Abstract
This study investigated the viscoelastic behavior and structural evolution of silicate glasses with the molar composition 70SiO2·(30 − x) Na2O·xK2O, where the molar ratio r = x/30 varied between 0, 0.25, 0.5, and 0.75. A notable “V”-shaped trend in relaxation activation energy (ΔHGt) was observed, with the energy reaching a minimum of 163.14 kJ/mol at r = 0.5. This trend exhibited a synergistic mixed alkali effect that significantly affected the viscoelastic properties of the glass. Raman spectroscopy analysis revealed dynamic structural reorganization within the [SiO4] network, transitioning from Q4 to Q3 for r < 0.5 and reverting to Q4 for r > 0.5 as the K2O content increased. These structural transformations provide atomic-scale evidence for the observed viscoelastic behavior. The findings offer critical insights into the mixed alkali effect on viscoelasticity, establishing a theoretical foundation for optimizing clad materials in optical fiber imaging arrays.
1. Introduction
Viscoelasticity refers to the deformation behavior of materials that exhibit both viscosity and elasticity under external forces. The viscoelastic behavior of glass during the thermal forming process directly affects the forming precision and optical performance of high-precision glass components [1,2]. Optical fiber imaging arrays are two-dimensional imaging materials fabricated from three distinct glasses (optical fiber core glass, optical fiber clad glass, and light-absorbing glass) via thermoforming processes—including stretching, fusion pressing, and twisting—at the forming temperature. During high-temperature forming, the viscoelasticity of these three glasses and the compatibility between their viscoelastic properties critically affect the imaging quality, manifesting as geometric distortions and dark spots, which significantly reduce the product qualification rate and result in substantial economic losses [3].
From a microscopic perspective, an optical fiber imaging array consists of millions of glass fibers with diameters ranging from 4 to 10 μm, with each fiber serving as an individual imaging unit. In recent years, with the increasing demand for high-resolution and high-contrast imaging in advanced optoelectronic detection equipment, the performance requirements for optical fiber imaging arrays have continuously increased. However, internal defects in the arrays remain a significant factor limiting the imaging quality for detection and identification [4,5,6,7,8]. The optical fiber clad glass, which is composed of a multi-component silicate glass, can confine light within the fiber core through total internal reflection, ensuring that light signals do not leak during transmission [9]. During high-temperature forming, the clad glass undergoes deformation, including elongation, void filling, and twisting adjustments, influenced by the stretching, pressing, and twisting forces. These deformations must match with the core glass to ensure array formation and maintain total internal reflection, while also significantly affecting the distribution and extent of internal defects in the array.
The deformation of multicomponent silicate glass is inherently time- and stress-dependent, exhibiting nonlinear coupling between elastic recovery and viscous flow. After the external force is removed, the deformation cannot fully recover, and so the relationship between internal stress and deformation is not one-to-one, showing viscoelastic characteristics. Therefore, understanding the viscoelasticity during the fabrication process of fiber optic imaging arrays is crucial to avoid defects. However, studying the viscoelasticity of multi-component silicate glass is a challenging task due to the complex coupling of multiple components and factors, as well as the uncertainty between the elastic and viscous behaviors of the glass. Typically, researchers reduce interference by limiting the number of oxides introduced or setting fixed boundary conditions, thus enabling a better study of the relationship between composition and viscoelastic properties. Currently, the research in this area is still limited.
Previous studies have investigated the viscoelasticity of multi-component silicate glasses. For example, Eisenberg and Takahashi [10] explored the viscoelasticity of silicate glasses and found that the stress relaxation activation energy above the glass transition temperature was likely related to the Si-O bond energy. Kitamura [11] studied the viscoelasticity of Li+ and Na+ binary alkali silicate glasses, analyzing the effect of Li+ and Na+ contents on viscoelasticity using parallel-plate viscosity methods and dynamic viscoelastic measurements. Zhang et al. [12] proposed a minimum uniaxial creep test (MUCT) based on TMA, and validated the accuracy of the results using finite element simulations. Research on phosphate glasses [13], chalcogenide glasses [14], and borate glasses [15] has also provided valuable insights for the study of silicate glass viscoelasticity.
It can be seen that the existing research mainly focuses on the viscoelasticity of individual ions (such as Li+ and Na+), with limited studies on the effect of mixed alkali ions on viscoelasticity. The mixed alkali effect is an important physicochemical reaction involving the complex influence of multiple alkali metal ions on the structure and properties of glass. Previous studies have shown that the mixed alkali effect significantly impacts glass properties such as the coefficient of thermal expansion, glass transition temperature, and electrical conductivity [16].
