Sorption and Desorption Isotherms of Lightweight Alkali-Activated Materials Modified with Silica Aerogel
Abstract
1. Introduction
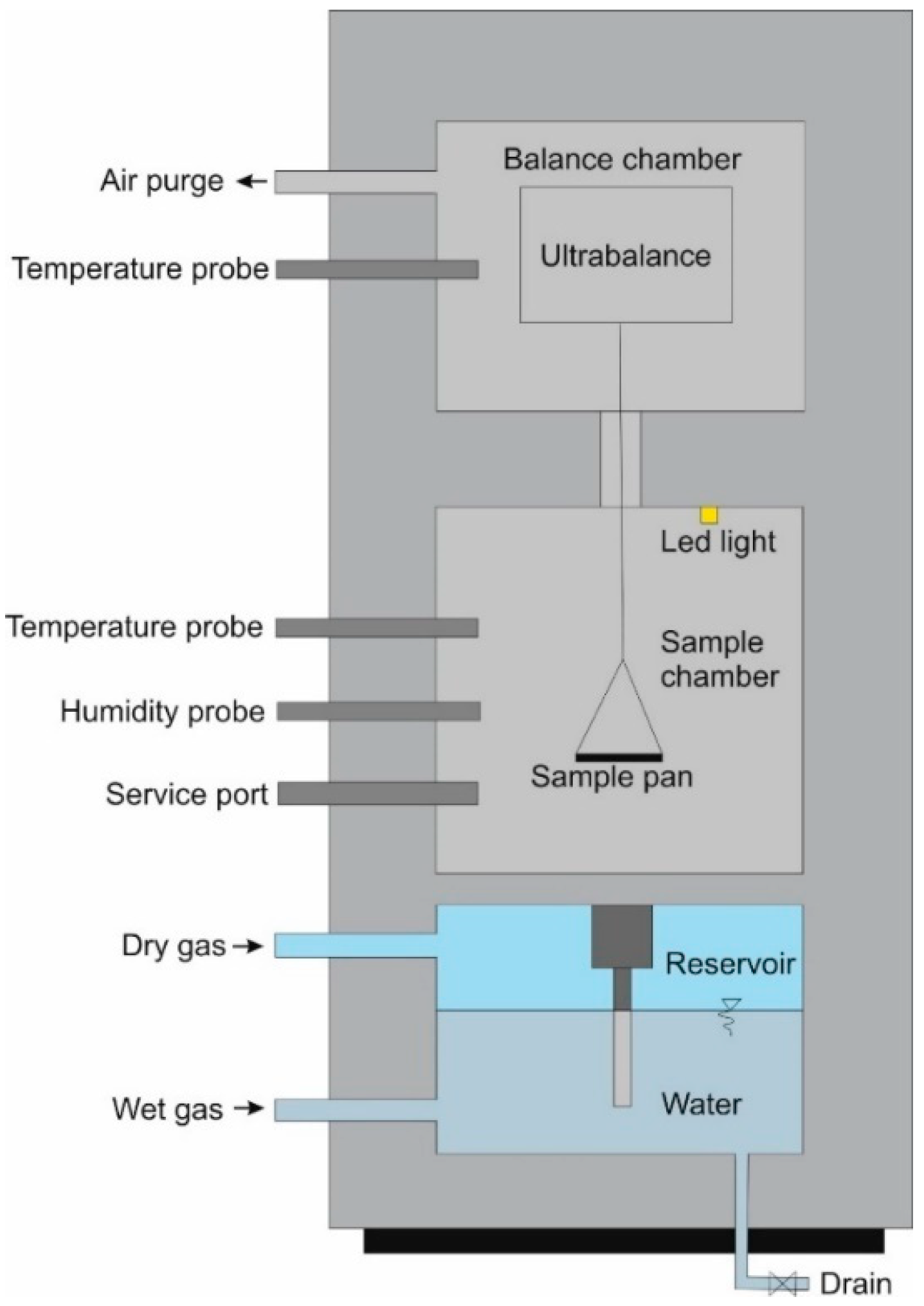
2. Materials and Methods
- (a)
- Using desiccators and weighing cups (desiccator method);
- (b)
- Using a climatic chamber (climatic chamber method).
