yoshino, Y.; Teruya, T.; Miyamoto, C.; Hirose, M.; Endo, S.; Ikari, A. Unraveling the Mechanisms Involved in the Beneficial Effects of Magnesium Treatment on Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 4994. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.H.; Bui, T.H.; Guseva, E.V.; Ta, A.T.; Nguyen, A.T.; Hoang, T.T.H.; Bui, X.V. Characterization of bioactive glass synthesized by sol-gel process in hot water. Crystals 2020, 10, 529. [Google Scholar] [CrossRef]
- Diba, M.; Tapia, F.; Boccaccini, A.R.; Strobel, L.A. Magnesium-containing bioactive glasses for biomedical applications. Int. J. Appl. Glass Sci. 2012, 3, 221–253. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Song, G.; Liu, T.; Cao, C.; Yang, Y.; Zhang, Y.; Hong, W. Incorporation of ZnO/bioactive glass nanoparticles into alginate/chitosan composite hydrogels for wound closure. ACS Appl. Bio Mater. 2019, 2, 5042–5052. [Google Scholar] [CrossRef] [PubMed]
- Özarslan, A.C.; Özel, C.; Okumuş, M.D.; Doğan, D.; Yücel, S. Development, structural and rheological characterization, and in vitro evaluation of the zinc-doped 45S5 bioactive glass-vaseline ointment for potential wound healing applications. J. Mater. Res. 2023, 38, 1557–1572. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chiou, Y.-J.; Chang, P.-J.; Chang, W.-M.; Yeh, Y.-C.; Chen, C.-Y.; Chang, Y.-K.; Lin, C.