Molecular Diffusion and Optical Properties of Implantable Collagen Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results
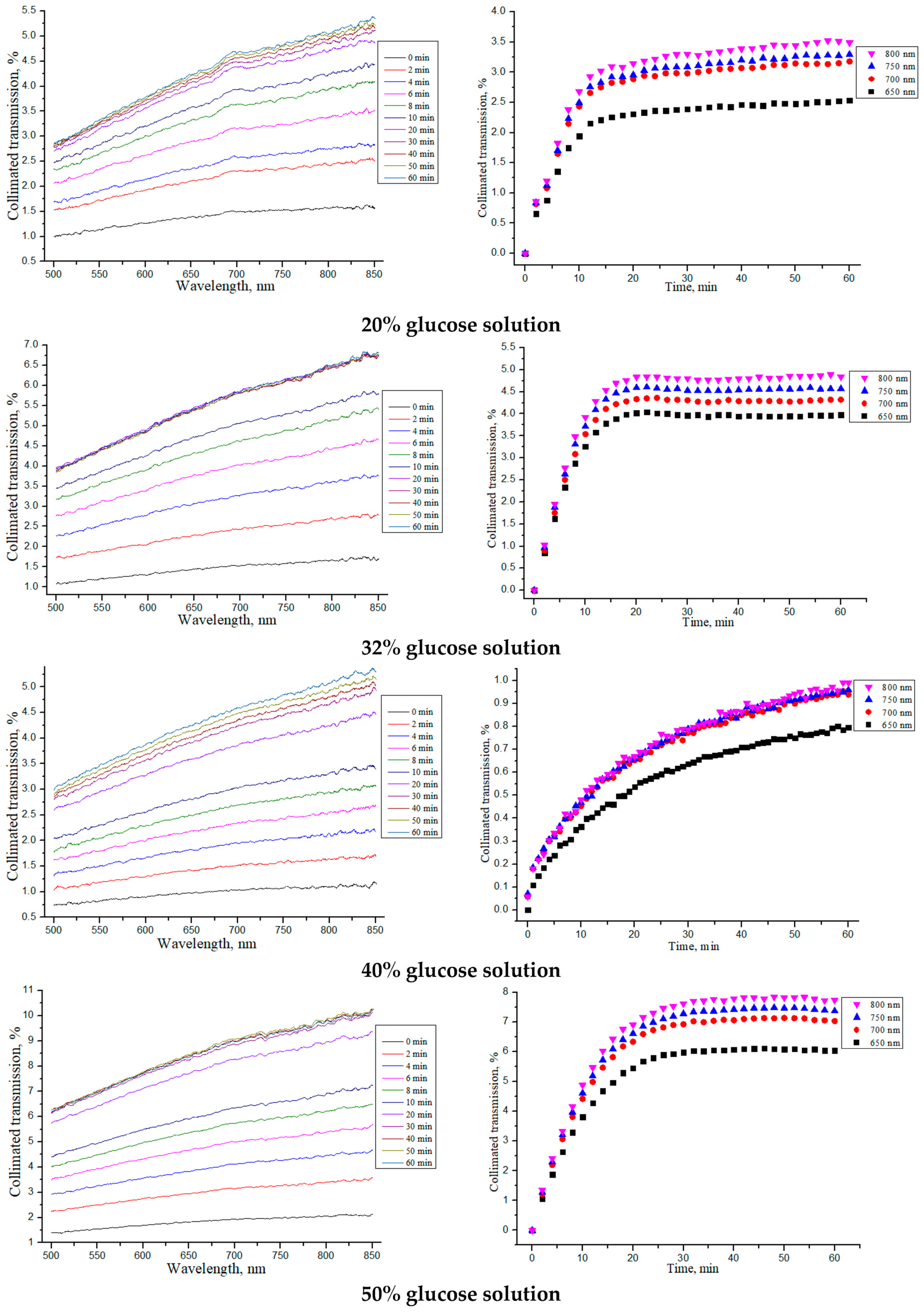
3.1. Determination of OCA Diffusion Coefficients in Collagen Materials
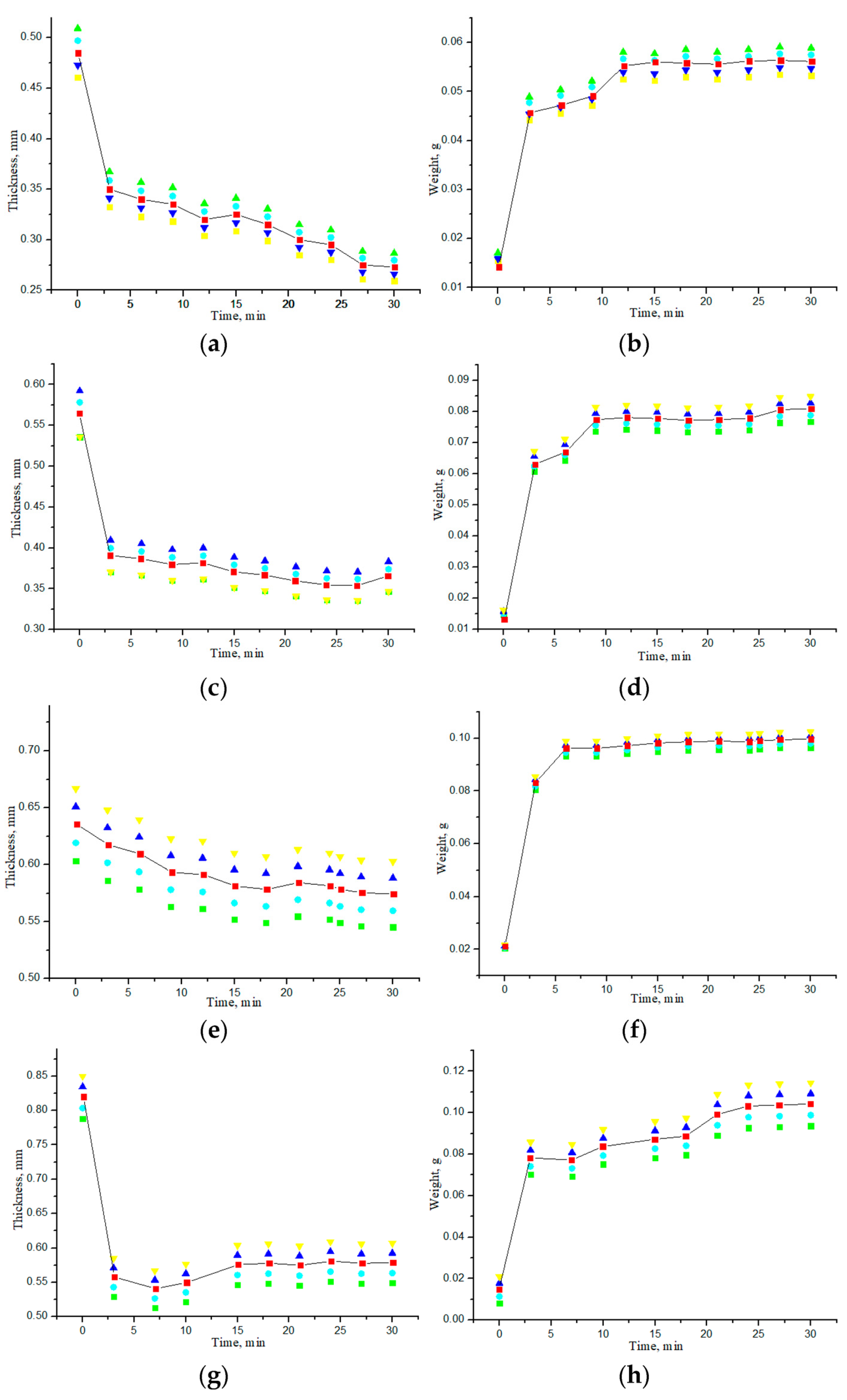
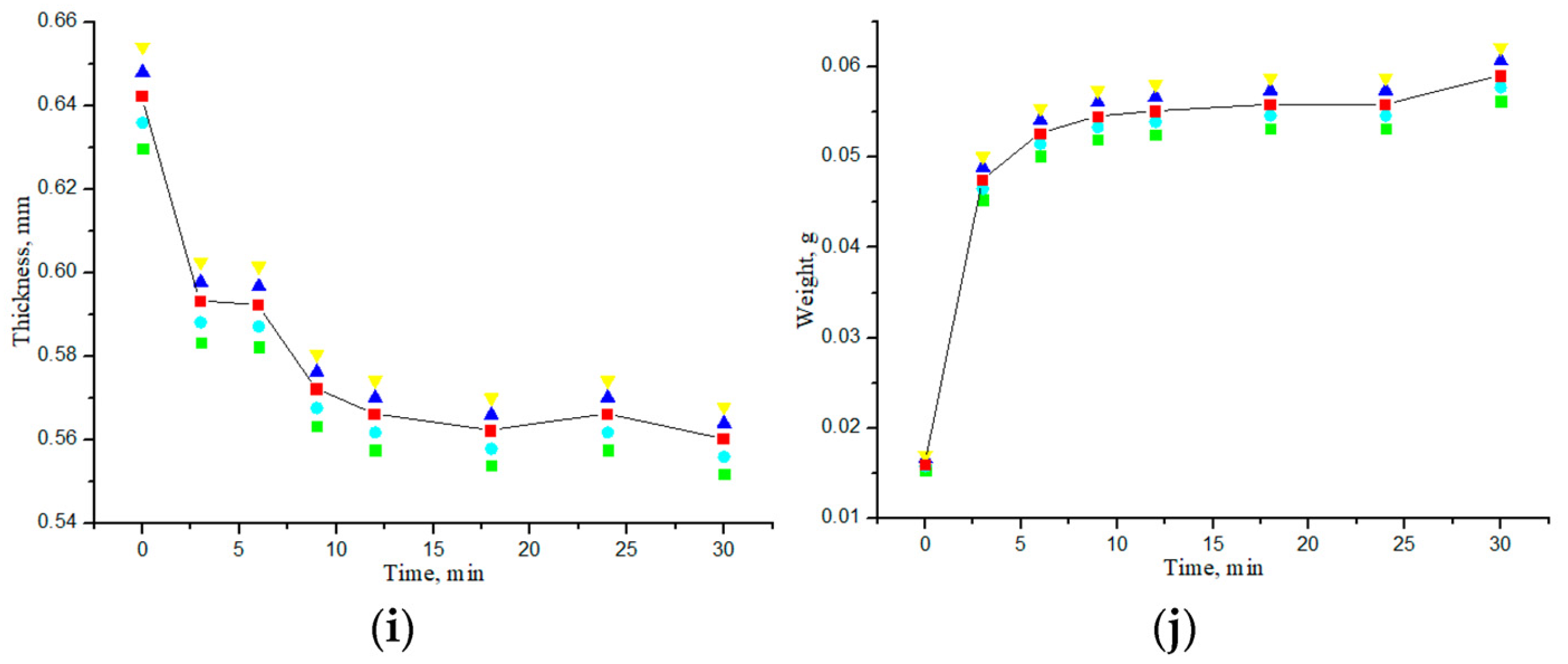
3.2. Study of Geometric and Weight Parameters of Samples Under Saline and Water Action
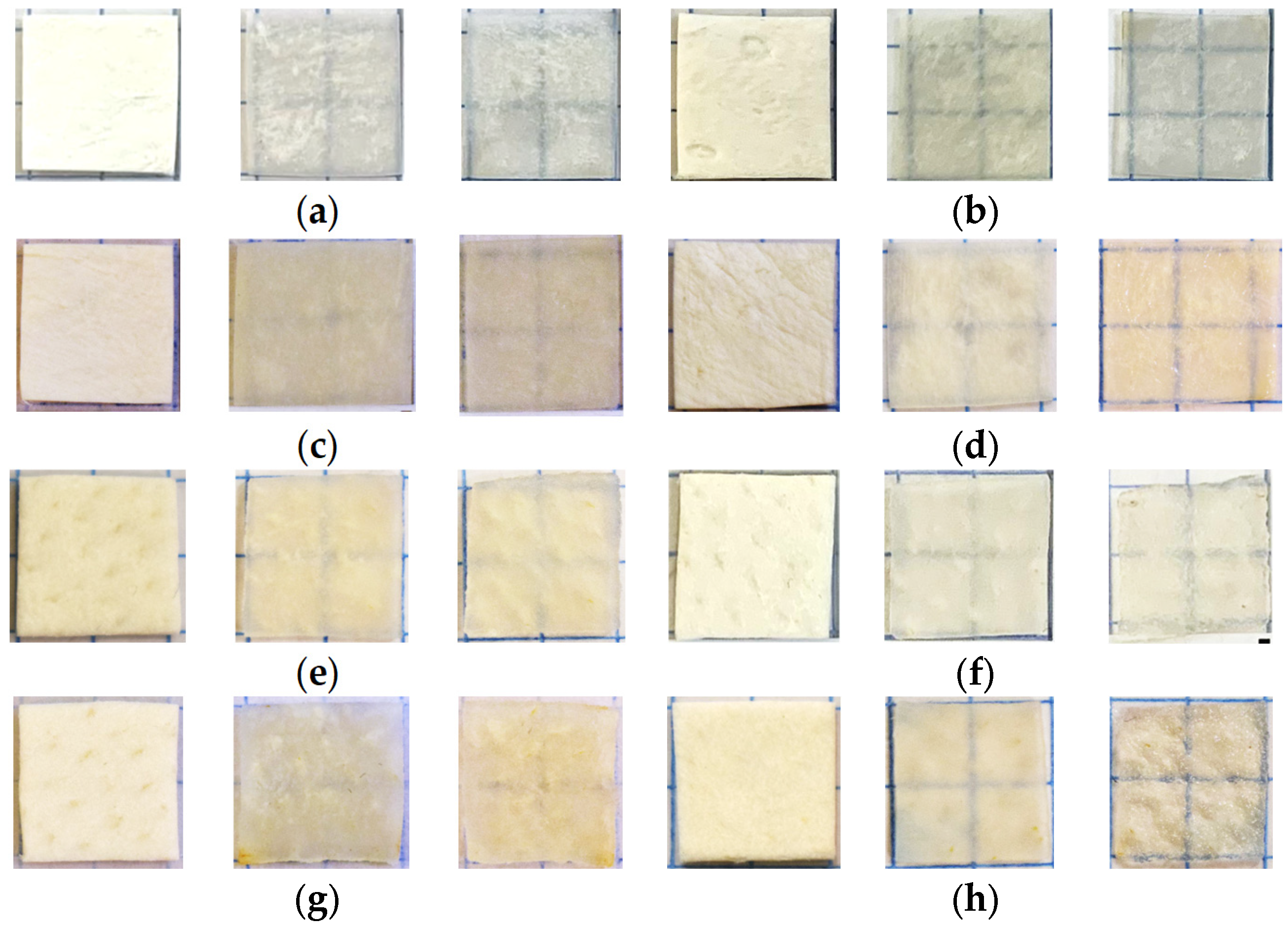
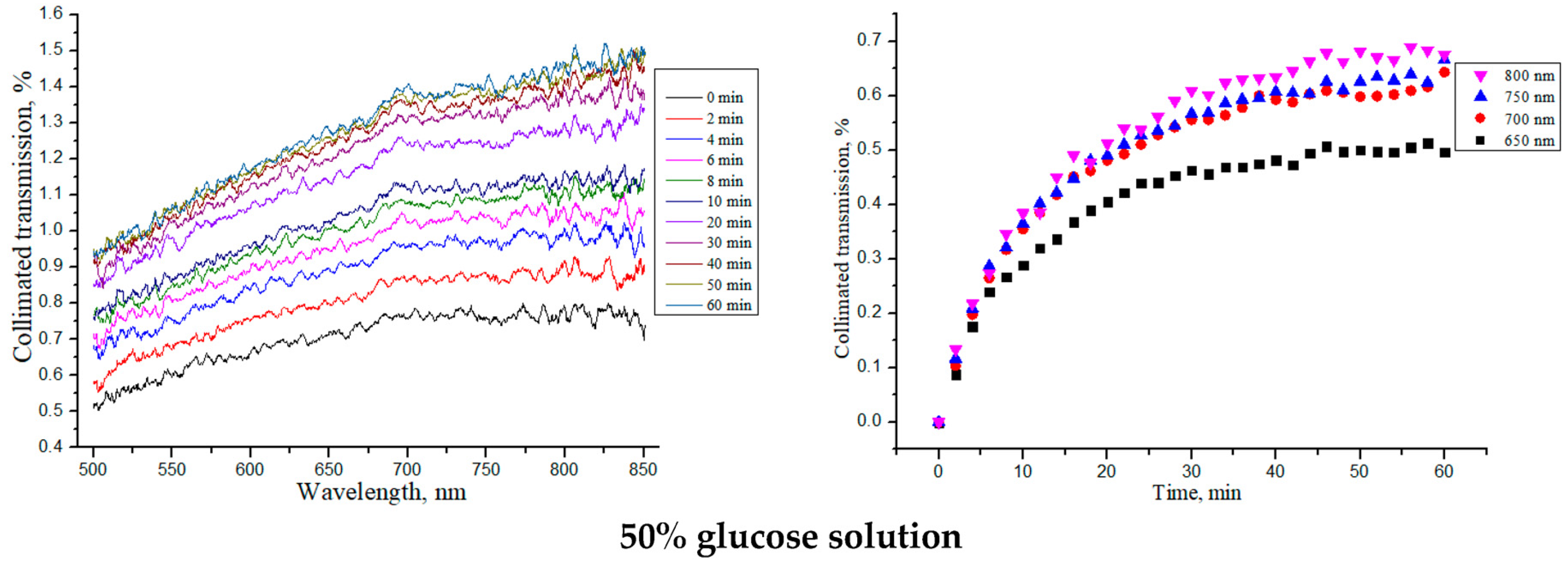
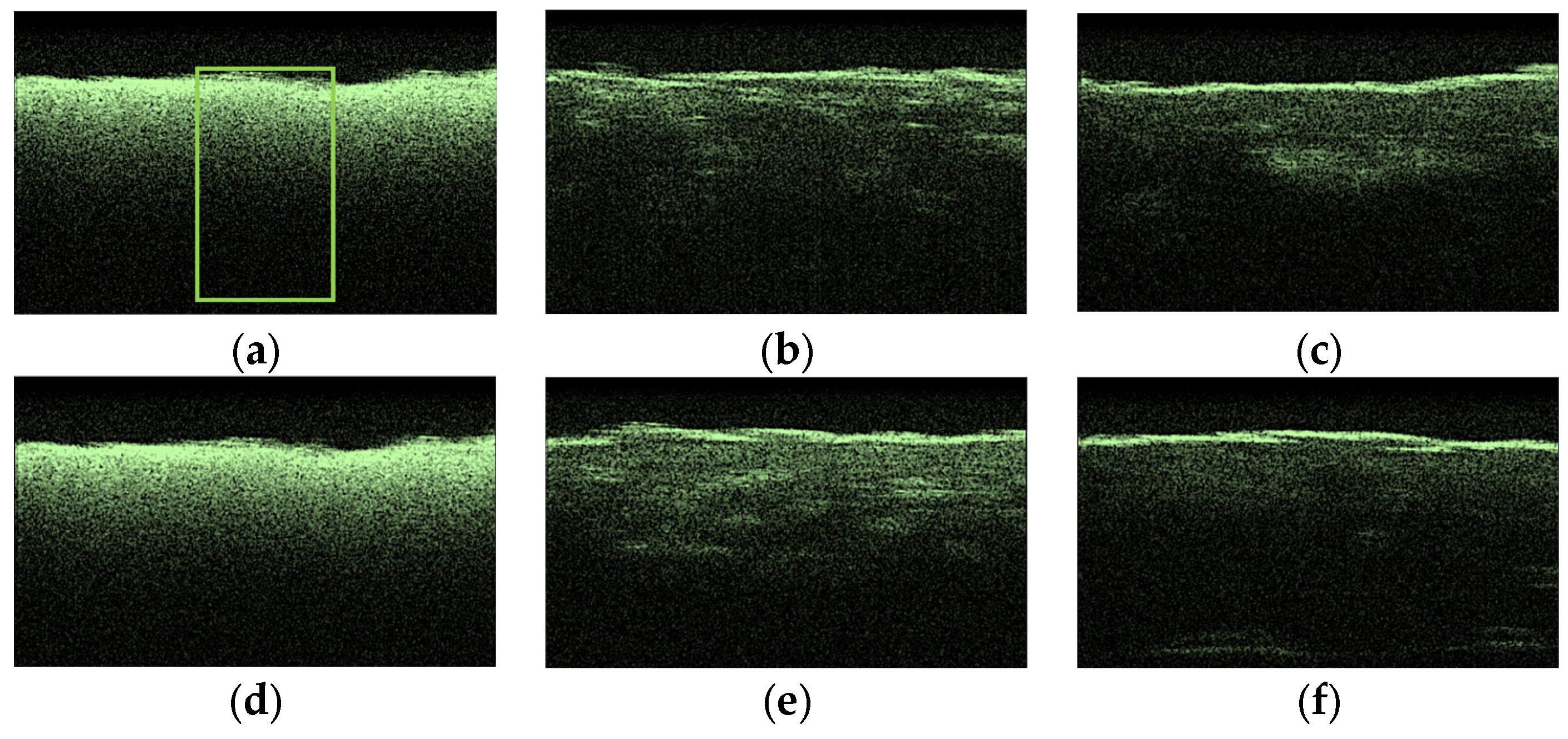
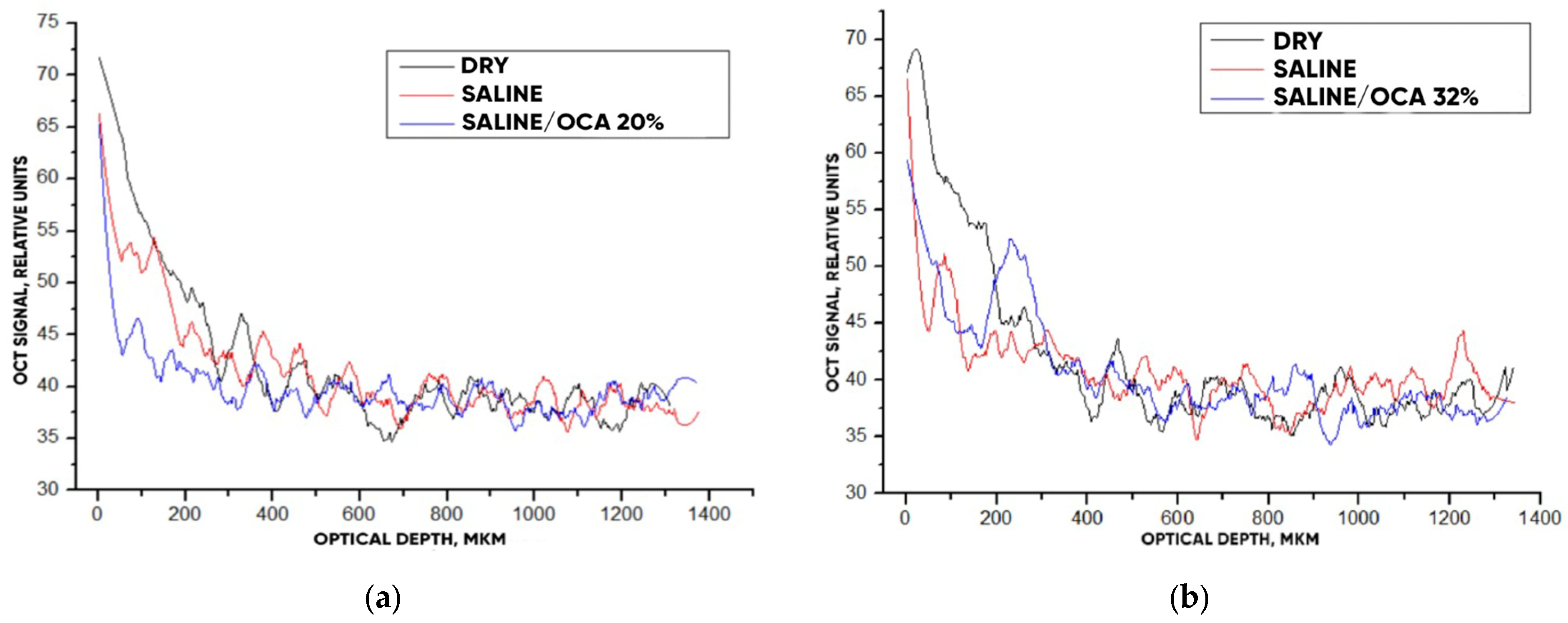
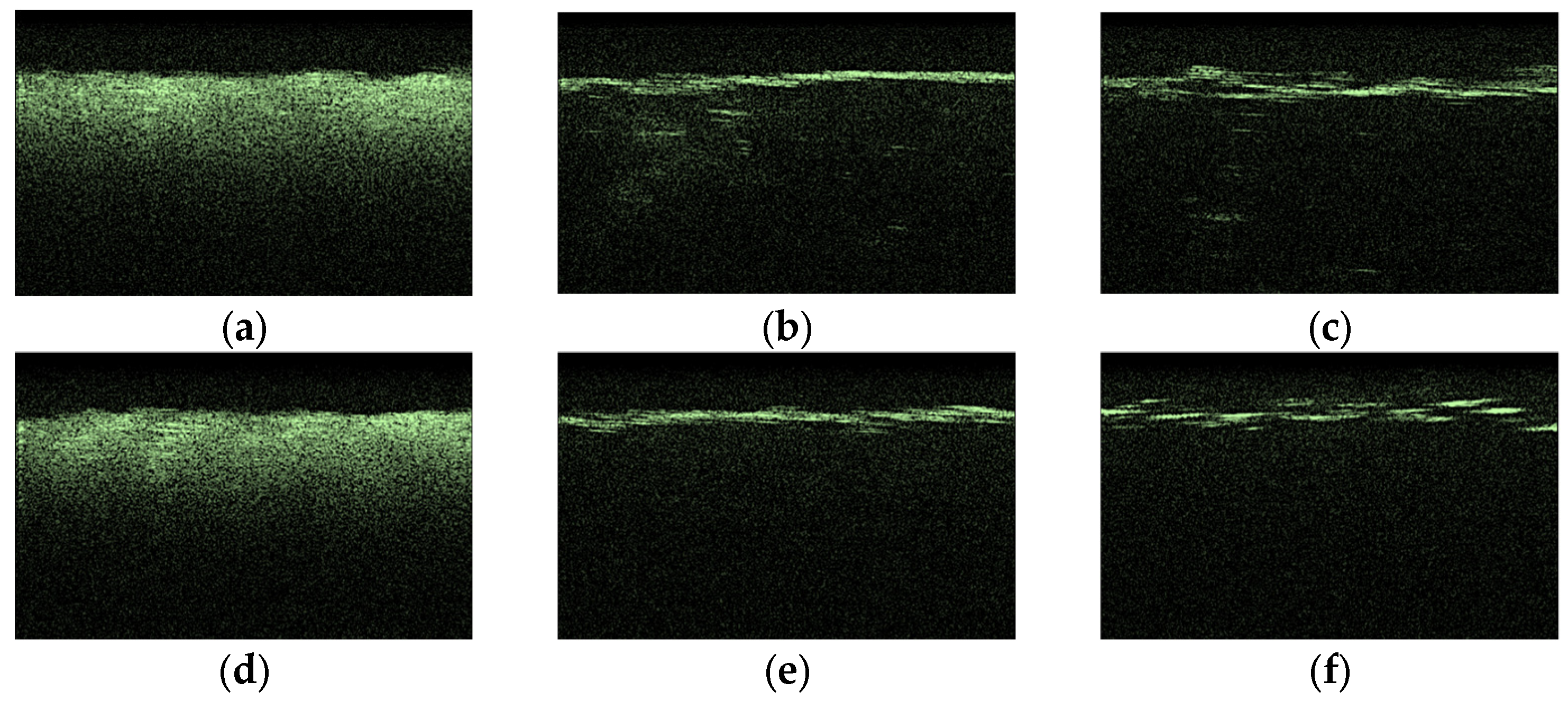
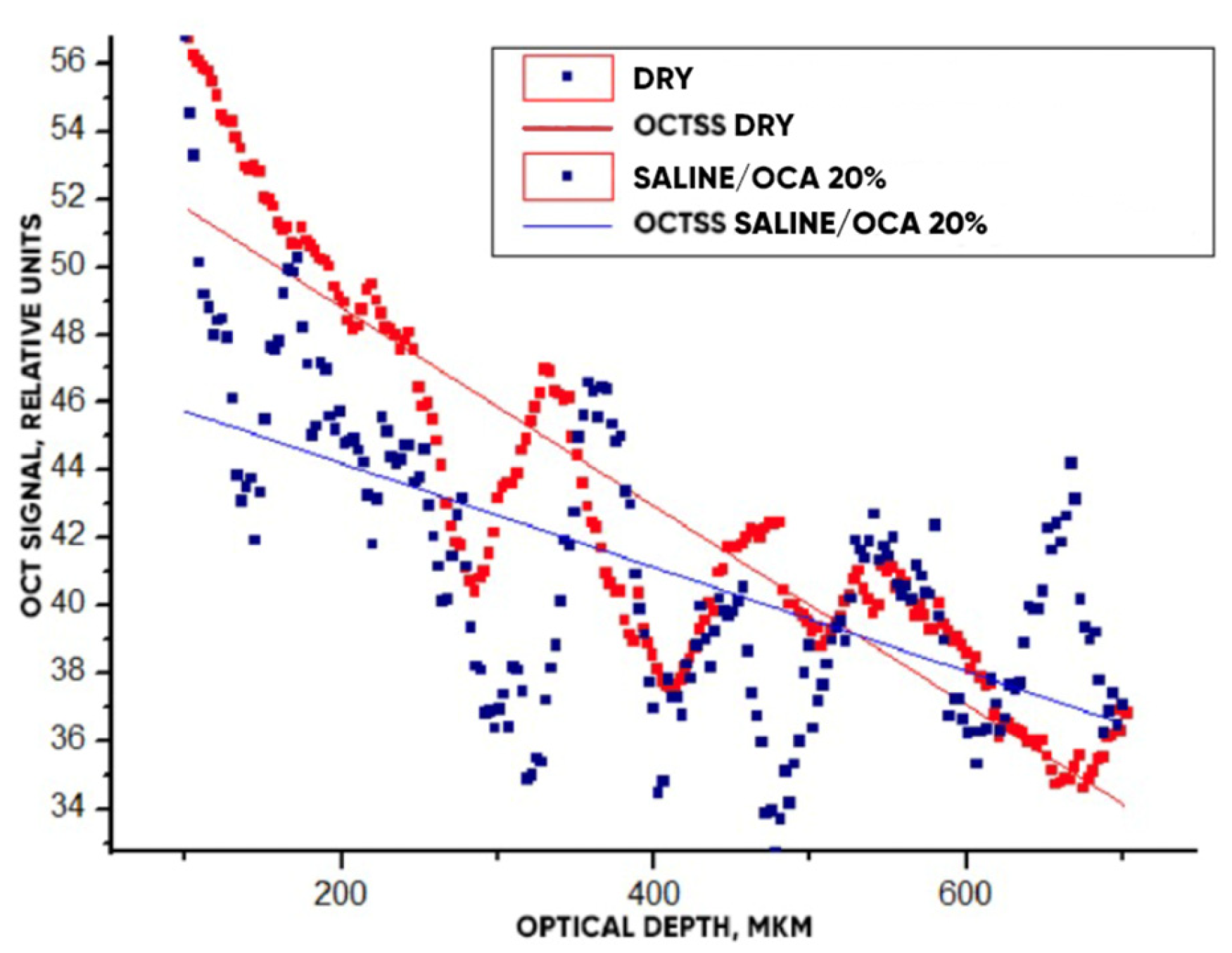
3.3. OCT-Monitoring of OCA Diffusion in Collagen Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| GAG | Glycosaminoglycans |
| OCA | Optical clearing agent |
| OCT | Optical coherence tomography |
| OCTSS | OCT signal slope |
| RNA | Ribonucleic acid |
| UV | Ultraviolet |
References
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Peijs, T. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Zeugolis, D.I. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mat. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. Collagen-based biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Pub: Cambridge, UK, 2018; pp. 127–150. [Google Scholar]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zhong Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 24, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.S.; Brand, D.D.; Czernuszka, J.T. Influence of telopeptides, fibrils and crosslinking on physicochemical properties of type I collagen films. J. Mater. Sci. Mater. Med. 2010, 21, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural polymeric scaffolds in bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.W. Decellularized extracellular matrix-based bioinks for engineering tissue-and organ-specific microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Barbeck, M. Barrier membranes for guided bone regeneration (gbr): A focus on recent advances in collagen membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible synthetic polymers for tissue engineering purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Boyce, S.T. Wound closure with EDC cross-linked cultured skin substitutes grafted to athymic mice. Biomaterials 2007, 28, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-linking of extruded collagen fibers-a biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. 2009, 89, 895–908. [Google Scholar] [CrossRef]
- Khew, S.T.; Yang, Q.J.; Tong, Y.W. Enzymatically crosslinked collagen-mimetic dendrimers that promote integrin-targeted cell adhesion. Biomaterials 2008, 29, 3034–3045. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics, 3rd ed.; PM 254; SPIE Press: Bellingham, WA, USA, 2015. [Google Scholar]
- Listewnik, P.; Ronowska, M.; Wąsowicz, M.; Tuchin, V.V.; Szczerska, M. Porous phantoms mimicking tissues—Investigation of optical parameters stability over time. Materials 2021, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Bardakova, K.N.; Grebenik, E.A.; Istranova, E.V.; Istranov, L.P.; Gerasimov, Y.V.; Grosheva, A.G.; Chailakhyan, R.K. Reinforced hybrid collagen sponges for tissue engineering. Bull. Exp. Biol. Med. 2018, 165, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zhao, Y. 3D bioprinting for biomedical applications. BME Front. 2023, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Bardakova, K.N.; Grebenik, E.A.; Minaev, N.V.; Churbanov, S.N.; Moldagazyeva, Z.; Krupinov, G.E.; Timashev, P.S. Tailoring the collagen film structural properties via direct laser crosslinking of star-shaped polylactide for robust scaffold formation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110300. [Google Scholar] [CrossRef] [PubMed]
- Grebenik, E.A.; Gafarova, E.R.; Istranov, L.P.; Istranova, E.V.; Ma, X.; Xu, J.; Guo, W.; Atala, A.; Timashev, P.S. Mammalian pericardium-based bioprosthetic materials in xenotransplantation and tissue engineering. Biotechnol. J. 2020, 15, e1900334. [Google Scholar] [CrossRef]