3. Results and Discussion
4. Conclusions
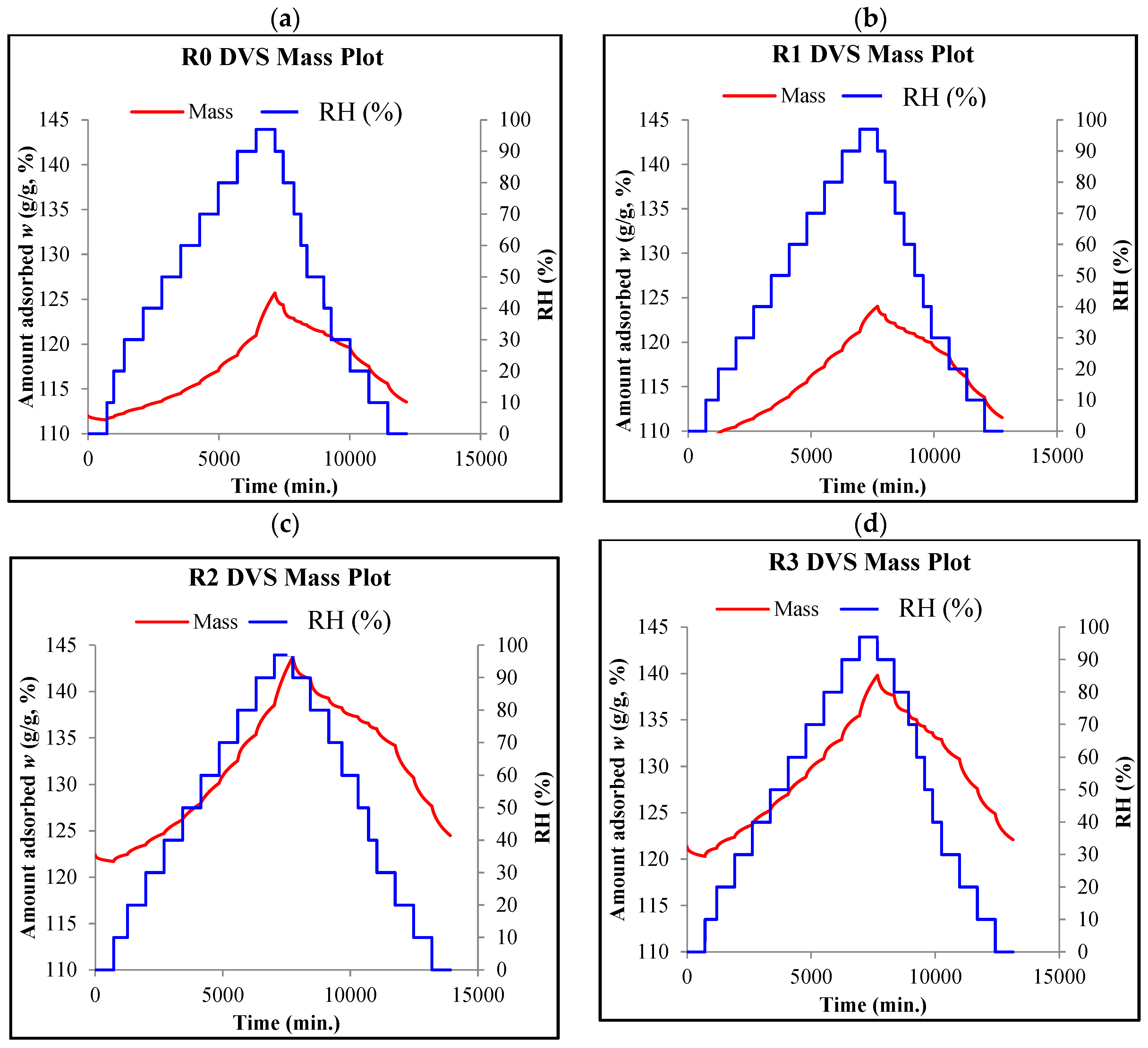
- The DVS method provided an effective determination of the equilibrium moisture values (w) for all the tested composites at each humidity level (RH).
- For each composite (R0–R3), ten measurement points were obtained in each sorption cycle and each desorption cycle, which provided the possibility of a precise determination of the course of both the sorption isotherms and desorption isotherms.
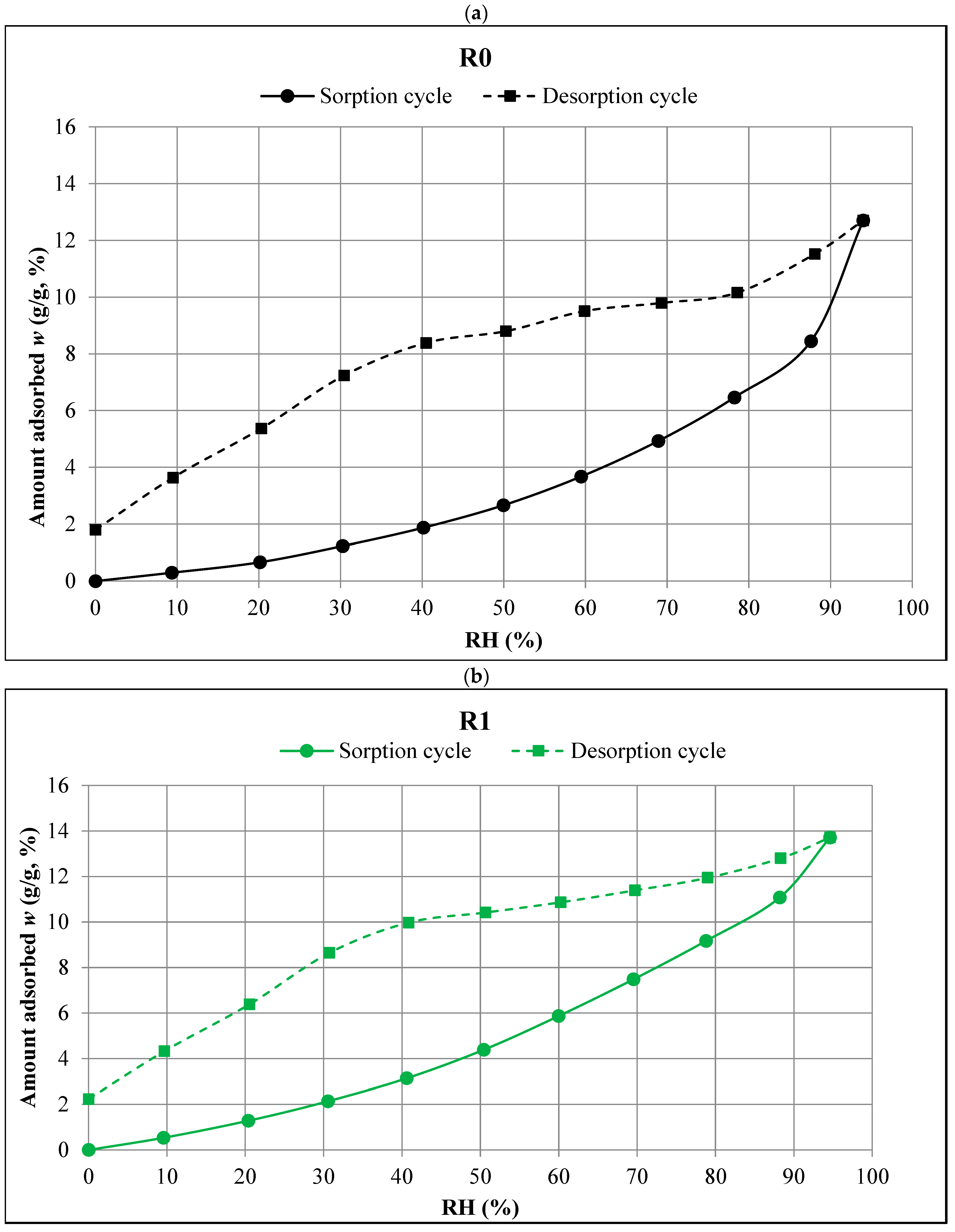
- All the tested composites exhibited Type III isotherms according to the Brunauer and IUPAC classifications. This type of isotherm occurs when the interactions between the adsorbate molecules are much stronger than those between the adsorbate and the adsorbent. Capillary condensation occurs in the pores of the adsorbent.