-K. In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition. Polymers 2024, 16, 2811. [Google Scholar] [CrossRef]
- Zhu, S.; Dai, Q.; Yao, L.; Wang, Z.; He, Z.; Li, M.; Wang, H.; Li, Q.; Gao, H.; Cao, X. Engineered multifunctional nanocomposite hydrogel dressing to promote vascularization and anti-inflammation by sustained releasing of Mg2+ for diabetic wounds. Compos. Part B Eng. 2022, 231, 109569. [Google Scholar] [CrossRef]
- Daguano, J.K.M.F.; Rogero, S.O.; Crovace, M.C.; Peitl, O.; Strecker, K.; Dos Santos, C. Bioactivity and cytotoxicity of glass and glass–ceramics based on the 3CaO·P2O5–SiO2–MgO system. J. Mater. Sci. Mater. Med. 2013, 24, 2171–2180. [Google Scholar] [CrossRef]
- Gabbai-Armelin, P.R.; Fernandes, K.R.; Magri, A.M.P.; Da Silva, A.C.; Fortulan, C.A.; Renno, A.C.M. Characterization and cytotoxicity evaluation of bio-inspired bioactive glass/collagen/magnesium composites. Mater. Chem. Phys. 2019, 228, 201–209. [Google Scholar] [CrossRef]
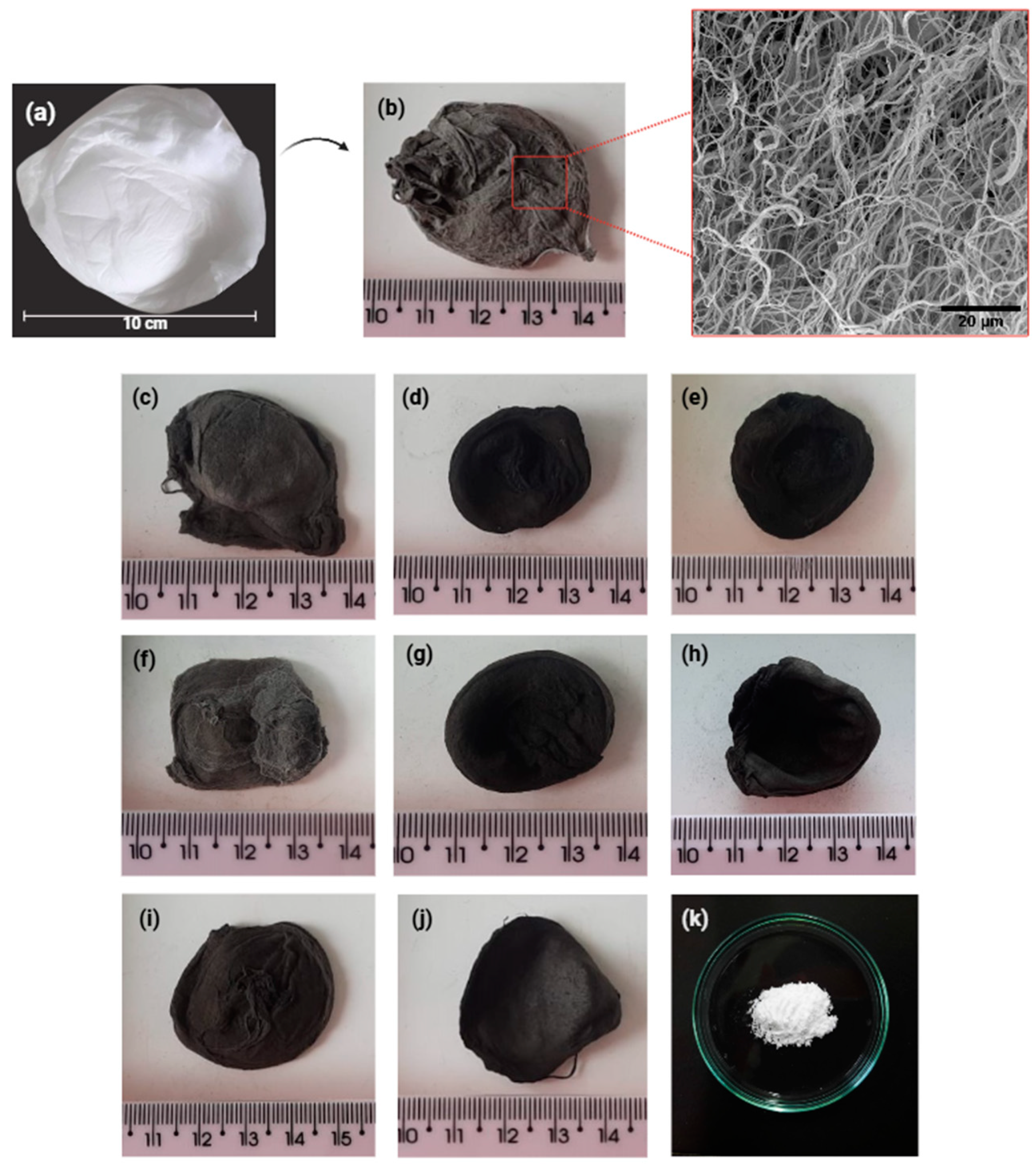
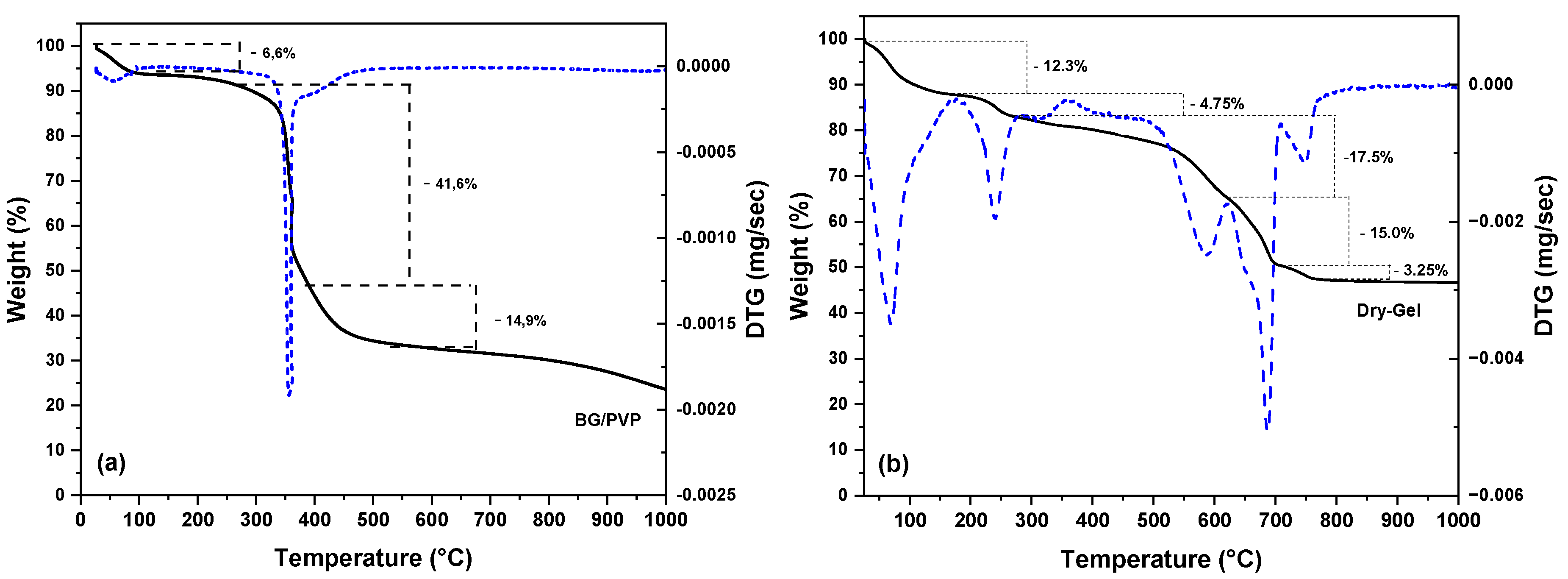
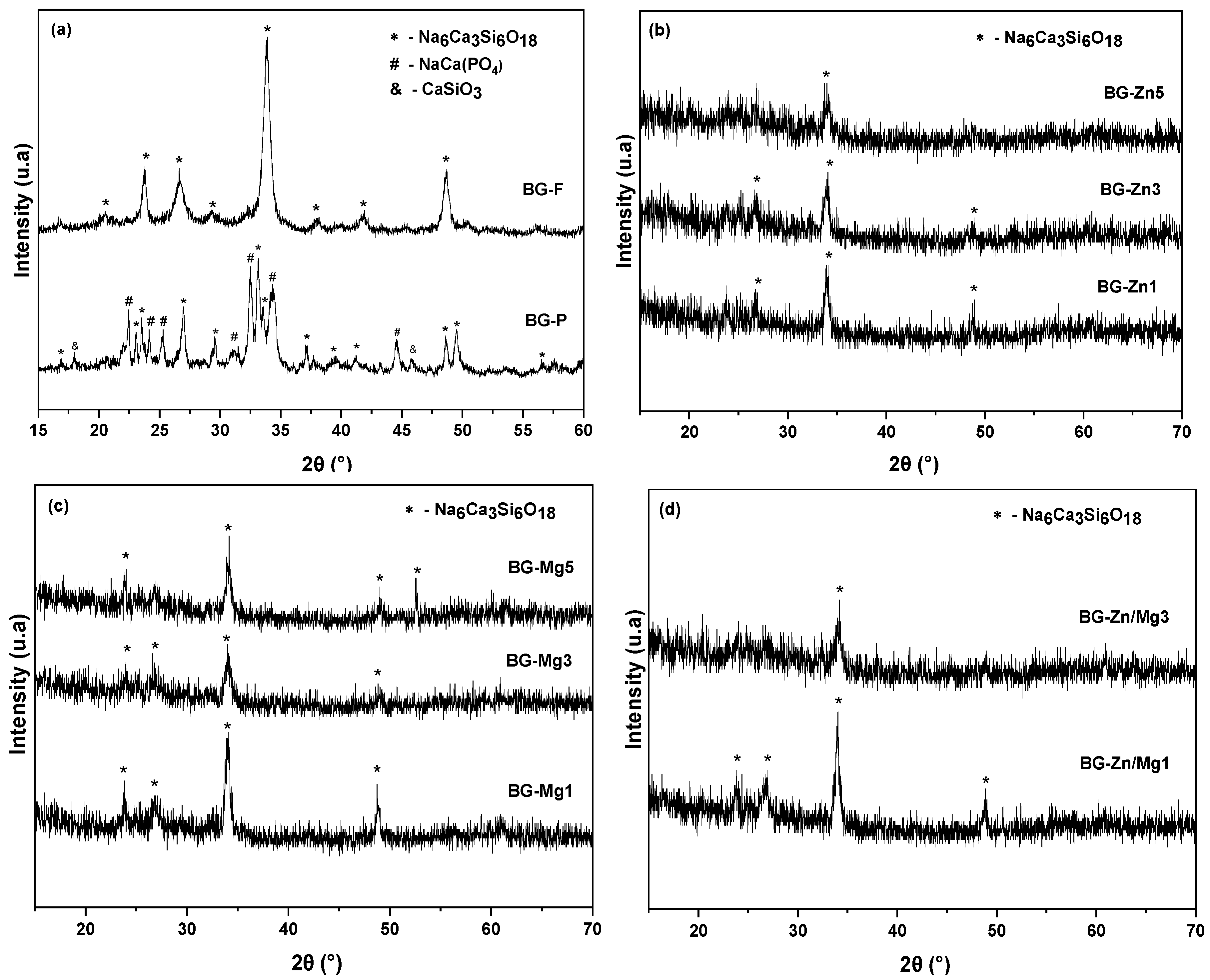
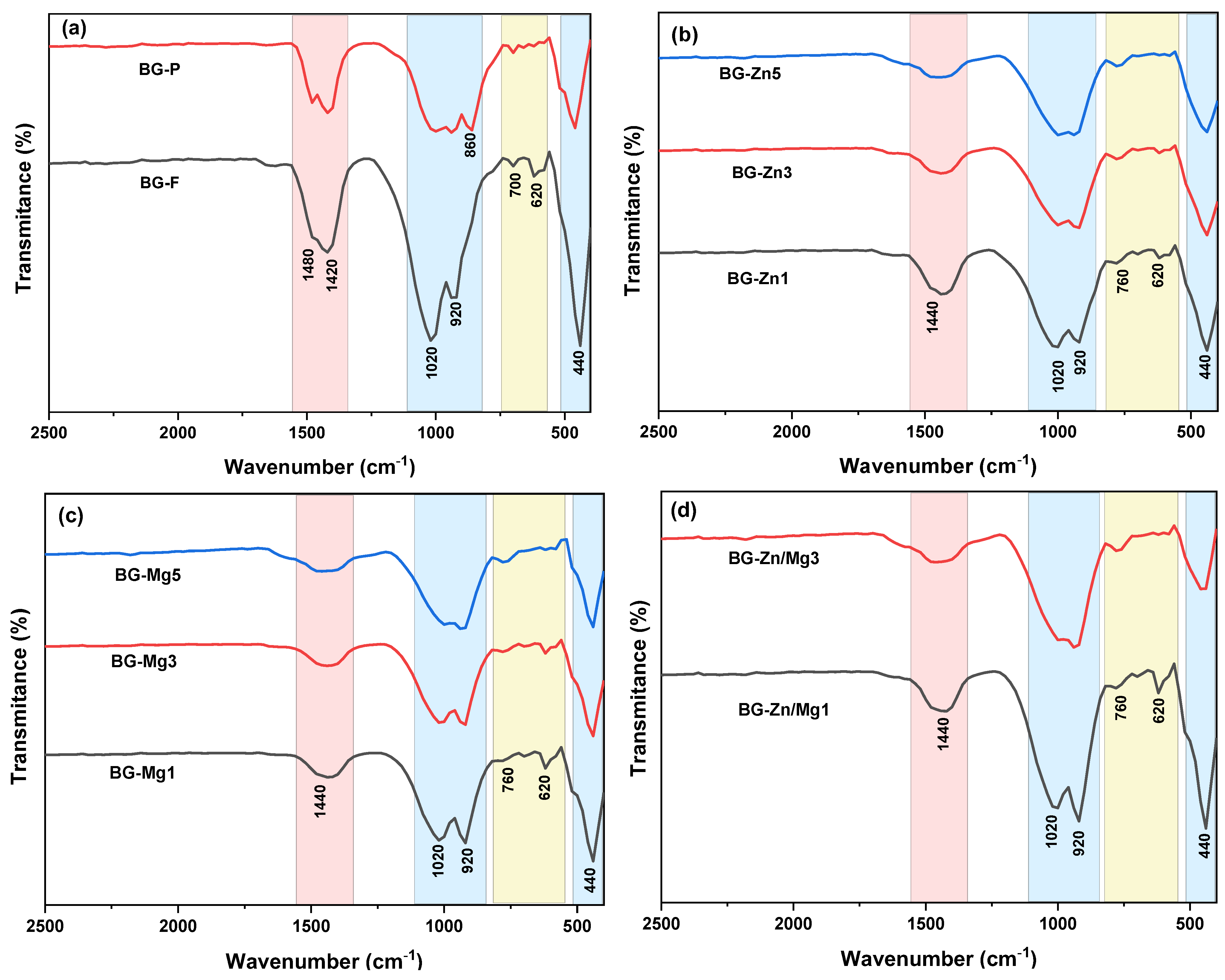


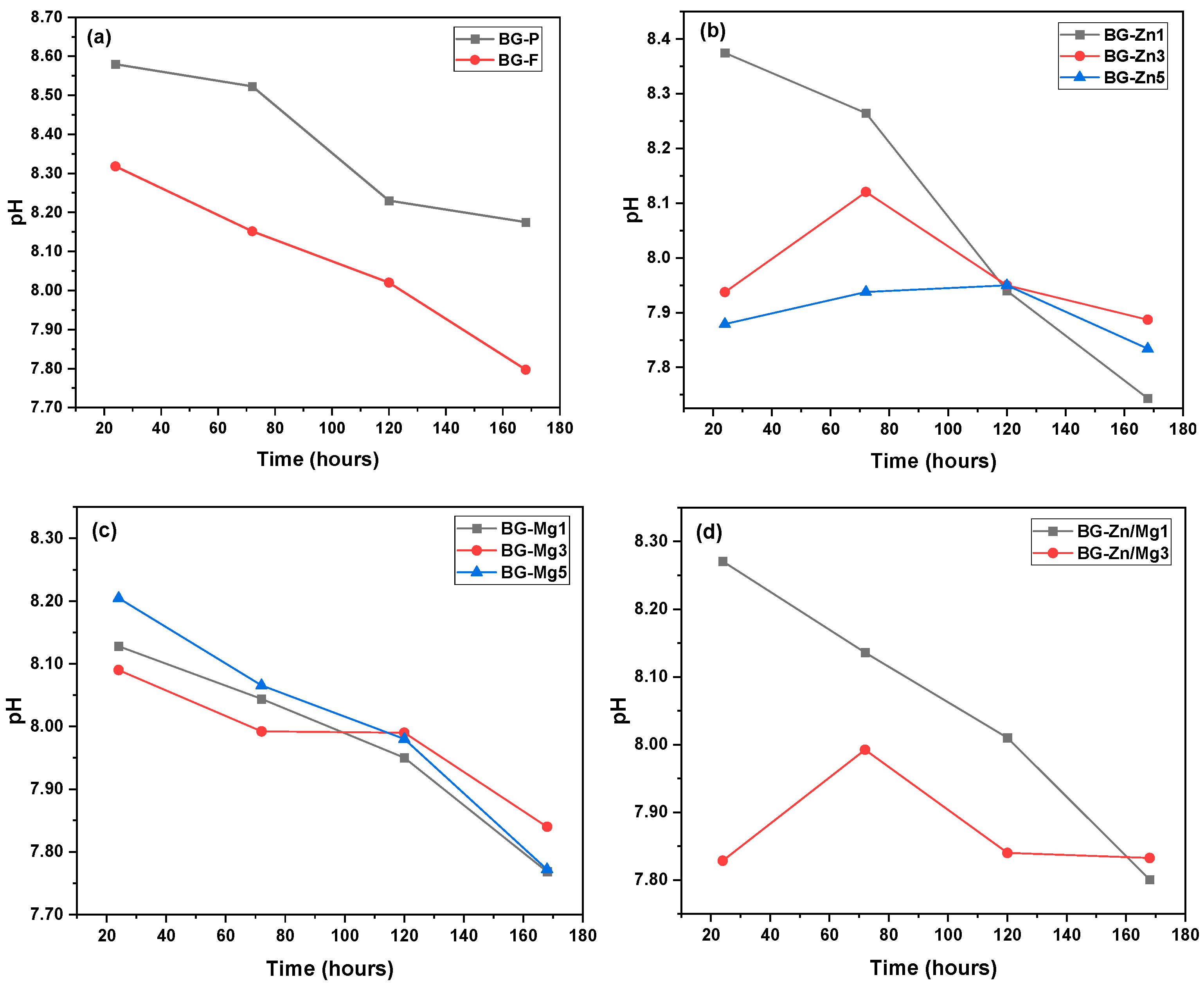
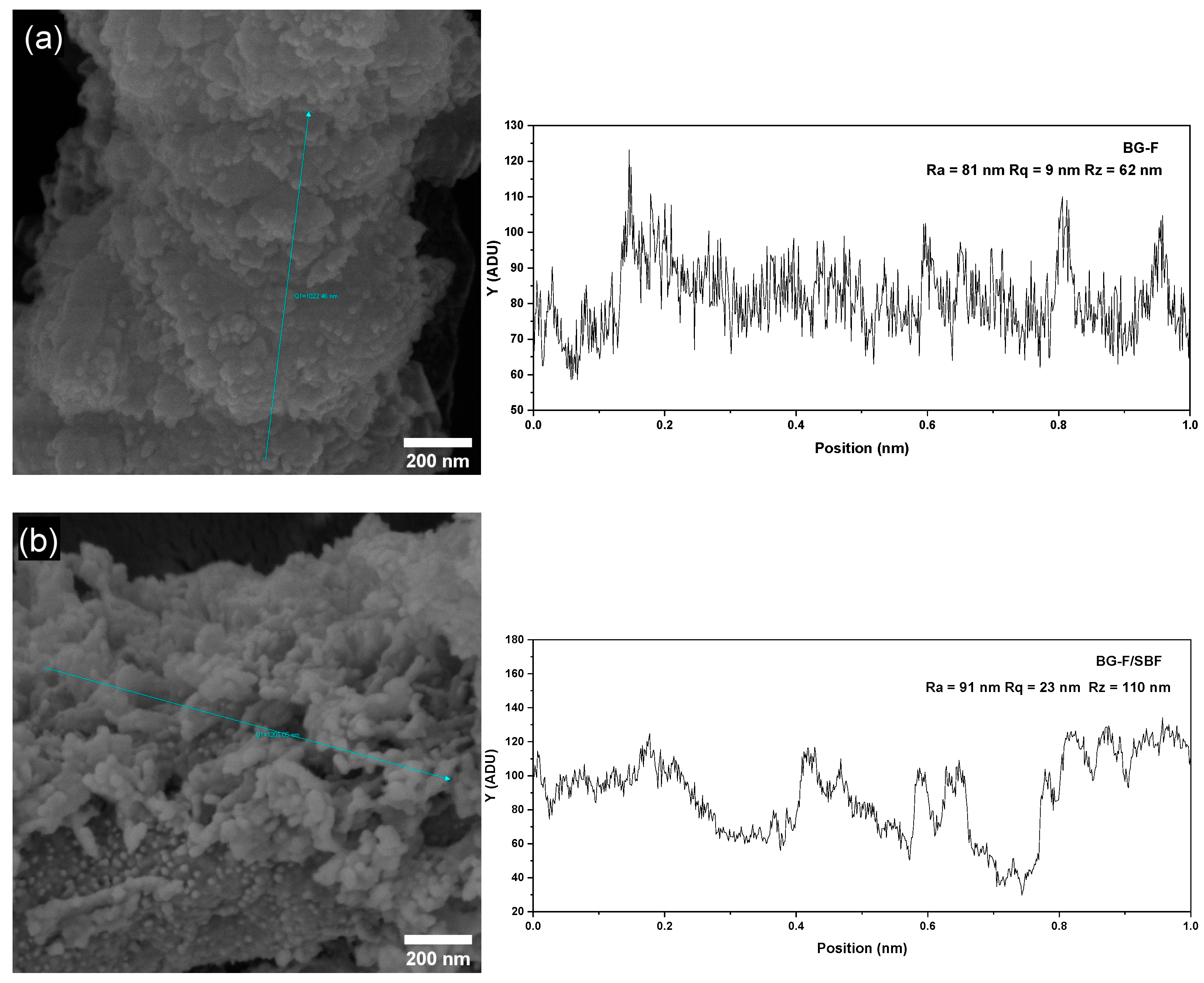
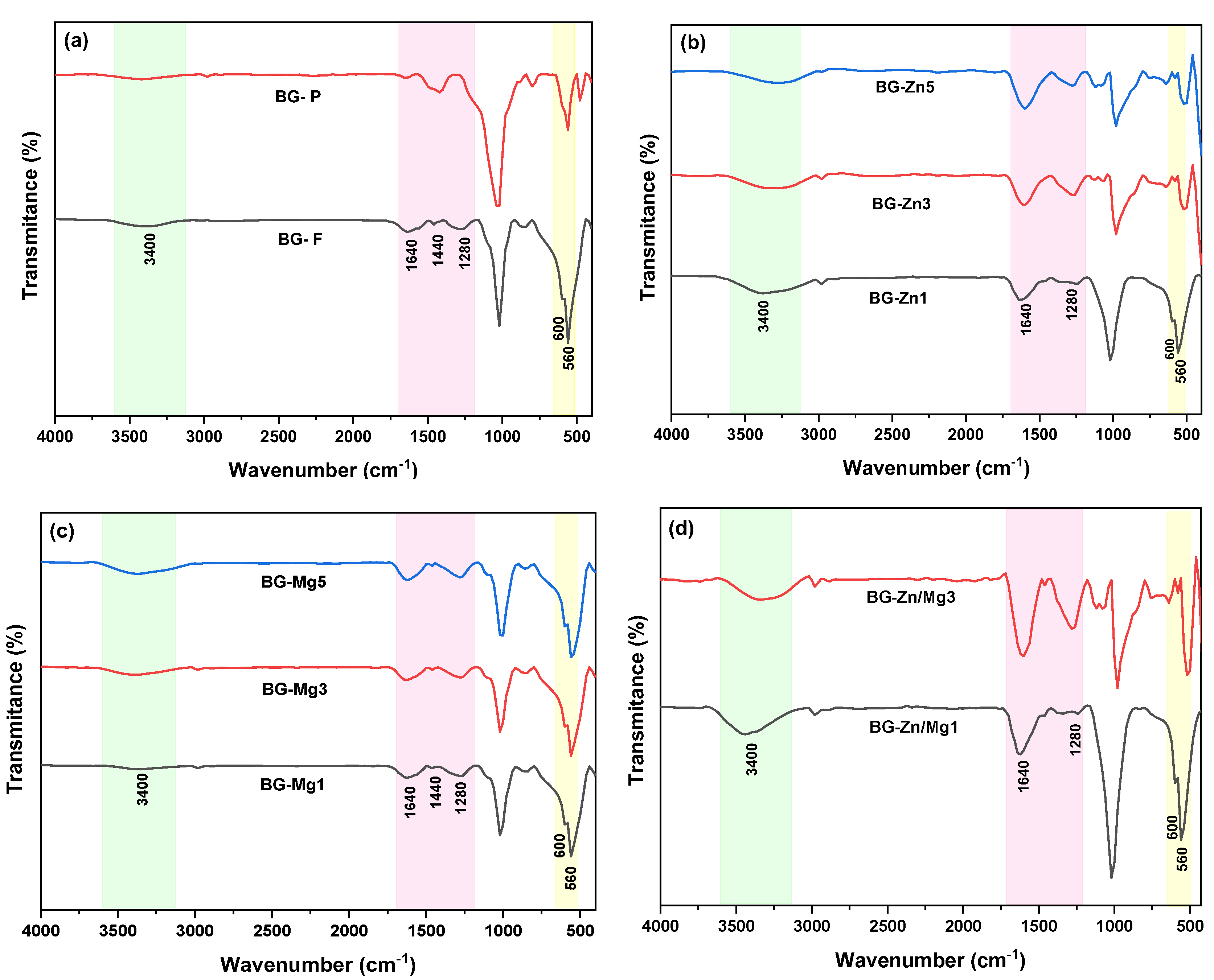
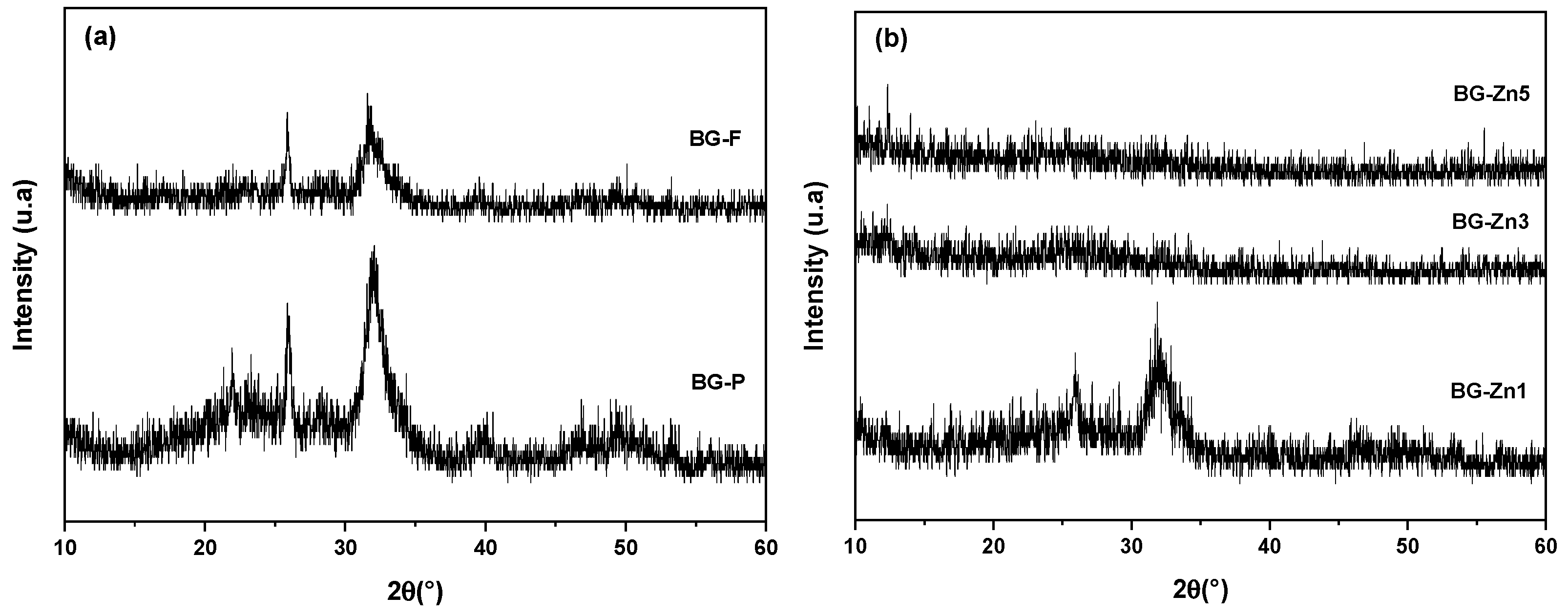
| Nome | SiO2 | P2O5 | CaO | Na2O | ZnO | MgO |
|---|---|---|---|---|---|---|