- Tuchina, D.K.; Timoshina, P.A.; Tuchin, V.V.; Bashkatov, A.N.; Genina, E.A. Kinetics of rat skin optical clearing at topical application of 40% glucose: Ex vivo and in vivo studies. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 7200508. [Google Scholar]
- Tuchina, D.K.; Shi, R.; Bashkatov, A.N.; Genina, E.A.; Zhu, D.; Luo, Q.; Tuchin, V.V. Ex vivo optical measurements of glucose diffusion kinetics in native and diabetic mouse skin. J. Biophoton. 2015, 8, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Born, M.; Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Beck, R.E.; Schultz, J.S. Hindrance of solute diffusion within membranes as measured with microporous membranes of known pore geometry. Biochim. Biophys. Acta 1972, 255, 273–303. [Google Scholar] [CrossRef]
- Siddique, J.I.; Anderson, D.M.; Bondarev, A. Capillary rise of a liquid into a deformable porous material. Phys. Fluids 2009, 21, 013106. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Du, H. Prediction of capillary suction in porous media based on micro-CT technology and B–C model. Open Phys. 2020, 18, 906–915. [Google Scholar] [CrossRef]
- Siddique, J.I.; Landis, F.A.; Mohyuddin, M.R. Dynamics of drainage of power-law liquid into a deformable porous material. Open J. Fluid Dyn. 2014, 4, 403–414. [Google Scholar] [CrossRef][Green Version]