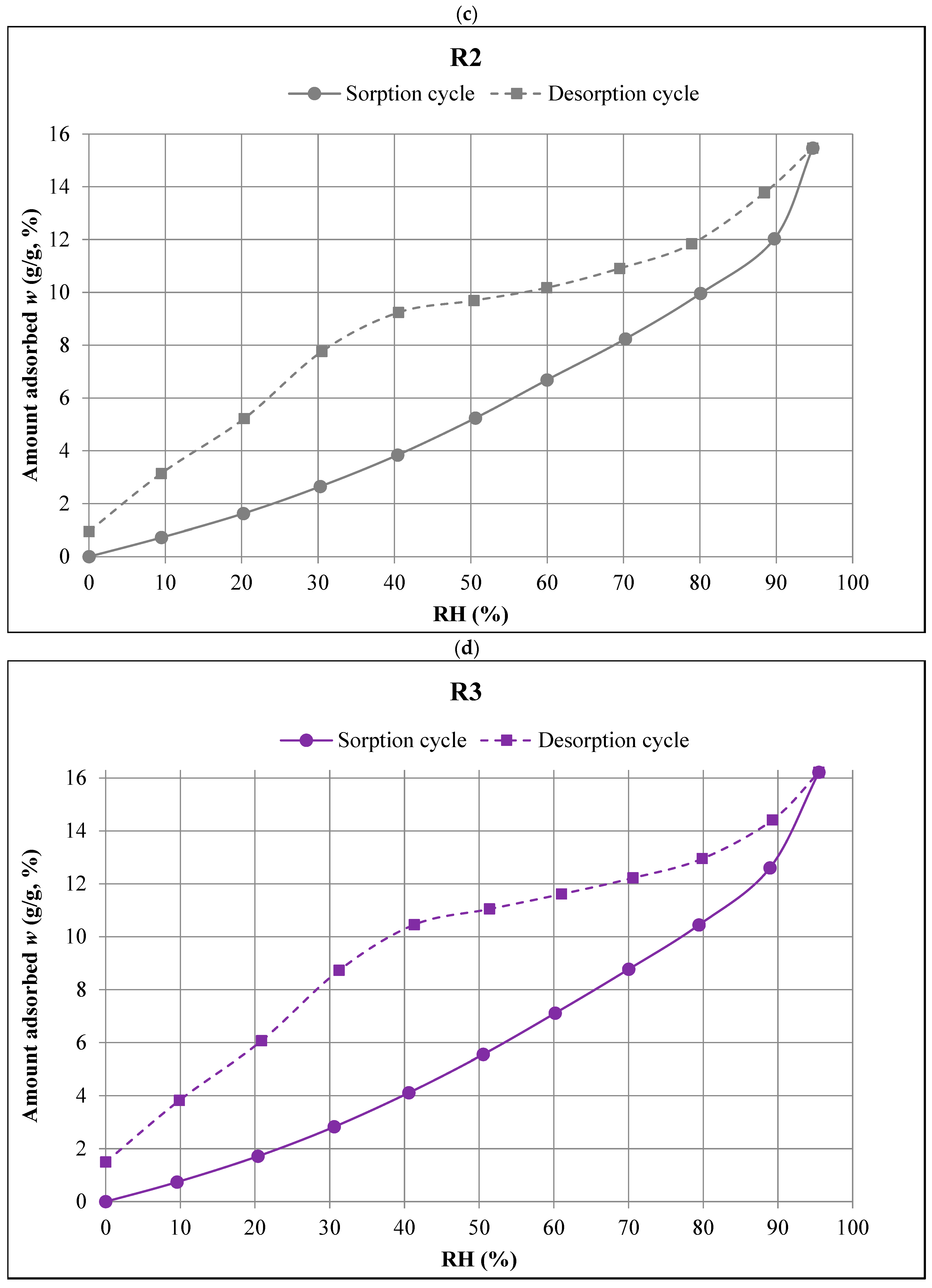
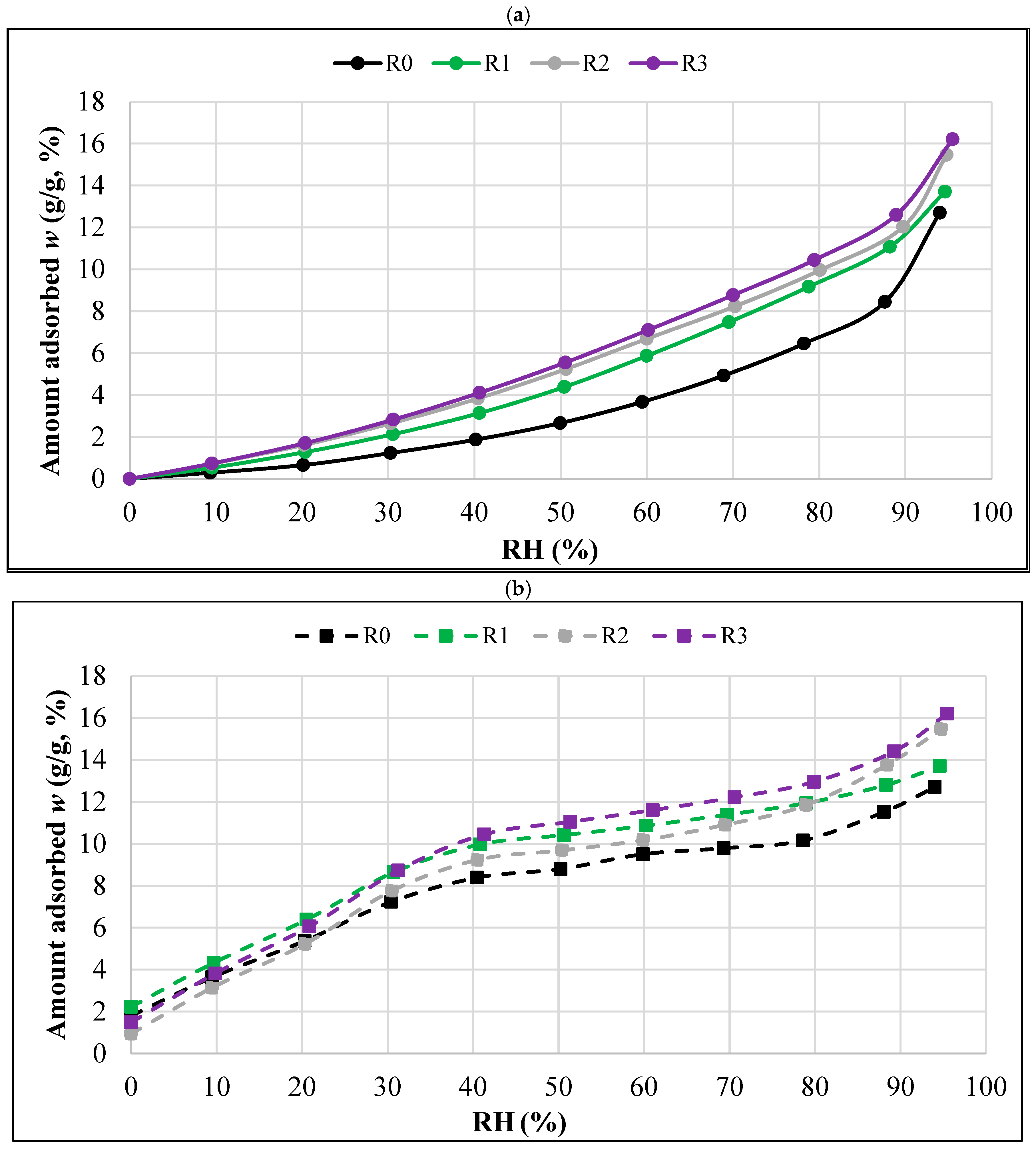
- The R0 composite, which did not contain aerogel, had the lowest sorption moisture values w in the entire humidity range tested (0–95%). Increasing the aerogel content led to higher moisture absorption. The R3 composite, with the highest aerogel content, showed the highest values of equilibrium sorption moisture w at all RH levels.
- In the range of the highest relative humidity (RH > 90%), the influence of capillary condensation is clearly visible, leading to an intense increase in the equilibrium sorption moisture w. It is most significant in the case of the R0 composite, with the most compact porosity structure.
- Capillary condensation also affected the course of the desorption isotherms of all the composites, leading to the more intense increase in the equilibrium values of w in the range RH > 80%.
- The desorption trends were similar to the sorption trends at RH > 30%, i.e., the lowest equilibrium values of desorption moisture w were found in the case of the R0 composite and the highest were in the case of the R3 composite.
- In the range of the lowest humidity (RH < 30%), no clear differences were observed between the desorption isotherms of the individual composites. However, in all the composites, there was a significant decrease in equilibrium moisture values w with a decrease in humidity. The changes in this range (RH = 30% → 0%) were much greater than those recorded in the measurements of the sorption isotherms.
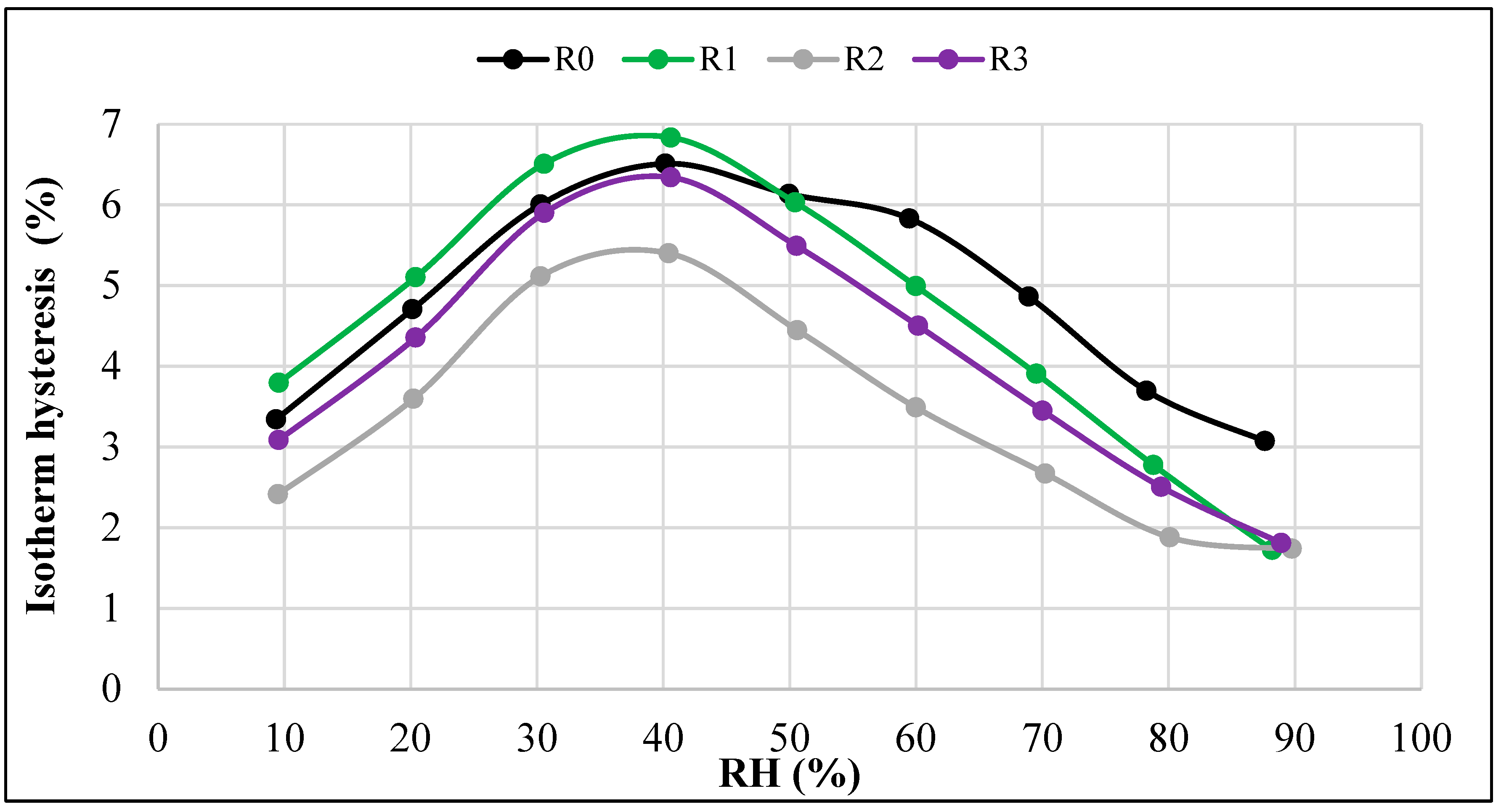
- The differences between the sorption and desorption equilibrium moisture w depended on the RH values. The largest differences, ranging from 5% to 7%, occurred at intermediate RH levels. The smallest differences were observed at both the lowest and highest RH levels.
