Ampoule Synthesis of Na-Doped Complex Bromide Cs2AgBiBr6 with Double Perovskite Structure
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis Technique
2.2. Analysis Methods
3. Results and Discussion
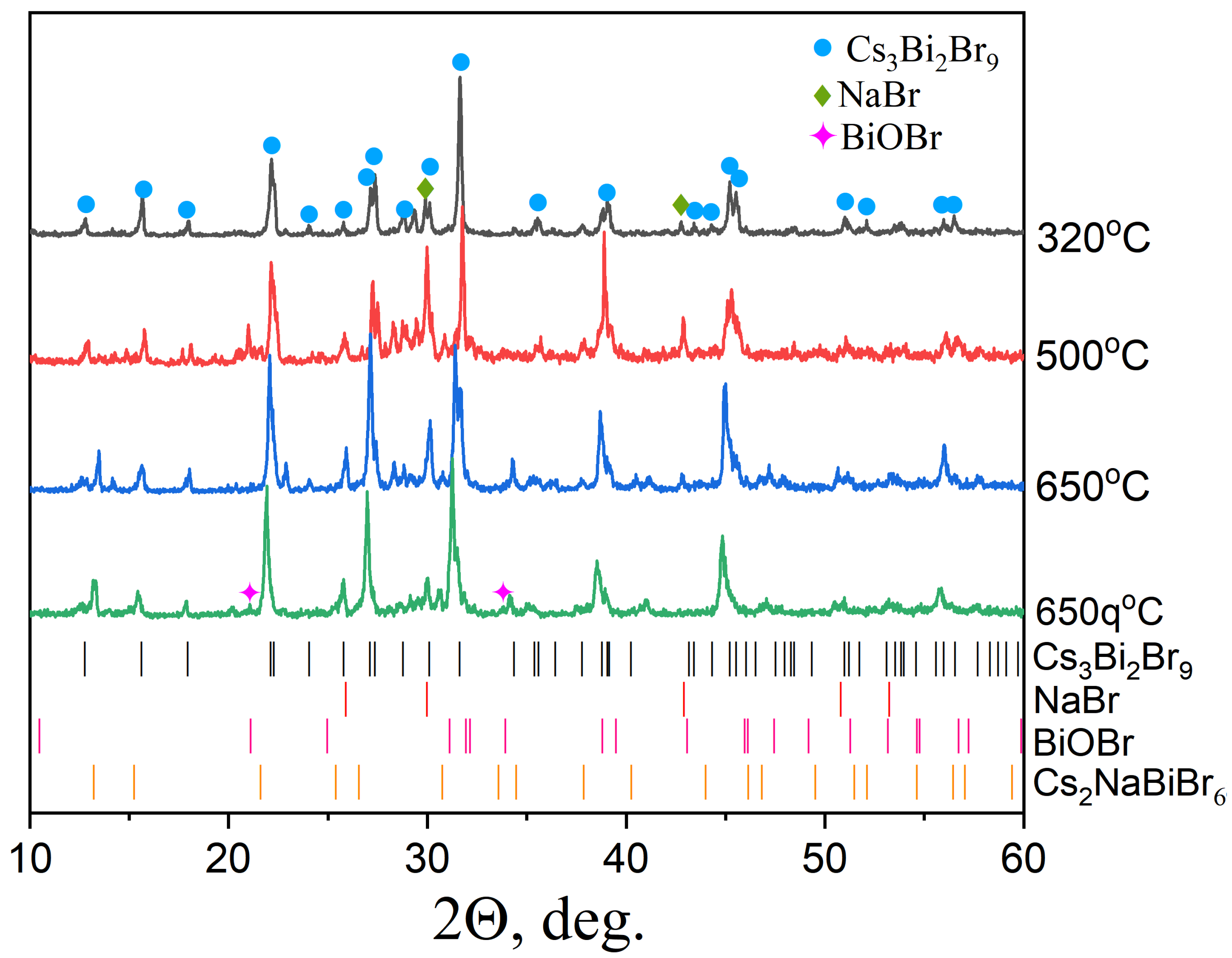
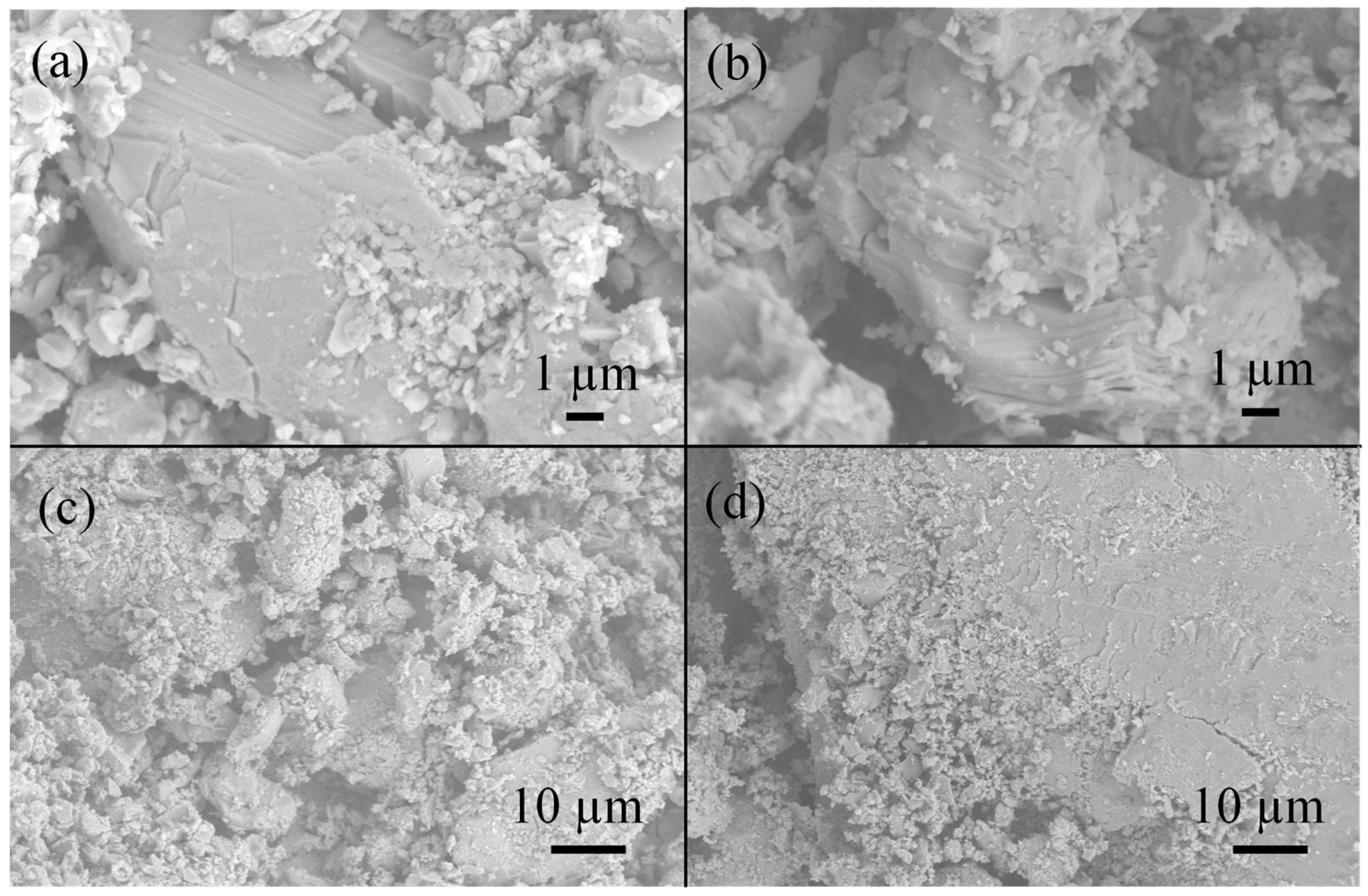
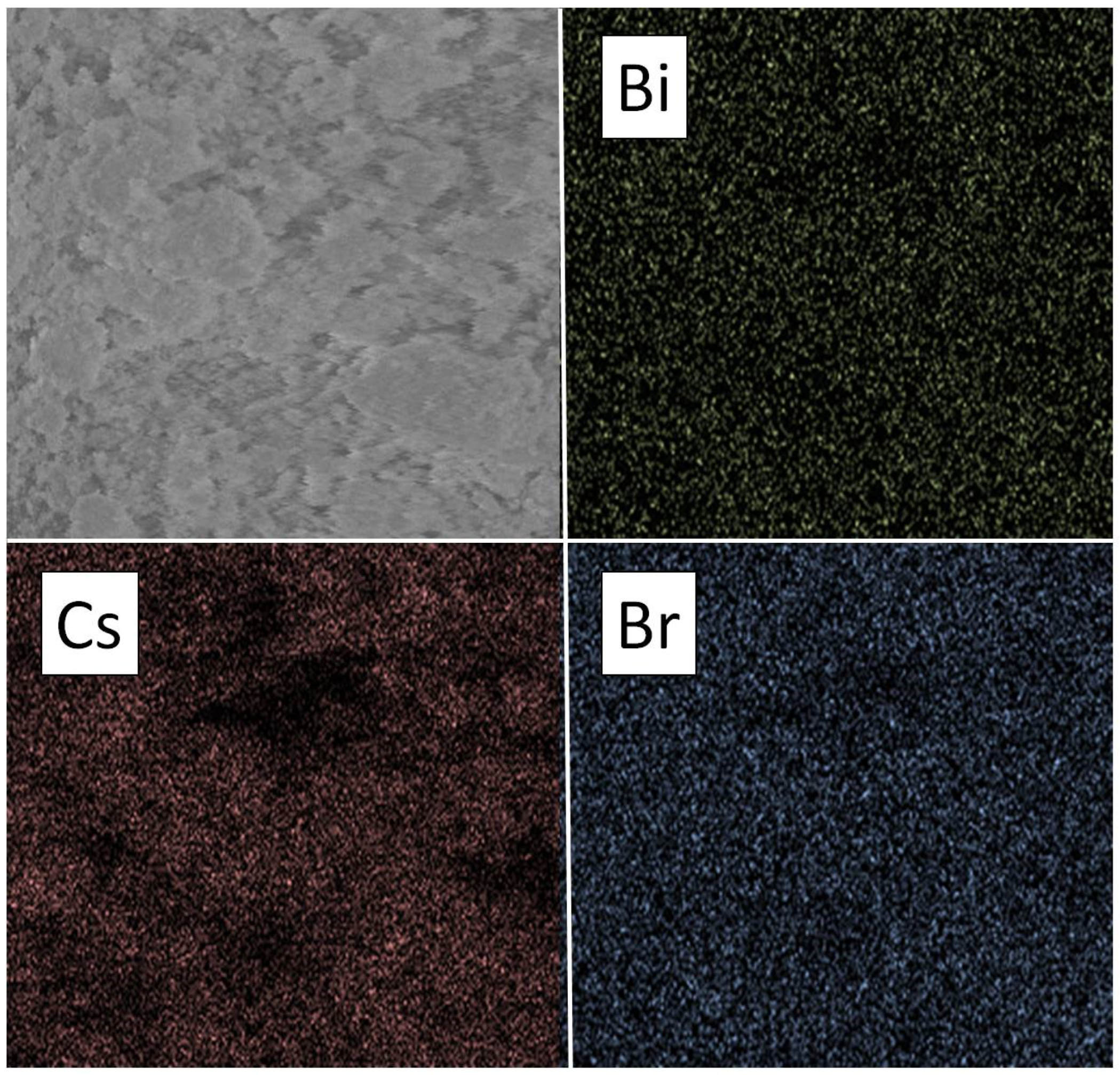
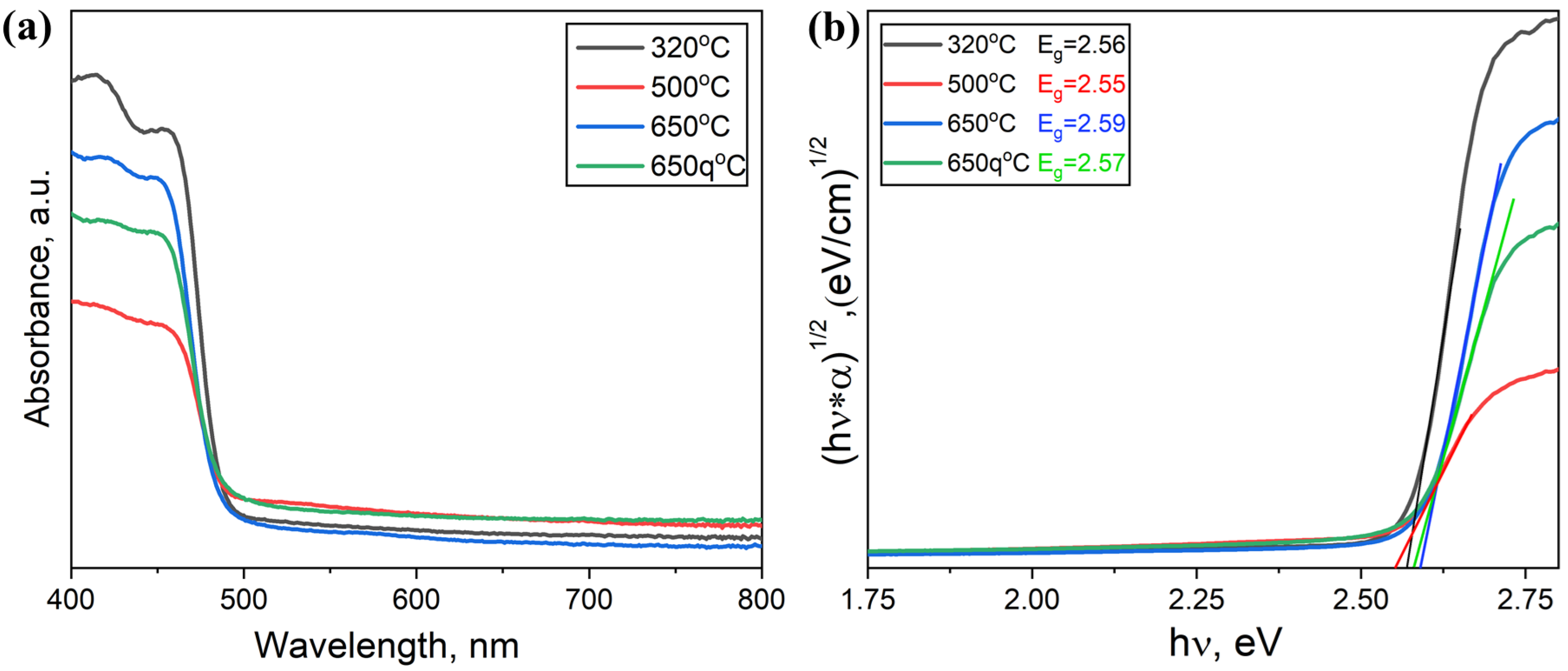
3.1. Samples of General Composition Cs2NaBiBr6
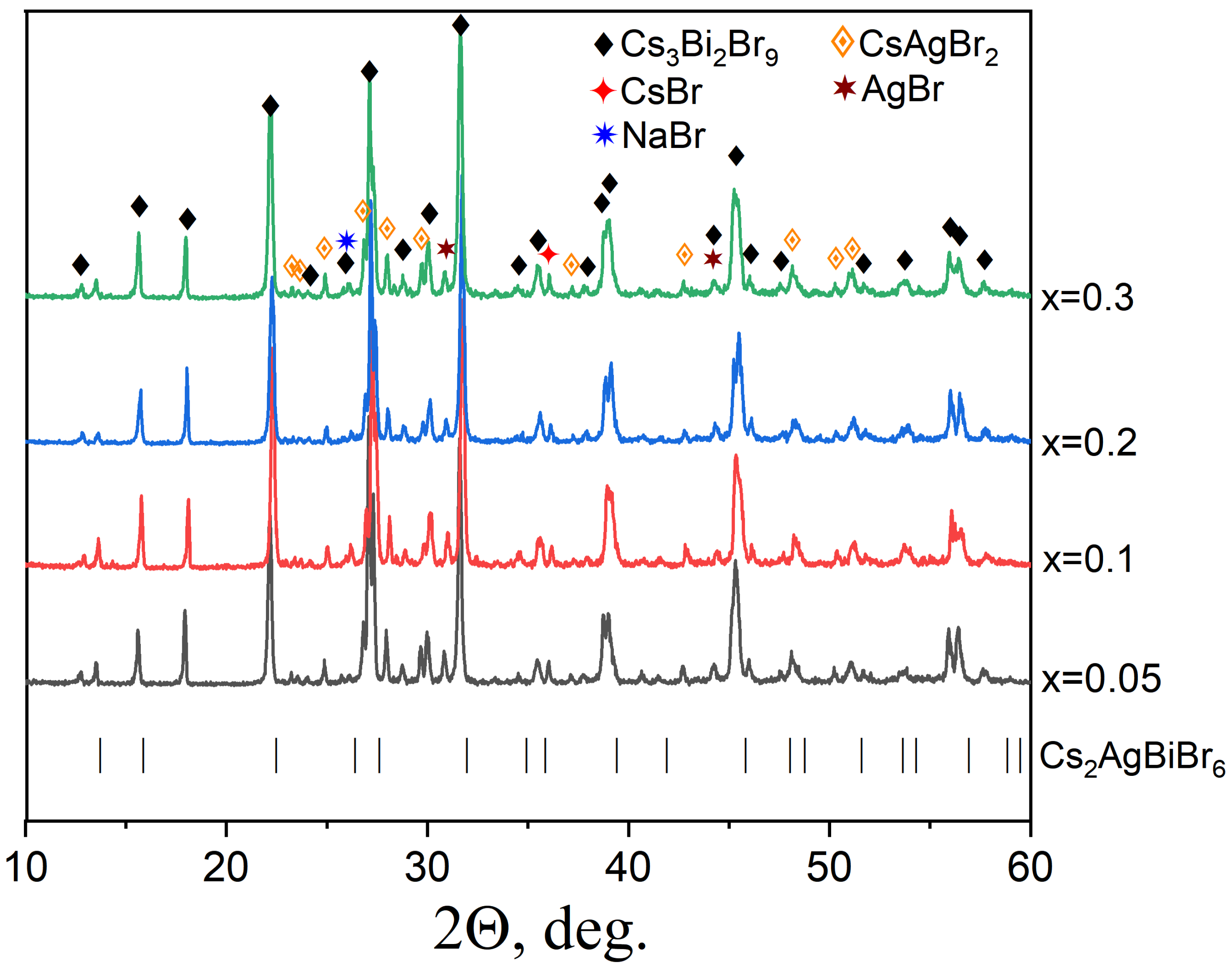
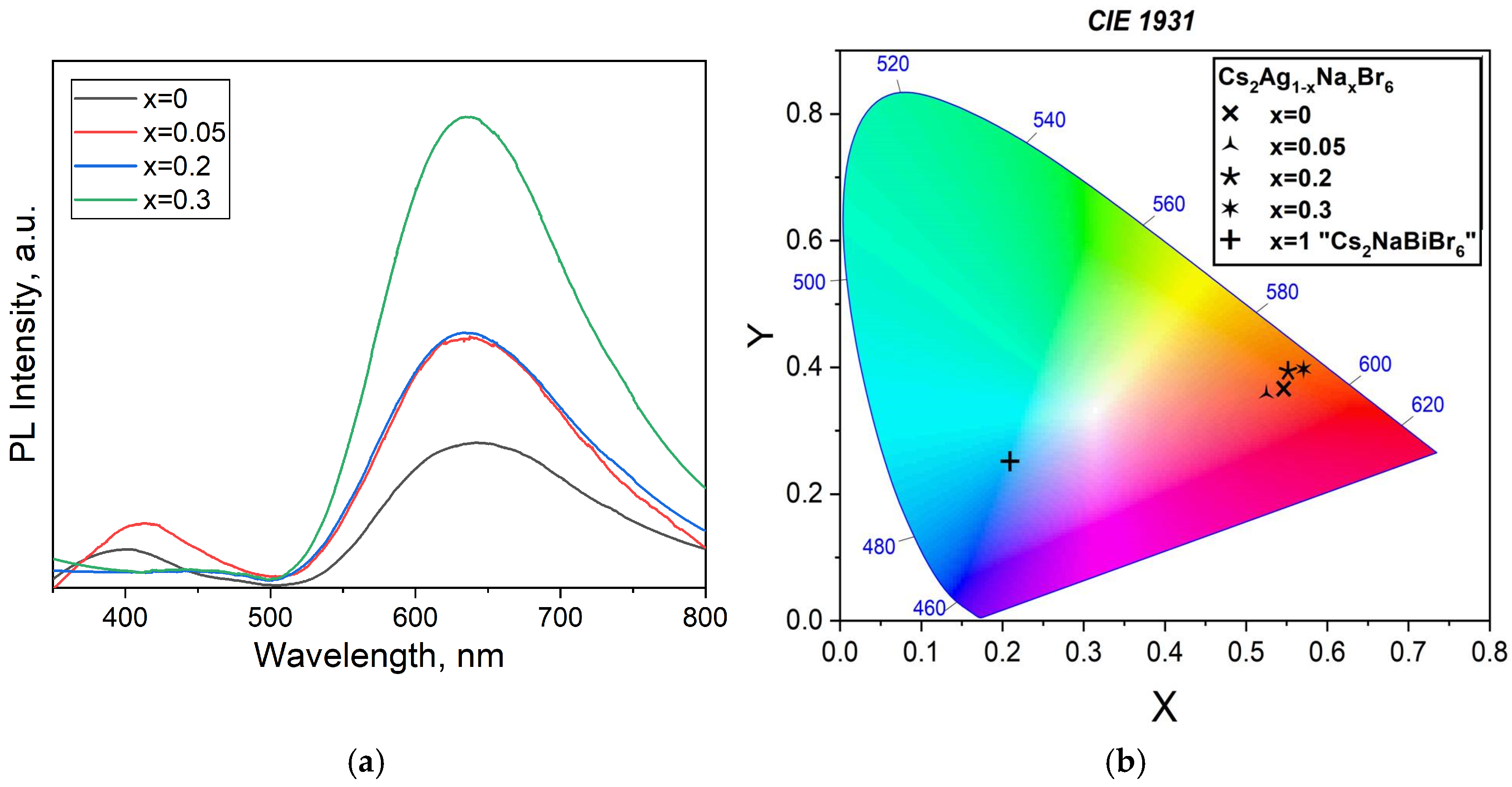
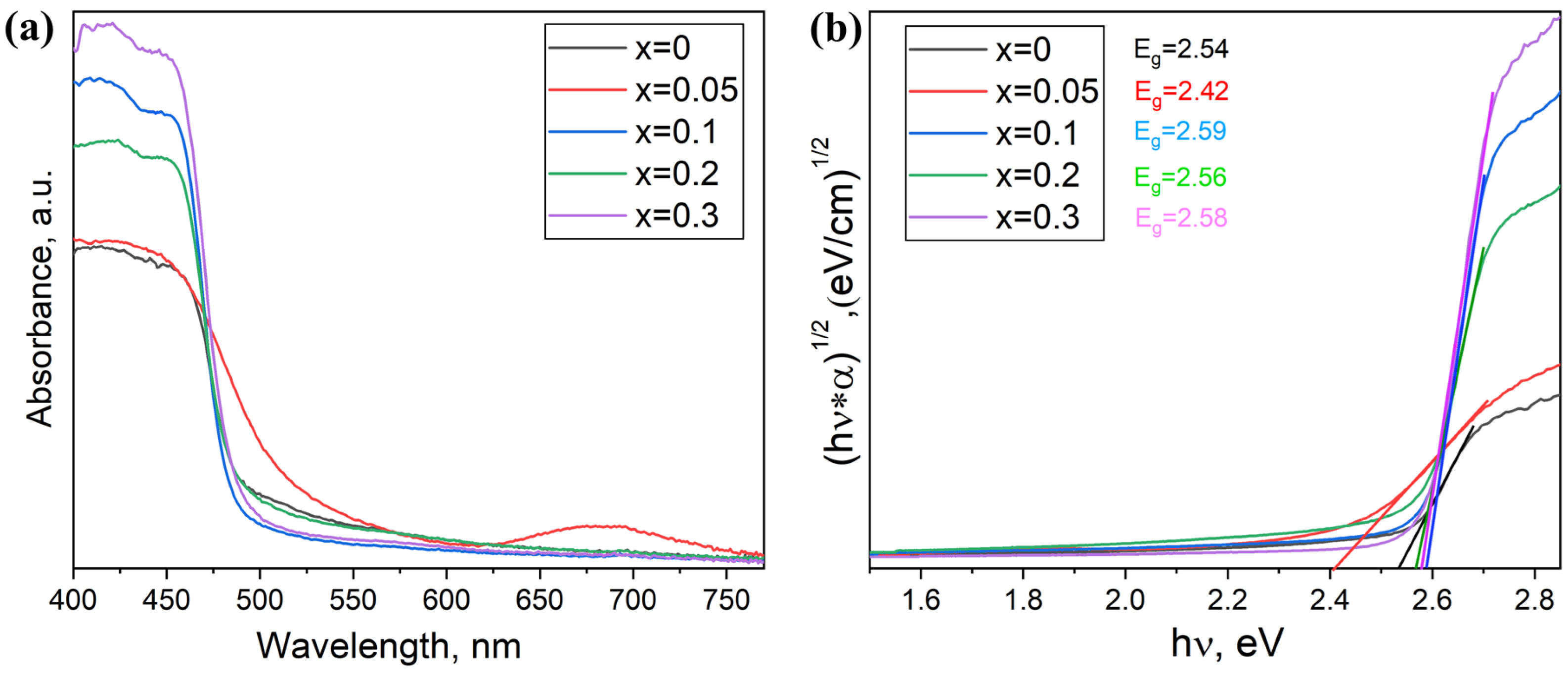
3.2. Samples of General Composition Cs2Ag1−xNaxBiBr6
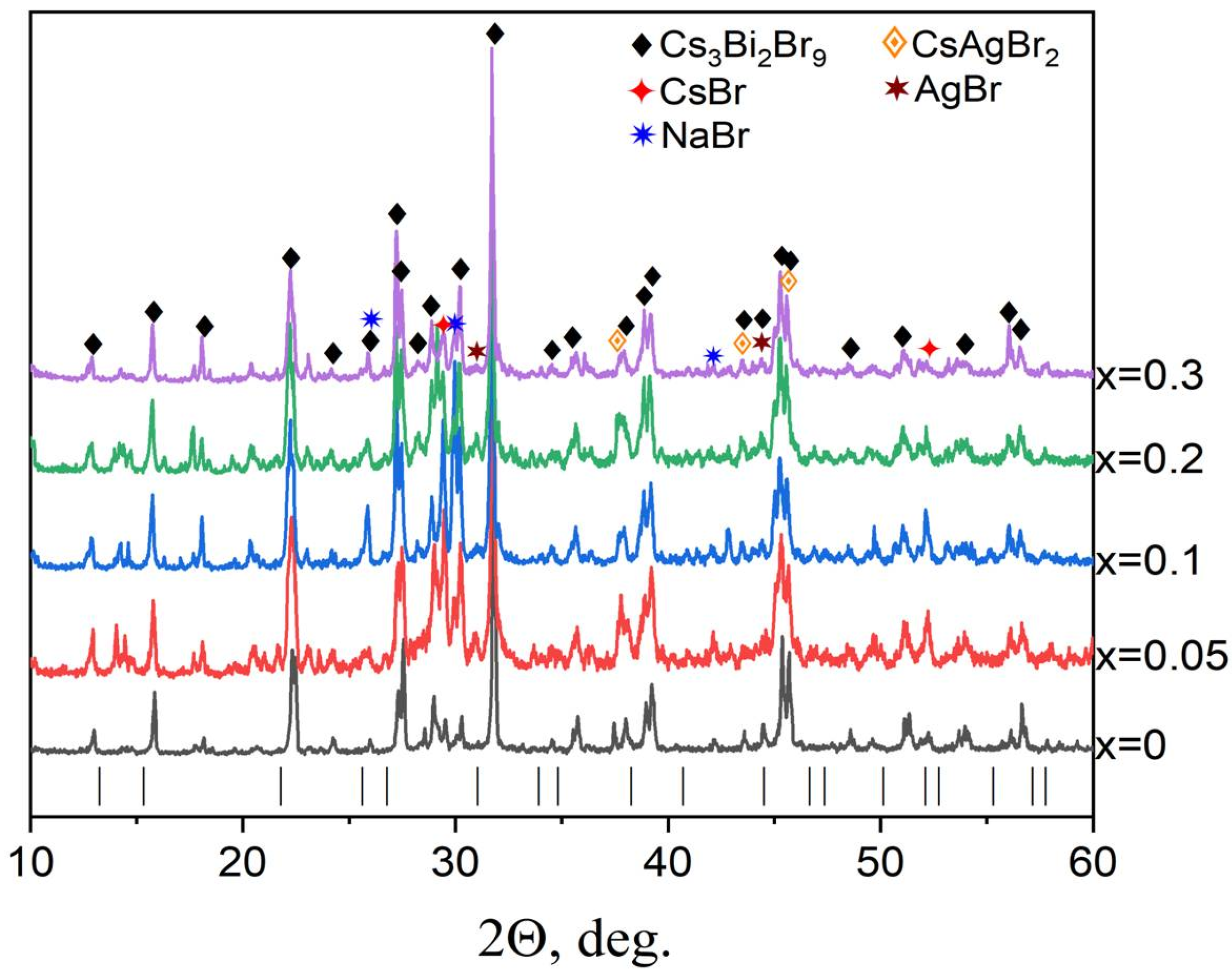
3.3. Samples of General Composition Cs2−2xNaxAgBiBr6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Cai, T.; Dube, L.; Chen, O. Synthesis of double perovskite and quadruple perovskite nanocrystals through post-synthetic transformation reactions. Chem. Sci. 2022, 13, 4874–4883. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Boschloo, G.; Wang, F.; Gao, F. Challenges and Progress in Lead-Free Halide Double Perovskite Solar Cells. Solar RRL 2023, 7, 2201112. [Google Scholar] [CrossRef]