| BG | 46.14 | 2.60 | 26.91 | 24.35 | - | - |
| BG-Zn1 | 46.14 | 2.60 | 25.91 | 24.35 | 1.00 | - |
| BG-Zn3 | 46.14 | 2.60 | 23.91 | 24.35 | 3.00 | - |
| BG-Zn3 | 46.14 | 2.60 | 21.91 | 24.35 | 5.00 | - |
| BG-Mg1 | 46.14 | 2.60 | 25.91 | 24.35 | - | 1.00 |
| BG-Mg3 | 46.14 | 2.60 | 23.91 | 24.35 | - | 3.00 |
| BG-Mg5 | 46.14 | 2.60 | 21.91 | 24.35 | - | 5.00 |
| BG-Zn/Mg1 | 46.14 | 2.60 | 24.91 | 24.35 | 1.00 | 1.00 |
| BG-Zn/Mg3 | 46.14 | 2.60 | 20.91 | 24.35 | 3.00 | 3.00 |
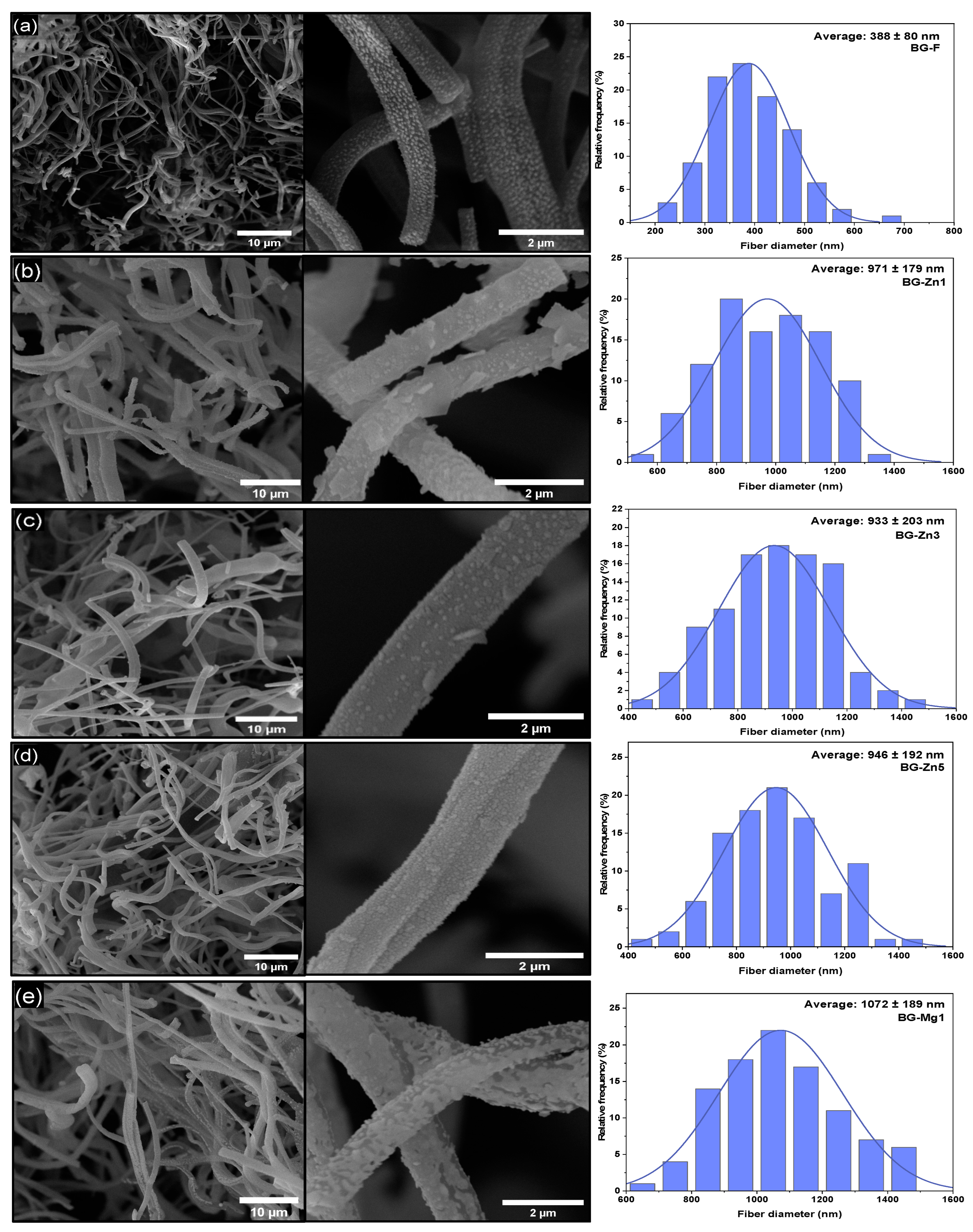
| Name | Average Diameter ± Standard Deviation (nm) | Tukey Comparison with Pure Fiber (p-Value) | Comparison Between Doping (p-Value) |
|---|---|---|---|
| BG-F | 388 ± 80 | - | - |