- The hysteresis was most pronounced in the R0 and R1 composites, i.e., those made without the addition of aerogel or with the lowest aerogel content. In the R0 composite, the hysteresis was more pronounced at RH > 50%, and in the R1 composite, the hysteresis was more evident at RH < 50%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimas, D.; Giannopoulou, I.; Panias, D. Polymerization in Sodium Silicate Solutions: A Fundamental Process in Geopolymerization Technology. J. Mater. Sci. 2009, 44, 3719–3730. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A Critical Review on Application of Alkali Activated Slag as a Sustainable Composite Binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly Ash/Blast Furnace Slag-Based Geopolymer as a Potential Binder for Mine Backfilling: Effect of Binder Type and Activator Concentration. Adv. Mater. Sci. Eng. 2019, 2019, 2028109. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Natali, M.E.; Rickard, W.D.A.; van Riessen, A. Room Temperature Alkali Activation of Fly Ash: The Effect of Na2O/SiO2 Ratio. Constr. Build. Mater. 2014, 69, 262–270. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, L.; Pan, Y.; Li, C.; Zhou, T.; Cheng, X. Facile Construction of the Aerogel/Geopolymer Composite with Ultra-Low Thermal Conductivity and High Mechanical Performance. RSC Adv. 2018, 8, 2350–2356. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Garbalińska, H.; Strzałkowski, J. Lightweight Alkali-Activated Composites Containing Sintered Fly Ash Aggregate and Various Amounts of Silica Aerogel. J. Build. Eng. 2023, 74, 106879. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Cui, Y. Study on Pore Structure and Thermal Conductivity of Aerogel Enhanced Porous Geopolymers. J. Therm. Anal. Calorim. 2022, 147, 1061–1070. [Google Scholar] [CrossRef]