- Ghosh, S.; Shankar, H.; Kar, P. Recent developments of lead-free halide double perovskites: A new superstar in the optoelectronic field. Mater. Adv. 2022, 3, 3742–3765. [Google Scholar] [CrossRef]
- Obada, D.O.; Akinpelu, S.B.; Abolade, S.A.; Okafor, E.; Ukpong, A.M.; Kumar, S.R.; Akande, A. Lead-Free Double Perovskites: A Review of the Structural, Optoelectronic, Mechanical, and Thermoelectric Properties Derived from First-Principles Calculations, and Materials Design Applicable for Pedagogical Purposes. Crystals 2024, 14, 86. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Sun, Q.; Wang, H.; Liu, F. Lead-Free Halide Perovskite Cs2AgBiBr6/Bismuthene Composites for Improved CH4 Production in Photocatalytic CO2 Reduction. ACS Appl. Energy Mater. 2022, 5, 12856–12864. [Google Scholar]
- Lei, H.W.; Hardy, D.; Gao, F. Lead-Free Double Perovskite Cs2AgBiBr6: Fundamentals, Applications, and Perspectives. Adv. Funct. Mater. 2021, 31, 2105898. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Aldamasy, M.H.; Frasca, C.; Abate, A.; Zhao, K.; Hu, Y. Advances in the Synthesis of Halide Perovskite Single Crystals for Optoelectronic Applications. Chem. Mater. 2023, 35, 2683–2712. [Google Scholar] [CrossRef]