In this study, Na⁺- and K⁺-doped 70 SiO2·(30 − x) Na2O·xK2O silicate glasses were prepared via the melting–quenching method to systematically investigate the mixed alkali effect on their viscoelastic behavior and structural evolution. The key parameters, including the glass transition temperature (Tg) and dilatometric softening temperature (Td), were measured to design uniaxial compression creep experiments, which elucidated the impact of Na⁺/K⁺ substitution on viscoelasticity. In addition, Raman spectroscopy was employed to analyze the compositional effects on the glass network structure, establishing correlations between the composition and viscoelastic properties. These findings provide theoretical foundations and practical guidelines for optimizing the material design and fabrication processes of optical fiber imaging arrays.
2. Materials and Methods
2.1. Preparation of Silicate Glass
A series of silicate glasses with the molar composition 70SiO2·(30 − x) Na2O·xK2O (where x = 0, 7.5, 15, 22.5, corresponding to the molar ratio r = [K2O]/([Na2O] + [K2O] = 0, 0.25, 0.5, 0.75) were prepared via the conventional melt–quenching method. Analytical purity grades of SiO2 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China)., Na2CO3 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and K2CO3 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were used as starting materials, with a total weight of 400 g. To ensure homogeneity, the weighed raw materials were placed in a zirconia ball-milling jar with zirconia grinding balls and mixed thoroughly for 5 h using an omnidirectional planetary ball mill (QXQM-2, Changsha Tianchuang Powder Technology Co., Ltd., Changsha, China). The homogenized mixture was transferred to a preheated platinum–rhodium alloy crucible at 1300 °C, held for 30 min, then heated to 1520 °C at 10 °C/min and maintained for 2 h to homogenize the melt and eliminate bubbles (as shown in Figure 1). Subsequently, the temperature was reduced to 1520 °C at 10 °C/min, held for 1 h, and the molten glass was cast into preheated graphite molds. The as-cast glasses were annealed at their respective glass transition temperatures for 3 h to relieve internal stresses, followed by furnace cooling. The annealed glasses were cut and polished to the required dimensions for further characterization.
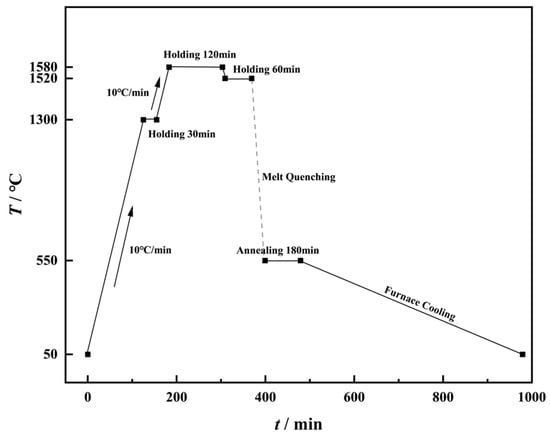
Figure 1.
Schematic diagram of the glass making process.
2.2. Sample Characterization and Testing
The glass transition temperature (Tg), dilatometric softening temperature (Td), and coefficient of thermal expansion (CTE, averaged over 30–300 °C) were measured using a thermal dilatometer (Netzsch DIL402 Classic, Selb, Germany) with a displacement resolution of 2 nm and a temperature accuracy of 0.1 K. The CTE was calculated using Equation (1):
where α is the CTE (1/°C or 1/K), L and L0 are the sample lengths at the test and initial temperatures, and T and T0 are the corresponding temperatures. The measurements were conducted under a nitrogen atmosphere at a heating rate of 5 K/min using cylindrical samples (φ6 × 50 mm).
The softening point temperature (Tf, corresponding to a viscosity of 106.6 Pa·s) was determined using a high-temperature viscometer (Orton PPV-1000, Westerville, OH, USA) following ASTM C1351M. Cylindrical samples (φ6 × 6 mm) were compressed between parallel plates under controlled heating. The viscosity (η) was calculated using Equation (2) [17]:
where m is the applied load (kg), g is gravitational acceleration (m/s2), t is time (s), h is the instantaneous sample height (m), and V is the sample volume (m3).