- Degefu, D.M.; Liao, Z.; Berardi, U.; Labbé, G. The Effect of Activator Ratio on the Thermal and Hygric Properties of Aerated Geopolymers. J. Build. Eng. 2022, 45, 103414. [Google Scholar] [CrossRef]
- Ngouloure, Z.N.M.; Kamseu, E.; Moungam, L.M.B.; Tchakoute, H.K.; Valentini, L.; Leonelli, C. Design of Porous Geopolymers for Hygrothermal Applications: Role of Nano and Meso Porosity. Silicon 2022, 14, 10045–10059. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef]
- Karim, A.N.; Johansson, P.; Sasic Kalagasidis, A. Knowledge Gaps Regarding the Hygrothermal and Long-Term Performance of Aerogel-Based Coating Mortars. Constr. Build. Mater. 2022, 314, 125602. [Google Scholar] [CrossRef]
- Berardi, U. Aerogel-Enhanced Systems for Building Energy Retrofits: Insights from a Case Study. Energy Build. 2018, 159, 370–381. [Google Scholar] [CrossRef]
- Stahl, T.; Ghazi Wakili, K.; Hartmeier, S.; Franov, E.; Niederberger, W.; Zimmermann, M. Temperature and Moisture Evolution beneath an Aerogel Based Rendering Applied to a Historic Building. J. Build. Eng. 2017, 12, 140–146. [Google Scholar] [CrossRef]
- Westgate, P.; Paine, K.; Ball, R.J. Physical and Mechanical Properties of Plasters Incorporating Aerogel Granules and Polypropylene Monofilament Fibres. Constr. Build. Mater. 2018, 158, 472–480. [Google Scholar] [CrossRef]
- Nosrati, R.H.; Berardi, U. Hygrothermal Characteristics of Aerogel-Enhanced Insulating Materials under Different Humidity and Temperature Conditions. Energy Build. 2018, 158, 698–711. [Google Scholar] [CrossRef]
- Feng, C.; Janssen, H.; Wu, C.; Feng, Y.; Meng, Q. Validating Various Measures to Accelerate the Static Gravimetric Sorption Isotherm Determination. Build. Environ. 2013, 69, 64–71. [Google Scholar] [CrossRef]
- EN ISO 12571:2021; Hygrothermal Performance of Building Materials and Products—Determination of Hygroscopic Sorption Properties. ISO: Geneva, Switzerland, 2021.