- Kamilov, R.K.; Yuldoshev, J.Z.; Knotko, A.V.; Grigorieva, A.V. Phase Equilibria in Ternary System CsBr-AgBr-InBr3. Materials 2023, 16, 559. [Google Scholar] [CrossRef]
- Kamilov, R.K.; Yuldoshev, J.Z.; Knotko, A.V.; Grigorieva, A.V. In Search of a Double Perovskite in the Phase Triangle of Bromides CsBr-CuBr-InBr3. Materials 2023, 16, 3744. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Cho, J. Melt synthesis of halide perovskites: Structural control and applications. Cryst. Growth Des. 2023, 23, 1456–1464. [Google Scholar]
- Tan, Z.; Wei, J.; Liu, S. High-frequency heating for perovskite synthesis: A breakthrough in rapid production. Adv. Mater. Interfaces 2024, 11, 2201567. [Google Scholar]
- Smith, J.; Brown, T. Advances in Lead-Free Perovskite Materials. J. Mater. Chem. A 2021, 9, 13456–13472. [Google Scholar]
- Zhang, Z.; Sun, Q.; Lu, Y.; Lu, F.; Mu, X.; Wei, S.-H.; Sui, M. Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell. Nat. Commun. 2022, 13, 3397. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Huang, Y.; Wang, F.; Kobera, F.L.; Xie, F.; Klarbring, J.; Abbrent, S.; Brus, J.; Yin, C.; Simak, S.I. Near-Infrared Light-Responsive Cu-Doped Cs2AgBiBr6. Adv. Funct. Mater. 2020, 30, 2005521. [Google Scholar] [CrossRef]