Uniaxial compressive creep tests were performed using a thermomechanical analyzer (TMA, NETZSCH 402F1 Hyperion, Selb, Germany) with a displacement resolution of 0.125 nm and load resolution of 0.01 mN. Polished cylindrical samples (φ5 × 5 mm) were heated to target temperatures under nitrogen at 5 °C/min, equilibrated for 30 min, and subjected to a 2 N constant load for 90 min while recording their time-dependent deformation.
The nonlinear mechanical behavior of viscoelastic materials arises from their time-dependent stress–strain relationships, primarily manifested as two phenomena: creep (time-dependent strain accumulation under constant stress) and stress relaxation (time-dependent stress decay under constant strain). To quantify these behaviors, creep compliance J(t) = ε(t)/σ0 (strain response to constant stress σ0) and the shear relaxation modulus G(t) = σ(t)/ε0 (stress decay under constant strain E0) were introduced. To analyze the deformation accurately, true stress (σtrue) and true strain (εtrue) were calculated using Equations (3) and (4):
where F is the instantaneous load, A0 is the initial cross-sectional area, and L0 and ΔL are the initial height and deformation, respectively. Following compression creep tests, the creep compliance J(t) was obtained. Based on linear viscoelastic theory and referencing the methodologies of Arai and Zhang et al. [18,19], the creep curves J(t) were fitted using a four-element Kelvin model and calculated using Equation (5):
In this section, E0 represents the initial Young modulus, while Ei and τi characterize the elastic modulus and relaxation time constant of the i-th Kelvin unit, respectively. Finally, the shear relaxation modulus G(t) was derived via Laplace transform, where G(t) was defined as the stress relaxation under constant strain.
Raman spectra were acquired at room temperature using a Horiba HR Evolution spectrometer (Irvine, CA, USA) with a 532 nm semiconductor green laser (100 mW power) in the backscattering geometry. The spectra (200–1600 cm−1) were collected with a 1 cm−1 resolution, 15 s acquisition time, and were averaged over 200 scans to enhance the signal-to-noise ratio.
3. Results and Discussion
3.1. Viscoelastic Analysis
3.1.1. Thermodynamic Analysis
The state and mechanical properties of glass evolve with temperature. Below the glass transition temperature (Tg), glass behaves predominantly as an elastic solid, while above the softening temperature (Tf), it transitions to a viscous fluid. Between Tg and Tf, glass exhibits complex viscoelastic behavior [20,21]. Thus, identifying its characteristic temperatures is critical for understanding its viscoelasticity. Figure 2a displays the original thermal expansion curve, with the inset illustrating the determination of Tg and the dilatometric softening temperature (Td). Figure 2b shows the composition dependence of Tg, Td, and the coefficient of thermal expansion (CTE) as a function of r (the molar ratio of K2O/(Na2O K2O)). As r increases (i.e., K2O progressively replaces Na2O), Tg and Td exhibit a “V”-shaped trend—initially decreasing, reaching minima at r = 0.5, then increasing. Conversely, CTE follows an inverse trend, peaking at r = 0.5 with values of 429.2 °C (Tg), 489.3 °C (Td), and 158.63 × 10−7/°C (CTE), which is consistent with prior studies [22]. Similarly, Tf, measured via parallel-plate viscometry (Figure 2c), shows a “V”-shaped dependence, reaching a minimum of 599.2 °C at r = 0.5 (Figure 2d). Notably, the composition-dependent trends of Tg, Td, CTE, and Tf are well fitted by quadratic polynomial equations (R2 > 0.99), indicating a synergistic dual-variable regulation mechanism.
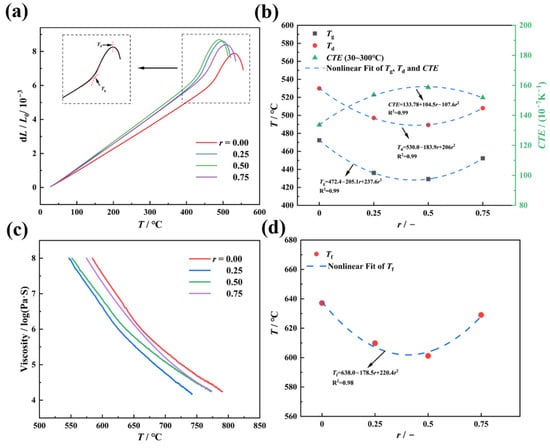
Figure 2.