- Garbalińska, H.; Bochenek, M.; Malorny, W.; von Werder, J. Comparative Analysis of the Dynamic Vapor Sorption (DVS) Technique and the Traditional Method for Sorption Isotherms Determination—Exemplified at Autoclaved Aerated Concrete Samples of Four Density Classes. Cem. Concr. Res. 2017, 91, 97–105. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V. Water Vapour Sorption Experiments on Hardened Cementitious Materials: Part I: Essential Tool for Analysis of Hygral Behaviour and Its Relation to Pore Structure. Cem. Concr. Res. 2007, 37, 414–437. [Google Scholar] [CrossRef]
- Kumar, A.; Ketel, S.; Vance, K.; Oey, T.; Neithalath, N.; Sant, G. Water Vapor Sorption in Cementitious Materials—Measurement, Modeling and Interpretation. Transp. Porous Media 2014, 103, 69–98. [Google Scholar] [CrossRef]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; Van Vlierberghe, S.; Dubruel, P.; Lodewyckx, P.; De Belie, N. The Influence of Different Drying Techniques on the Water Sorption Properties of Cement-Based Materials. Cem. Concr. Res. 2014, 64, 54–62. [Google Scholar] [CrossRef]
- De Belie, N.; Kratky, J.; Van Vlierberghe, S. Influence of Pozzolans and Slag on the Microstructure of Partially Carbonated Cement Paste by Means of Water Vapour and Nitrogen Sorption Experiments and BET Calculations. Cem. Concr. Res. 2010, 40, 1723–1733. [Google Scholar] [CrossRef]
- Wu, M.; Johannesson, B.; Geiker, M. A Study of the Water Vapor Sorption Isotherms of Hardened Cement Pastes: Possible Pore Structure Changes at Low Relative Humidity and the Impact of Temperature on Isotherms. Cem. Concr. Res. 2014, 56, 97–105. [Google Scholar] [CrossRef]
- Pavlík, Z.; Žumár, J.; Medved, I.; Černý, R. Water Vapor Adsorption in Porous Building Materials: Experimental Measurement and Theoretical Analysis. Transp. Porous Media 2012, 91, 939–954. [Google Scholar] [CrossRef]
- McGregor, F.; Heath, A.; Fodde, E.; Shea, A. Conditions Affecting the Moisture Buffering Measurement Performed on Compressed Earth Blocks. Build. Environ. 2014, 75, 11–18. [Google Scholar] [CrossRef]
- Fabbri, A.; McGregor, F.; Costa, I.; Faria, P. Effect of Temperature on the Sorption Curves of Earthen Materials. Mater. Struct. 2017, 50, 253. [Google Scholar] [CrossRef]
- McGregor, F.; Heath, A.; Shea, A.; Lawrence, M. The Moisture Buffering Capacity of Unfired Clay Masonry. Build. Environ. 2014, 82, 599–607. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Norton, A.; Newman, G. The Water Vapor Sorption Behavior of Natural Fibers. J. Appl. Polym. Sci. 2009, 112, 1524–1537. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Jalaludin, Z.; Curling, S.F.; Anandjiwala, R.D.; Norton, A.J.; Newman, G. The Dynamic Water Vapour Sorption Behaviour of Natural Fibres and Kinetic Analysis Using the Parallel Exponential Kinetics Model. J. Mater. Sci. 2011, 46, 479–489. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Norton, A.J.; Newman, G. The Water Vapour Sorption Properties of Sitka Spruce Determined Using a Dynamic Vapour Sorption Apparatus. Wood Sci. Technol. 2010, 44, 497–514. [Google Scholar] [CrossRef]
- Laborel-Préneron, A.; Magniont, C.; Aubert, J.-E. Hygrothermal Properties of Unfired Earth Bricks: Effect of Barley Straw, Hemp Shiv and Corn Cob Addition. Energy Build. 2018, 178, 265–278. [Google Scholar] [CrossRef]