- Sheshenin, S.V.; Artamonova, N.B. The simulation of the nonlinear consolidation of porous media. PNRPU Mech. Bull. 2022, 1, 167–176. [Google Scholar] [CrossRef]
- Molteni, C.; Parrinello, M. Glucose in aqueous solution by first principles molecular dynamics. J. Am. Chem. Soc. 1998, 120, 2168–2171. [Google Scholar] [CrossRef]
- Sung, H.W.; Chang, W.H.; Ma, C.Y.; Lee, M.H. Crosslinking of biological tissues using genipin and/or carbodiimide. J. Biomed. Mater. Res. A 2003, 64, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.K.; Lai, J.Y.; Cheng, H.Y.; Tsai, C.C.; Yeh, L.K. Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials 2010, 25, 6647–6658. [Google Scholar] [CrossRef]
- Larin, K.V.; Tuchin, V.V. Functional imaging and assessment of the glucose diffusion rate in epithelial tissues in optical coherence tomography. Quant. Electr. 2008, 38, 551–556. [Google Scholar] [CrossRef]
- Oliveira, L.; Tuchin, V.V. The Optical Clearing Method: A New Tool for Clinical Practice and Biomedical Engineering; Springer Nature: Basel, Switzerland, 2019. [Google Scholar]
- Yanina, I.Y.; Trunina, N.A.; Tuchin, V.V. Photoinduced cell morphology alterations quantified within adipose tissues by spectral optical coherence tomography. J. Biomed. Opt. 2013, 18, 111407. [Google Scholar] [CrossRef]