- Li, Z.; Kavanagh, S.R.; Napari, M.; Palgrave, R.G.; Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Davies, D.W.; Laitinen, M.; Julin, J.; Isaacs, M.A.; et al. Bandgap lowering in mixed alloys of Cs2Ag(SbxBi1−x)Br6 double perovskite thin films. J. Mater. Chem. A 2020, 8, 21780–21788. [Google Scholar] [CrossRef]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef]
- Dakshinamurthy, A.C.; Sudakar, C. Photoinduced degradation of thermally stable Cs2AgBiBr6 double perovskites by micro-Raman studies. Mater. Adv. 2022, 3, 5813–5817. [Google Scholar] [CrossRef]
- Babaei, M. Investigation of the properties of lead-free Cs2NaBiX6 (X = I and Br) double perovskites using density functional theory (DFT). Opt. Quantum Electron. 2024, 56, 1449. [Google Scholar] [CrossRef]
- Pistor, P.; Meyns, M.; Guc, M.; Wang, H.-C.; Marques, M.A.-L.; Alcobé, X.; Cabot, A.; Izquierdo-Roca, V. Advanced Raman spectroscopy of Cs2AgBiBr6 double perovskites and identification of Cs3Bi2Br9 secondary phases. Scr. Mater. 2020, 184, 24–29. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Zhao, H. Enhanced optical properties of lead-free double perovskite Cs2AgBiBr6 nanocrystals by doping of Na ions. Solid State Commun. 2023, 373–374, 115288. [Google Scholar] [CrossRef]
- Wu, Y.; Meng, Y.; Hu, Q.; Shen, S.; Zhang, C.; Bian, A.; Dai, J. A-Site Ion Doping in Cs2AgBiBr6 Double Perovskite Films for Improved Optical and Photodetector Performance. Crystals 2024, 14, 1068. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, Y.; Li, W.; Zhao, C.; Mai, W. Reducing current fluctuation of Cs3Bi2Br9 perovskite photodetectors for diffuse reflection imaging with wide dynamic range. Sci. Bull. 2020, 65, 1371–1379. [Google Scholar]
- Elattar, A.; Kobera, L.; Kangsabanik, J.; Suzuki, H.; Abbrent, S.; Nishikawa, T.; Thygesen, K.S.; Brus, J.; Hayashi, Y. Structure modulation for bandgap engineered vacancy-ordered Cs3Bi2Br9 perovskite structures through copper alloying. J. Mater. Chem. C 2022, 10, 12863–12872. [Google Scholar] [CrossRef]
- Aragon, A.G.; Wiggins, T.E.; Ma, X.; Geyer, S.M. Lead-free Cs3Bi2Br9 and Cs3Bi2−xSbxBr9 nanocrystals as photocatalysts with enhanced activity for the degradation of rhodamine in aqueous environments. J. Photochem. Photobiol. A Chem. 2023, 436, 114391. [Google Scholar] [CrossRef]