- Cagnon, H.; Aubert, J.E.; Coutand, M.; Magniont, C. Hygrothermal Properties of Earth Bricks. Energy Build. 2014, 80, 208–217. [Google Scholar] [CrossRef]
- Åhs, M.S. Sorption Scanning Curves for Hardened Cementitious Materials. Constr. Build. Mater. 2008, 22, 2228–2234. [Google Scholar] [CrossRef]
- Espinosa, R.M.; Franke, L. Inkbottle Pore-Method: Prediction of Hygroscopic Water Content in Hardened Cement Paste at Variable Climatic Conditions. Cem. Concr. Res. 2006, 36, 1954–1968. [Google Scholar] [CrossRef]
- Anderberg, A.; Wadsö, L. Method for Simultaneous Determination of Sorption Isotherms and Diffusivity of Cement-Based Materials. Cem. Concr. Res. 2008, 38, 89–94. [Google Scholar] [CrossRef]
- Saeidpour, M.; Wadsö, L. Evidence for Anomalous Water Vapor Sorption Kinetics in Cement Based Materials. Cem. Concr. Res. 2015, 70, 60–66. [Google Scholar] [CrossRef]
- Strzałkowski, J.; Stolarska, A.; Kożuch, D.; Dmitruk, J. Hygrothermal and Strength Properties of Cement Mortars Containing Cenospheres. Cem. Concr. Res. 2023, 174, 107325. [Google Scholar] [CrossRef]
- Strzałkowski, J.; Stolarska, A. Preliminary Studies on a Lightweight Porous Cement-Based Composite—Gel Concrete. J. Phys. Conf. Ser. 2023, 2654, 012069. [Google Scholar] [CrossRef]
- Stolarska, A.; Rucińska, T. Evaluation of Physical Characteristics and Sorption of Cement Mortars with Recycled Ceramic Aggregate. Materials 2021, 14, 7852. [Google Scholar] [CrossRef]
- Poyet, S.; Trentin, K.; Amblard, E. The Use of Sorption Balance for the Characterization of the Water Retention Curve of Cement-Based Materials. J. Adv. Concr. Technol. 2016, 14, 354–367. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V.; Chaussadent, T. Texture and Moisture Characterization of Hardened Cement Pastes and Concretes from Water Vapour Sorption Measurements. In The Modelling of Microstructure and Its Potential for Studying Transport Properties and Durability; Jennings, H., Kropp, J., Scrivener, K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1996; pp. 241–255. ISBN 978-94-015-8646-7. [Google Scholar]
- Wang, Z.; Li, K. Understanding Water Vapor Sorption Hysteresis and Scanning Behaviors of Hardened Cement Pastes: Experiments and Modeling. Cem. Concr. Res. 2024, 177, 107435. [Google Scholar] [CrossRef]
- Seng, B.; Magniont, C.; Lorente, S. Characterization of a Precast Hemp Concrete Block. Part II: Hygric Properties. J. Build. Eng. 2019, 24, 100579. [Google Scholar] [CrossRef]
- Garbalińska, H.; Bochenek, M. Experimentelle Ermittlung der Desorptionsisothermen von Porenbeton unterschiedlicher Dichte mit der Standardmethode SSS und mit der DVS-Technik. Bauphysik 2017, 39, 191–196. [Google Scholar] [CrossRef]
- Wang, J.; Yio, M.H.N.; Zhou, T.; Wong, H.S.; Davie, C.T.; Masoero, E. Water Sorption Isotherms and Hysteresis of Cement Paste at Moderately High Temperature, up to 80 °C. Cem. Concr. Res. 2023, 165, 107076. [Google Scholar] [CrossRef]
- DVS Intrinsic PLUS Brochure. Available online: https://surfacemeasurementsystems.com/product/dvs-intrinsic-plus/ (accessed on 9 October 2024).

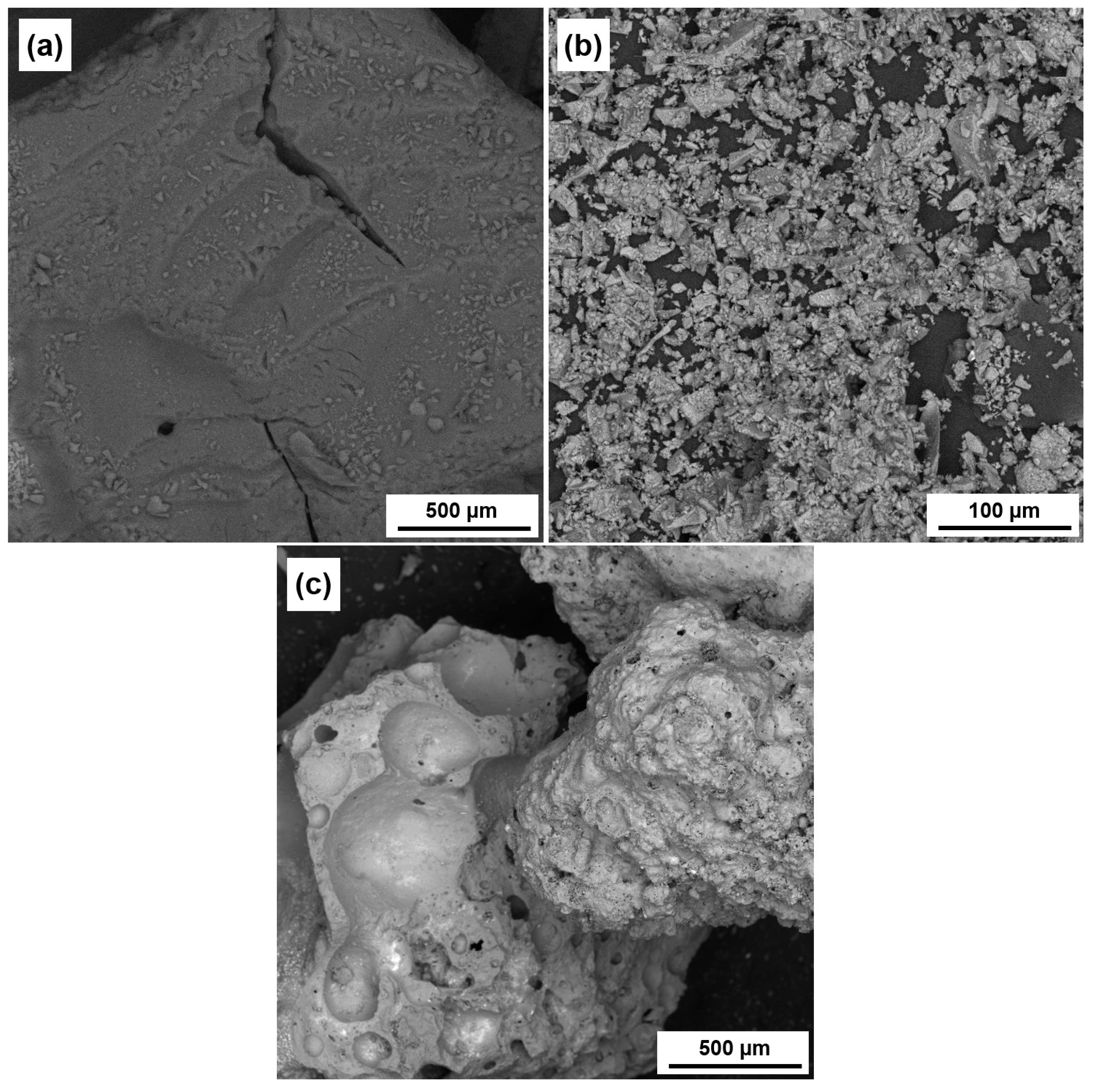
| Chemical Element Content, wt.% by EDS Analysis | |||||
|---|---|---|---|---|---|