- Carneiro, I.; Carvalho, S.; Henrique, R.; Oliveira, L.M.; Tuchin, V.V. A robust ex vivo method to evaluate the diffusion properties of agents in biological tissues. J. Biophoton. 2019, 12, e201800333. [Google Scholar] [CrossRef]
- Martins, I.S.; Silva, H.F.; Lazareva, E.N.; Chernomyrdin, N.V.; Zaytsev, K.I.; Oliveira, L.M.; Tuchin, V.V. Measurement of tissue optical properties in a wide spectral range: A review. Biomed. Opt. Express 2022, 14, 249–298. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Pinheiro, M.R.; Tuchina, D.K.; Timoshina, P.A.; Carvalho, M.I.; Oliveira, L.M. Light in evaluation of molecular diffusion in tissues: Discrimination of pathologies. Adv. Drug Deliv. Rev. 2024, 212, 115420. [Google Scholar] [CrossRef] [PubMed]






| λ, nm Glucose Solution, % | 546 | 589 | 644 | 656 | 680 | 930 |
|---|---|---|---|---|---|---|
| 20 | 1.3644 | 1.3628 | 1.3611 | 1.3608 | 1.3601 | 1.3565 |
| 32 | 1.3778 | 1.3761 | 1.3746 | 1.3742 | 1.3733 | 1.3693 |
| 40 | 1.3852 | 1.3839 | 1.3821 | 1.3817 | 1.3809 | 1.3769 |
| 50 | 1.3997 | 1.3980 | 1.3961 | 1.3958 | 1.3950 | 1.3910 |
| Concentration of Glucose Solution | l, mm (Dry/Saline/OCA) | τ, min | D, cm2/c | P, cm/c |
|---|---|---|---|---|