- Messaoudi, I.S.; Zaoui, A.; Ferhat, M. Band-gap and phonon distribution in alkali halides. Phys. Status Solidi B 2014, 252, 490–495. [Google Scholar] [CrossRef]
- Mitzi, D.B. (Ed.) Lead-Free Halide Perovskites: Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2020; 310p. [Google Scholar]
- Gao, M.; Zhang, C.; Lian, L.; Guo, J.; Xia, Y.; Pan, F.; Su, X.; Zhang, J.; Li, H.; Zhang, D. Controlled synthesis and photostability of blue emitting Cs3Bi2Br9 perovskite nanocrystals by employing weak polar solvents at room temperature. J. Mater. Chem. C 2019, 7, 3688–3695. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, J.; Wu, S.; Liu, C.; Xing, F.; Xu, J.; Di, Y.; Yu, L.; Dong, L.; Gan, Z. Growth and Optical Properties of Lead-Free Cs3Bi2Br9 Perovskite Microplatelets. Phys. Status Solidi B 2022, 259, 2100593. [Google Scholar] [CrossRef]
- Saparov, B.; Hong, F.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Cs2NaBiBr6: Optical and electronic properties of a lead-free double perovskite. J. Mater. Chem. C 2017, 5, 4452–4458. [Google Scholar]