Thermal expansion and mid-temperature viscosity tests were conducted for 70SiO2 (30 − x) Na2O·xK2O glasses with varying molar ratios r, where r is equal to the molar ratio of [K2O]/([Na2O] + [K2O]). (a) Thermal expansion curves, with the inset illustrating the method of determining Tg and Td from the expansion data; (b) variation in Tg, Td, and the coefficient of thermal expansion CTE as a function of r; (c) mid-temperature viscosity curves; and (d) variation in Tf with r.
These phenomena can be attributed to dynamic structural changes in the glass network. At low r values, the partial substitution of Na+ by K+ disrupts the Na+-dominated network topology, increasing local structural strain and mismatch. This enhances network flexibility while introducing heterogeneous ion migration paths due to the “site memory effect” of mixed alkali ions. Specifically, alkali ions occupy sites with differing chemical environments and energy states, creating kinetic barriers for ion migration. Consequently, K+ incorporation disrupts uniform ion distribution, reducing Tg, Td, and Tf, while increasing CTE [23]. At r = 0.5, structural strain and mismatch are maximized, corresponding to the extremal values of Tg Td, Tf, and CTE. As the K+ content further increases, it becomes the dominant network-modifying ion, gradually restoring structural homogeneity and reducing migration barriers. This leads to rising Tg, Td, and Tf, alongside declining CTE [24,25]. Within the Tg–Tf range, the mechanical response of glass is governed by the interplay between network rearrangement and ion migration, resulting in intricate viscoelastic behavior. A detailed understanding of viscoelastic constitutive relationships in this regime is essential for optimizing the thermoforming processes and enhancing thermal stability.
3.1.2. Creep and Stress Relaxation Analysis
Figure 3a–d show the uniaxial compressive creep deformation curves of the glasses near their Td. As experimental temperature increases, the deformation magnitude under constant load grows, while the deformation rate decreases over time. The derived creep compliance J(t) is shown in Figure 3e–h. At lower experimental temperatures, J(t) exhibits linear growth, transitioning to nonlinear behavior at elevated temperatures.
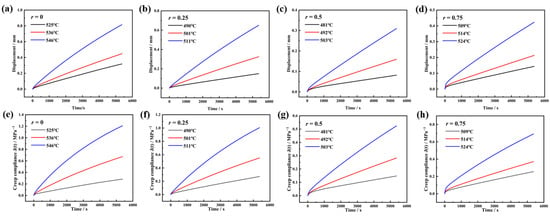
Figure 3.
The history of height displacements and the calculated creep compliance curves of 70SiO2·(30 − x) Na2O·xK2O glass with different molar ratios r are measured, where r is equal to the molar ratio of [K2O]/([Na2O] + [K2O]). (a–d) Displacement history. (e–h) Creep compliance curves.
Figure 4a–d present the shear relaxation modulus G(t) calculated via Laplace transform for the glass systems near their dilatometric softening temperature Td. The G(t) curves exhibit a rapid decay to near-zero equilibrium values within extremely short time scales under constant stress at elevated temperatures. All G(t) curves at different temperatures can be superimposed by shifting along the logarithmic time axis, confirming that the system obeys the time–temperature superposition principle (TTSP) [26]. Specifically, the relaxation rate varies with temperature according to the TTSP. With an increasing creep test temperature, all G(t) curves shift toward shorter timescales, indicating accelerated relaxation rates. The distance of this shift is referred to as the shift factor lgαT, which obeys the Arrhenius-type Narayanaswamy equation [27], as shown in Equation (6):
where R is the molar gas constant, 8.314 J·K−1·mol−1, and T0 is the initial temperature, which in this study refers to the glass’s dilatometric softening temperature Td, measured in Kelvin (K). ΔHGt represents the relaxation activation energy, which quantitatively characterizes the minimum energy required to overcome the barrier for network rearrangement.
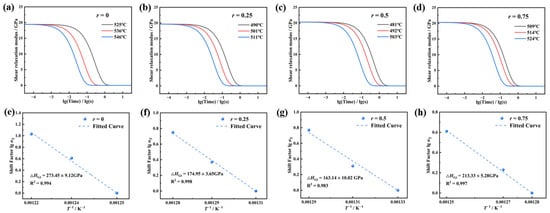
Figure 4.
The relationship between the shear relaxation modulus G(t), the displacement factor lg αT, and the reciprocal of temperature T of 70SiO2·(30 − x) Na2O·xK2O glass with different molar ratios r, where r is equal to the molar ratio of [K2O]/([Na2O] + [K2O]). (a–d) Shear relaxation modulus G(t) curve. (e–h) Displacement factor lgαT and the reciprocal of temperature T.