| 20% | (0.470 ± 0.146)/ (0.308 ± 0.157)/ (0.392 ± 0.07) | 8.1 ± 1.2 | (0.28 ± 0.12) × 10−6 | (0.71 ± 0.07) × 10−4 |
| 32% | (0.343 ± 0.070)/ (0.204 ± 0.075)/ (0.304 ± 0.078) | 7.6 ± 0.8 | (0.18 ± 0.13) × 10−6 | (0.61 ± 0.11) × 10−4 |
| 40% | (0.469 ± 0.030)/ (0.292 ± 0.050)/ (0.420 ± 0.047) | 10.8 ± 1.3 | (0.23 ± 0.15) × 10−6 | (0.57 ± 0.14) × 10−4 |
| 50% | (0.466 ± 0.043)/ (0.287 ± 0.034)/ (0.426 ± 0.065) | 11.8 ± 1.8 | (0.24 ± 0.07) × 10−6 | (0.65 ± 0.09) × 10−4 |
| Concentration of Glucose Solution | l, mm (Dry/Saline/OCA) | τ, min | D, cm2/c | P, cm/c |
|---|---|---|---|---|
| 20% | (0.791 ± 0.153)/ (0.605 ± 0.086)/ (0.744 ± 0.087) | 7.3 ± 1.4 | (1.43 ± 0.41) × 10−6 | (2.06 ± 0.22) × 10−4 |
| 32% | (0.831 ± 0.061)/ (0.624 ± 0.145)/ (0.766 ± 0.042) | 6.9 ± 1.1 | (1.52 ± 0.32) × 10−6 | (2.32 ± 0.27) × 10−4 |
| 40% | (0.860 ± 0.041)/ (0.627 ± 0.077)/ (0.806 ± 0.116) | 11.8 ± 2.2 | (0.82 ± 0.12) × 10−6 | (1.080 ± 0.22) × 10−4 |
| 50% | (0.703 ± 0.254)/ (0.472 ± 0.368)/ (0.657 ± 0.032) | 8.8 ± 1.3 | (1.58 ± 0.41) × 10−6 | (2.54 ± 0.17) × 10−4 |
| Sample | τ, min | D, cm2/c | P, cm/c |
|---|---|---|---|
| “PER uncrosslinked” | 11.9 ± 0.8 | (0.22 ± 0.05) × 10−6 | (0.55 ± 0.34) × 10−4 |
| “SPILAK” | 7.5 ± 0.9 | (1.41 ± 0.05) × 10−6 | (1.77 ± 0.27) × 10−4 |
| Sample | Dry Weight, mg | Weight with Saline, mg | Weight with Saline/OCA, mg |
|---|---|---|---|