| BG-Zn1 | 971 ± 179 | p < 0.001 | p = 0.910 (Zn3) p = 0.993 (Zn5) |
| BG-Zn3 | 933 ± 203 | p < 0.001 | p =0.910 (Zn1) p= 0.999 (Zn3) |
| BG-Zn5 | 946 ± 192 | p < 0.001 | p =0.910 (Zn1) p= 0.999 (Zn3) |
| BG-Mg1 | 1072 ± 189 | p < 0.001 | p = 0.281 (Mg3) p = 0.999 (Mg5) |
| BG-Mg3 | 1139 ± 202 | p < 0.001 | p = 0.281 (Mg3) p = 0.652 (Mg5) |
| BG-Mg5 | 1088 ± 257 | p < 0.001 | p = 0.999 (Mg1) p = 0.652 (Mg3) |
| BG-Zn/Mg1 | 998 ± 179 | p < 0.001 | p = 1 (Zn/Mg3) |
| BG-Zn/Mg3 | 1005 ± 234 | p < 0.001 | p = 1 (ZnMg1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, K.C.; Andrade, M.G.d.S.; Araujo, R.N.d.; Abreu Junior, A.R.d.; Sobral, M.V.; Gonçalves, J.C.R.; Sousa, B.V.; Neves, G.A.; Menezes, R.R. PVP