Figure 4e–h show the relationship between the shift factor lgαT and the reciprocal of temperature for r = 0, 0.25, 0.5, and 0.75, and the corresponding ΔHGt values are obtained by fitting with Equation (4). When r = 0, ΔHGt is approximately 273.45 kJ/mol, which is in close agreement with the dissociation energy of the Na-O bond (270 ± 4 kJ/mol [28]), indicating that the Na+ migration dominates the relaxation process in this composition. As K+ gradually replaces Na+, ΔHGt exhibits a trend of initially decreasing and then increasing, reaching a minimum of 163.14 kJ/mol at r = 0.5, which is significantly lower than in the single alkali system. When r = 0.75, it increases to 213.33 kJ/mol, which is slightly lower than the dissociation energy of the K-O bond (271.5 ± 12.6 kJ/mol [28]). This trend is highly consistent with the “V”-shaped variation in Tg, Td, and Tf, and also reveals that the mixed alkali effect significantly alters the network rearrangement barrier by modulating the ion migration synergy. Further analysis with chemical bond energies shows that the Si–O bond dissociation energy (798 kJ/mol [29]) is much higher than that of the R–O bonds (R = Na/K), and that the ionic bond characteristics of R–O bonds allow for greater bond length and bond angle tolerance. In contrast, the covalent nature of Si–O bonds make them more rigid. Therefore, at high temperatures, the viscoelastic relaxation process of glass is dominated by R–O bonds. By controlling the r value to optimize ΔHGt, the gradient matching of the viscoelastic behavior of the core and clad glasses during hot forming can be optimized, providing a theoretical foundation for material design in the development of ultra-low-defect fiber optic imaging arrays.
3.2. Structural Origins of Viscoelastic Behavior
To elucidate the structural evolution induced by Na2O substitution with K2O, the Raman spectra of the 70SiO2·(30 − x) Na2O·xK2O glasses with varying r are analyzed (Figure 5a). Two dominant vibrational bands are observed: a low-frequency band at 400–600 cm⁻1 and a high-frequency band at 1000–1200 cm⁻1. The 400–600 cm⁻1 band corresponds to the symmetric stretching vibrations of Si–O bonds, which can be deconvoluted into two peaks at 520 cm⁻1 (D1, planar four-membered rings) and 606 cm⁻1 (D2, planar three-membered rings) [30,31,32]. Notably, the D2 peak intensity increases with the K⁺ content. The high-frequency band (1000–1200 cm⁻1) arises from asymmetric Si–O stretching vibrations and is highly sensitive to the distribution of [SiO4] structural units [22,33,34,35]. Based on the number of bridging oxygens (BOs) per [SiO4] unit, the structural units are classified as Q1–Q4, corresponding to peaks at −870 cm⁻1 (Q1), −950 cm⁻1 (Q2), −1100 cm⁻1 (Q3), and −1150 cm⁻1 (Q4). The 1050 cm⁻1 peak reflects the contributions from both Q3 and Q4. The wavenumbers of the Raman spectra corresponding to the glass structural groups are shown in Table 1. For the 70SiO2·(30 − x) Na2O·xK2O glasses, the network is dominated by Q3 and Q4 units. Figure 5b–e present the deconvolution results of the high-frequency band using Gaussian fitting, and Figure 6 summarizes the relative area ratios of these structural units as a function of r.
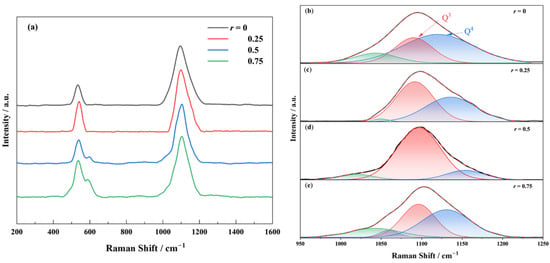
Figure 5.
The Raman spectra of 70SiO2·(30 − x) Na2O·xK2O glass with different molar ratios r and the diagram of the results of Gaussian function fitting of the characteristic peak at 950–1250 cm−1 are obtained, where r is equal to the molar ratio of [K2O]/([Na2O] + [K2O]). (a) Raman spectra; and (b–e) r = 0, 0.25, 0.5, 0.75, respectively, in which the black line is the original Raman spectrum, the red region (1100 cm−1) corresponds to the Si-O stretching vibration in Q3 unit, the blue region (1150 cm−1) corresponds to the Si-O stretching vibration in Q4 unit, and the green region (1050 cm−1) is determined by the Si-O stretching vibration of Q3 and Q4.