| “PER uncrosslinked” | 14 ± 1 | 60 ± 5 | 75 ± 3 |
| “SPILAK” | 16 ± 1 | 83 ± 7 | 119 ± 9 |
| “PER Uncrosslinked”, S, mm2 | “SPILAK”, S, mm2 | |
|---|---|---|
| Dry sample | 45.7 ± 2.4 | 45.5 ± 2.6 |
| Saline | 45.6 ± 2.4 | 42.8 ± 2.4 |
| 20% glucose | 45.3 ± 1.3 | 44.9 ± 6.2 |
| 32% glucose | 51.6 ± 3.1 | 51.6 ± 2.7 |
| 40% glucose | 47.1 ± 5.3 | 44.8 ± 8.8 |
| 50% glucose | 44.4 ± 4.2 | 40.1 ± 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atsigeida, S.V.; Tuchina, D.K.; Timashev, P.S.; Tuchin, V.V. Molecular Diffusion and Optical Properties of Implantable Collagen Materials. Materials 2025, 18, 1035. https://doi.org/10.3390/ma18051035
Atsigeida SV, Tuchina DK, Timashev PS, Tuchin VV. Molecular Diffusion and Optical Properties of Implantable Collagen Materials. Materials. 2025; 18(5):1035. https://doi.org/10.3390/ma18051035
Chicago/Turabian StyleAtsigeida, Sofya V., Daria K. Tuchina, Peter S. Timashev, and Valery V. Tuchin. 2025. "Molecular Diffusion and Optical Properties of Implantable Collagen Materials" Materials 18, no. 5: 1035. https://doi.org/10.3390/ma18051035
APA StyleAtsigeida, S. V., Tuchina, D. K., Timashev, P. S., & Tuchin, V. V. (2025). Molecular Diffusion and Optical Properties of Implantable Collagen Materials. Materials, 18(5), 1035. https://doi.org/10.3390/ma18051035