| Silica Aerogel | GGBFS | Fly Ash-Based Aggregate | |||
| Oxygen | 52.27 | Oxygen | 31.96 | Oxygen | 52.06 |
| Silicon | 36.48 | Silicon | 11.40 | Silicon | 17.53 |
| Carbon | 11.25 | Carbon | 10.01 | Carbon | 6.54 |
| Calcium | 37.35 | Calcium | 8.44 | ||
| Aluminum | 3.07 | Aluminum | 6.95 | ||
| Potassium | 0.66 | Potassium | 3.26 | ||
| Magnesium | 2.85 | Magnesium | 1.56 | ||
| Iron | 1.21 | Iron | 1.53 | ||
| Sulfur | 0.60 | Sodium | 1.38 | ||
| Titanium | 0.75 | ||||
| Measured RH [%] | R0 w [%] | Measured RH [%] | R1 w [%] | Measured RH [%] | R2 w [%] | Measured RH [%] | R3 w [%] |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.000 | 0.00 | 0.000 | 0.00 | 0.000 | 0.0 | 0.000 |
| 9.34 | 0.295 | 9.56 | 0.532 | 9.49 | 0.726 | 9.5 | 0.737 |
| 20.14 | 0.663 | 20.38 | 1.281 | 20.21 | 1.626 | 20.4 | 1.714 |
| 30.27 | 1.234 | 30.55 | 2.136 | 30.28 | 2.649 | 30.6 | 2.828 |
| 40.15 | 1.876 | 40.59 | 3.140 | 40.41 | 3.840 | 40.6 | 4.113 |
| 49.95 | 2.666 | 50.43 | 4.390 | 50.60 | 5.243 | 50.5 | 5.556 |
| 59.47 | 3.677 | 59.98 | 5.871 | 59.98 | 6.686 | 60.2 | 7.111 |
| 68.91 | 4.931 | 69.52 | 7.484 | 70.23 | 8.239 | 70.0 | 8.772 |
| 78.23 | 6.463 | 78.78 | 9.170 | 80.08 | 9.959 | 79.4 | 10.445 |
| 87.61 | 8.448 | 88.19 | 11.079 | 89.74 | 12.033 | 88.9 | 12.600 |
| 94.00 | 12.703 | 94.59 | 13.712 | 94.77 | 15.470 | 95.5 | 16.211 |
| Measured RH [%] | R0 w [%] | Measured RH [%] | R1 w [%] | Measured RH [%] | R2 w [%] | Measured RH [%] | R3 w [%] |
|---|---|---|---|---|---|---|---|
| 0.00 | 1.798 | 0.00 | 2.228 | 0.00 | 0.948 | 0.0 | 1.492 |
| 9.49 | 3.638 | 9.66 | 4.327 | 9.47 | 3.141 | 9.9 | 3.824 |
| 20.30 | 5.370 | 20.53 | 6.385 | 20.33 | 5.223 | 20.9 | 6.070 |
| 30.44 | 7.238 | 30.72 | 8.644 | 30.49 | 7.762 | 31.2 | 8.728 |
| 40.48 | 8.387 | 40.84 | 9.974 | 40.53 | 9.240 | 41.3 | 10.454 |
| 50.24 | 8.800 | 50.65 | 10.420 | 50.40 | 9.691 | 51.4 | 11.048 |
| 59.87 | 9.507 | 60.22 | 10.866 | 59.93 | 10.177 | 61.0 | 11.611 |
| 69.31 | 9.794 | 69.71 | 11.391 | 69.49 | 10.910 | 70.6 | 12.221 |
| 78.59 | 10.160 | 78.99 | 11.947 | 78.92 | 11.841 | 79.9 | 12.950 |
| 88.06 | 11.521 | 88.30 | 12.803 | 88.44 | 13.773 | 89.3 | 14.411 |
| 94.00 | 12.703 | 94.59 | 13.712 | 94.77 | 15.470 | 95.5 | 16.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbalińska, H.; Stolarska, A.; Strzałkowski, J.; Ślosarczyk, A. Sorption and Desorption Isotherms of Lightweight Alkali-Activated Materials Modified with Silica Aerogel. Materials 2025, 18, 1338. https://doi.org/10.3390/ma18061338
Garbalińska H, Stolarska A, Strzałkowski J, Ślosarczyk A. Sorption and Desorption Isotherms of Lightweight Alkali-Activated Materials Modified with Silica Aerogel. Materials. 2025; 18(6):1338. https://doi.org/10.3390/ma18061338
Chicago/Turabian StyleGarbalińska, Halina, Agata Stolarska, Jarosław Strzałkowski, and Agnieszka Ślosarczyk. 2025. "Sorption and Desorption Isotherms of Lightweight Alkali-Activated Materials Modified with Silica Aerogel" Materials 18, no. 6: 1338. https://doi.org/10.3390/ma18061338
APA StyleGarbalińska, H., Stolarska, A., Strzałkowski, J., & Ślosarczyk, A. (2025). Sorption and Desorption Isotherms of Lightweight Alkali-Activated Materials Modified with Silica Aerogel. Materials, 18(6), 1338. https://doi.org/10.3390/ma18061338