Table 1.
The wavenumbers of the Raman spectra and the corresponding glass structural groups.
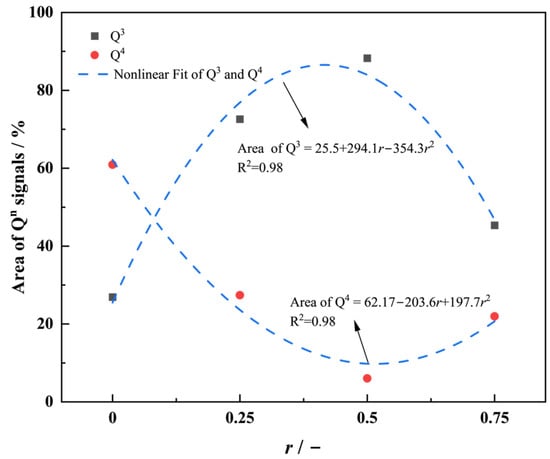
Figure 6.
Q3 and Q4 unit area ratio versus r curve.
As r increases, the glass network undergoes significant restructuring. At a low K2O content, the incorporation of K⁺ (larger ionic radius, lower field strength) disrupts the network by destabilizing Si–O–Si bond angles, breaking BO bonds in Q4 units and converting them into Q3 units, thereby decreasing the degree of network polymerization. At r = 0.5, the synergistic perturbation from mixed alkali ions maximizes network depolymerization, with Q3 reaching its highest relative proportion (88%). By further increasing the K⁺ content, K⁺ will act as a dominant network modifier, occupying non-bridging oxygen (NBO) sites due to its lower field strength. This promotes BO reformation, increasing Q4 content and enhancing the degree of network polymerization, stability, and the mechanical strength of the network.
This structural reorganization mechanism explains the nonlinear variation in viscoelastic parameters (e.g., ΔHGt). By precisely tuning r, the network topology of clad glass can be optimized to balance formability and dimensional stability. Near r = 0.5, the moderately depolymerized Q3-dominated network ensures sufficient rheological flow during thermoforming while maintaining structural integrity upon cooling, enabling the synergistic control of defects in fiber optic imaging arrays.
4. Conclusions
This study systematically investigates the effects of K2O substitution for Na2O on the viscoelastic behavior of silicate glasses by tuning the molar ratio r = [K2O]/([Na2O] + [K2O] (0, 0.25, 0.5, 0.75). The glass transition temperature (Tg), dilatometric softening temperature (Td), and softening point temperature (Tf) exhibit a distinct “V”-shaped dependence on r, reaching minima at r = 0.5: Tg = 429.2 °C, Td = 489.3 °C, Tf = 599.2 °C. The coefficient of thermal expansion (CTE) follows an inverse trend, peaking at r = 0.5 (158.63 × 10⁻7/°C). This nonlinear evolution highlights the significant regulatory role of the mixed alkali effect on the thermodynamic and mechanical properties of silicate glasses. The trend of ΔHGt follows the “V”-shaped behavior observed for Tg, Td, and Tf; initially decreasing and then increasing with the increasing r. The ΔHGt values are 273.45, 174.95, 163.14, and 213.33 kJ/mol, reaching a minimum at r = 0.5. Additionally, when r = 0, ΔHGt is approximately 273.45 kJ/mol, consistent with the Na-O bond dissociation energy ((270 ± 4) kJ/mol). As r increases, ΔHGt falls below the bond dissociation energies of the Na-O and K-O bonds, indicating that the mixed alkali effect reduces the energy barrier for ion migration and structural rearrangement. Raman spectroscopy further confirms that ΔHGt is governed by alkali–oxygen (R-O) bond dynamics rather than the rigid Si-O network. These results provide critical insights into the tailoring of the viscoelastic behavior of multi-alkali silicate glasses through compositional design, enabling optimized thermoforming processes for high-performance fiber optic imaging arrays. In future work, we plan to integrate the prepared sodium–potassium mixed silicate glasses with existing core and absorber glasses to fabricate an optical fiber imaging array. This will allow us to investigate the influence of viscoelastic compatibility among glass components on the performance of the optical fiber imaging array, thereby further enhancing its imaging quality and improving the overall performance of night vision and detection devices.