as an Oxygen Vacancy-Inducing Agent in the Development of Black 45S5 Bioactive Glass Fibrous Scaffolds Doped with Zn and Mg Using A-HSBS. Materials 2025, 18, 1340. https://doi.org/10.3390/ma18061340
Costa KC, Andrade MGdS, Araujo RNd, Abreu Junior ARd, Sobral MV, Gonçalves JCR, Sousa BV, Neves GA, Menezes RR. PVP as an Oxygen Vacancy-Inducing Agent in the Development of Black 45S5 Bioactive Glass Fibrous Scaffolds Doped with Zn and Mg Using A-HSBS. Materials. 2025; 18(6):1340. https://doi.org/10.3390/ma18061340
Chicago/Turabian StyleCosta, Keila C., Maria Geórgia da S. Andrade, Rondinele N. de Araujo, Adegildo R. de Abreu Junior, Marianna V. Sobral, Juan Carlos R. Gonçalves, Bianca V. Sousa, Gelmires A. Neves, and Romualdo R. Menezes. 2025. "PVP as an Oxygen Vacancy-Inducing Agent in the Development of Black 45S5 Bioactive Glass Fibrous Scaffolds Doped with Zn and Mg Using A-HSBS" Materials 18, no. 6: 1340. https://doi.org/10.3390/ma18061340
APA StyleCosta, K. C., Andrade, M. G. d. S., Araujo, R. N. d., Abreu Junior, A. R. d., Sobral, M. V., Gonçalves, J. C. R., Sousa, B. V., Neves, G. A., & Menezes, R. R. (2025). PVP as an Oxygen Vacancy-Inducing Agent in the Development of Black 45S5 Bioactive Glass Fibrous Scaffolds Doped with Zn and Mg Using A-HSBS. Materials, 18(6), 1340. https://doi.org/10.3390/ma18061340