- Becker, T.; Stolarczyk, J.K. (Eds.) Halide Perovskites: Photovoltaics, Light Emission, and Beyond; Wiley: Hoboken, NJ, USA, 2021; 450p. [Google Scholar]
- Schade, L.; Wright, A.D.; Johnson, R.D.; Dollmann, M.; Wenger, B.; Nayak, P.K.; Prabhakaran, D.; Herz, L.M.; Nicholas, R.; Snaith, H.J.; et al. Structural and Optical Properties of Cs2AgBiBr6 Double Perovskite. ACS Energy Lett. 2019, 4, 299–305. [Google Scholar] [CrossRef]
- Wang, C.; Ding, Y.; Liu, B.; Roeffaers, M.B.J. Crystal structure engineering of metal halide perovskites for photocatalytic organic synthesis. Chem. Commun. 2023, 59, 3122–3125. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, M.; Chang, B.; Sun, H.; Zheng, K.; Pullerits, T.; Chi, Q. Lead-free double halide perovskite Cs3BiBr6 with well-defined crystal structure and high thermal stability for optoelectronics. J. Mater. Chem. C 2019, 7, 3369–3374. [Google Scholar] [CrossRef]
- Cao, F.; Li, Z.; Liu, X.; Shi, Z.; Fang, X. Air Induced Formation of Cs3Bi2Br9/Cs3BiBr6 Bulk Heterojunction and Its Dual-band Photodetection Abilities for Light Communication. Adv. Funct. Mater. 2022, 32, 2206151. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosirova, N.K.; Kamilov, R.K.; Ibrohimov, M.M.; Lepnev, L.S.; Astafurov, M.O.; Knotko, A.V.; Grigorieva, A.V. Ampoule Synthesis of Na-Doped Complex Bromide Cs2AgBiBr6 with Double Perovskite Structure. Materials 2025, 18, 1197. https://doi.org/10.3390/ma18061197
Nosirova NK, Kamilov RK, Ibrohimov MM, Lepnev LS, Astafurov MO, Knotko AV, Grigorieva AV. Ampoule Synthesis of Na-Doped Complex Bromide Cs2AgBiBr6 with Double Perovskite Structure. Materials. 2025; 18(6):1197. https://doi.org/10.3390/ma18061197
Chicago/Turabian StyleNosirova, Nigina K., Rustam K. Kamilov, Maqsudjon M. Ibrohimov, Leonid S. Lepnev, Mikhail O. Astafurov, Alexander V. Knotko, and Anastasia V. Grigorieva. 2025. "Ampoule Synthesis of Na-Doped Complex Bromide Cs2AgBiBr6 with Double Perovskite Structure" Materials 18, no. 6: 1197. https://doi.org/10.3390/ma18061197
APA StyleNosirova, N. K., Kamilov, R. K., Ibrohimov, M. M., Lepnev, L. S., Astafurov, M. O., Knotko, A. V., & Grigorieva, A. V. (2025). Ampoule Synthesis of Na-Doped Complex Bromide Cs2AgBiBr6 with Double Perovskite Structure. Materials, 18(6), 1197. https://doi.org/10.3390/ma18061197