Author Contributions
Writing—original draft, Data curation, F.W.; Writing–review and editing, Methodology, Y.H.; Resources, Project administration, H.T.; Investigation, Formal analysis, P.J.; Visualization, Formal analysis, Z.X.; Funding acquisition, Supervision, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 52072357, 52172007), the Youth Talent Support Project of Beijing Association for Science and Technology (No. BYESS2024388), the Major Scientific and Technological Research Projects in the National Building Materials Industry of the China Building Materials Federation (No. 202201JBGS11-01), and the Key Core Technology Research Projects of China National Building Materials Group (No. 2023SJYL07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shu, C.; Yin, S.; Li, Y.; Mao, Z.; Guo, X.; Huang, S. High-precision molding simulation prediction of glass lens profile for a new lanthanide optical glass. Ceram. Int. 2022, 48, 15800–15810. [Google Scholar] [CrossRef]
- Riande, E.; Diaz-Calleja, R.; Prolongo, M.; Masegosa, R.; Salom, C. Polymer Viscoelasticity: Stress and Strain in Practice; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Jiao, P.; Jia, J.; Fu, Y.; Zhang, L.; Wang, Y.; Wang, J.; Zhou, Y.; Shi, P.; Zhao, R.; Huang, Y. Detection of blemish for fiber-optic imaging elements. Opt. Eng. 2020, 59, 053105. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S.S.; Li, Z.W. Technological advances in U.S. military low-light night vision head-mounted systems. Infrared 2022, 43, 12–19. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, N.J.; Zhu, S.Q.; Zhang, L.X.; Cai, W. Research progress of extreme ultraviolet detectors. Chin. J. Lasers 2024, 51, 0701008. (In Chinese) [Google Scholar] [CrossRef]
- Du, J. Challenges in molecular dynamics simulations of multicomponent oxide glasses. In Molecular Dynamics Simulations of Disordered Materials; Massobrio, C., Du, J., Bernasconi, M., Salmon, P.S., Eds.; Springer: Cham, Switzerland, 2015; pp. 157–180. [Google Scholar] [CrossRef]
- Ma, X.; Ren, H.; Liu, Y.; Wang, Z.; Liu, H.; Wang, S. Research progress of high-resolution polymer fiber optic faceplates. Proc. SPIE 2024, 13282, 8–13. [Google Scholar] [CrossRef]
- Jiao, P.; Huang, Y.; Wang, Y.; Wang, J.; Fu, Y.; Du, Y.; Zhang, L.; Zhao, Y.; Zhang, X.; Jia, J. Influencing mechanism of fiber deformation on mesh pattern noise in inverting-image fiber-optic arrays. Opt. Fiber Technol. 2023, 76, 103219. [Google Scholar] [CrossRef]
- Ma, X.X.; Ren, H.Y.; Qiao, H.; Wang, Z.Z.; Zhang, B.Q.; Wang, S.Z. Advanced preparation technology and imaging performance of polymer fiber optic faceplates. Acta Opt. Sin. 2024, 44, 2306003. (In Chinese) [Google Scholar] [CrossRef]
- Eisenberg, A.; Takahashi, K. Viscoelasticity of silicate polymers and its structural implications. J. Non-Cryst. Solids 1970, 3, 279–293. [Google Scholar] [CrossRef]
- Kitamura, N. Compression creep and dynamic viscoelastic studies of binary sodium and lithium silicate glasses around deformation temperature. J. Ceram. Soc. Jpn. 2017, 125, 721–727. [Google Scholar] [CrossRef]
- Zhang, Z.; Ispas, S.; Kob, W. Origin of the non-linear elastic behavior of silicate glasses. Acta Mater. 2021, 231, 117855. [Google Scholar] [CrossRef]
- Zheng, Q.; Mauro, J.C. Viscosity of glass-forming systems. J. Am. Ceram. Soc. 2017, 100, 6–25. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, H.; Deng, Y.; Yan, J.; Jiang, Y.; Chen, G.; Zu, Q.; Sun, L. A novel viscosity-temperature model of glass-forming liquids by modifying the Eyring viscosity equation. Appl. Sci. 2020, 10, 428. [Google Scholar] [CrossRef]
- Wang, L.; Gong, W.; Cao, X.W.; Yu, Y.H.; Juodkazis, S.; Chen, Q.D. Holographic laser fabrication of 3D artificial compound μ-eyes. Light Adv. Manuf. 2023, 4, 26. [Google Scholar] [CrossRef]
- Razum, M.; Marijan, S.; Filho, J.C.; Andrade, A.A.; Silva, A.C.A.; Dantas, N.O.; Pisk, J.; Šantić, A.; Pavić, L. Mixed-alkali effect and correlation to glass structure in ionically conductive P2O5-Al2O3-Na2O-K2O glass system. Coatings 2023, 13, 185. [Google Scholar] [CrossRef]
- Kitamura, N.; Suetsugu, T.; Nakano, K.; Higashi, N.; Yamaguro, T. Viscoelastic study of the creep behavior of GeS2-SbS3/2-SnS and GaS3/2-SbS3/2-SnS glasses around their deformation temperatures. J. Non-Cryst. Solids 2019, 517, 44–50. [Google Scholar] [CrossRef]
- Arai, M.; Kato, Y.; Kodera, T. Characterization of the thermo-viscoelastic property of glass and numerical simulation of the press molding of glass lens. J. Therm. Stress. 2009, 32, 1235–1255. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, S.; Liang, R.; Luo, H.; Xiao, H.; Yuan, N. New testing and calculation method for determination of viscoelasticity of optical glass. Opt. Express 2020, 28, 626–638. [Google Scholar] [CrossRef]
- Liu, G.; Fang, F. Determination accuracy enhancement of viscoelastic constitutive model of optical glass. Mater. Des. 2023, 236, 112513. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Le Losq, C.; Neuville, D.R. Effect of the Na/K mixing on the structure and the rheology of tectosilicate silica-rich melts. Chem. Geol. 2013, 346, 57–71. [Google Scholar] [CrossRef]
- Habasaki, J.; Okada, I.; Hiwatari, Y. MD study of the mixed alkali effect in a lithium–potassium metasilicate glass. J. Non-Cryst. Solids 1995, 183, 12–21. [Google Scholar] [CrossRef]
- Wilkinson, C.J.; Potter, A.R.; Welch, R.S.; Bragatto, C.; Zheng, Q.; Bauchy, M.; Affatigato, M.; Feller, S.A.; Mauro, J.C. Topological origins of the mixed alkali effect in glass. J. Phys. Chem. B 2019, 123, 7482–7489. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Takimoto, Y.; Hijiya, H.; Taniguchi, T.; Urata, S.; Inaba, S.; Fujita, S.; Obayashi, I.; Hiraoka, Y.; Kohara, S. Origin of the mixed alkali effect in silicate glass. NPG Asia Mater. 2019, 11, 75. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, S.; Huang, B.; Fang, D.; Liu, K. Accurate and efficient determination of glass thermo-viscoelastic properties using creep compliance master curve construction approach. J. Non-Cryst. Solids 2024, 631, 122930. [Google Scholar] [CrossRef]
- Narayanaswamy, O.S. A model of structural relaxation in glass. J. Am. Ceram. Soc. 1971, 54, 491–498. [Google Scholar] [CrossRef]
- McEwan, M.J.; Phillips, L.F. Dissociation energy of NaO2. Trans. Faraday Soc. 1966, 62, 1717–1720. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Henderson, G.S.; Bancroft, G.M.; Ho, R. Experimental evidence for Na coordination to bridging oxygen in Na-silicate glasses: Implications for spectroscopic studies and for the modified random network model. J. Non-Cryst. Solids 2015, 409, 139–148. [Google Scholar] [CrossRef]
- Schmitz, S.; Beltrán, A.M.; Cresswell, M.; Boccaccini, A.R. A structural comparison of ordered and non-ordered ion doped silicate bioactive glasses. Materials 2020, 13, 992. [Google Scholar] [CrossRef]
- Wang, F.; Dong, H.H.; Guo, M.T.; Shao, C.Y.; Yu, C.L.; Hu, L.L. Optical spectra and structural characteristics of Yb-doped silica glass. J. Chin. Ceram. Soc. 2022, 50, 991–1005. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, X.Z.; Han, T.; Yang, P.H.; Zu, C.K. Research progress of lithium-aluminosilicate glass. Bull. Chin. Ceram. Soc. 2022, 41, 2143–2152+2159. (In Chinese) [Google Scholar] [CrossRef]
- Kumar Yadav, A.; Singh, P. A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Le Losq, C.; Neuville, D.R. Molecular structure, configurational entropy and viscosity of silicate melts: Link through the Adam and Gibbs theory of viscous flow. J. Non-Cryst. Solids 2017, 463, 175–188. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Singh, K. Modification of silicate structural units by K2O for enhancing automobile windshield glass properties. J. Phys. Chem. Solids 2023, 181, 111523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).