An Acid–Base Proton Transfer Approach to Robust Superhydrophobic Self-Cleaning Surfaces for the Corrosion Protection of Magnesium
Abstract
1. Introduction
2. Materials and Methods
2.1. General Materials
2.2. Instruments
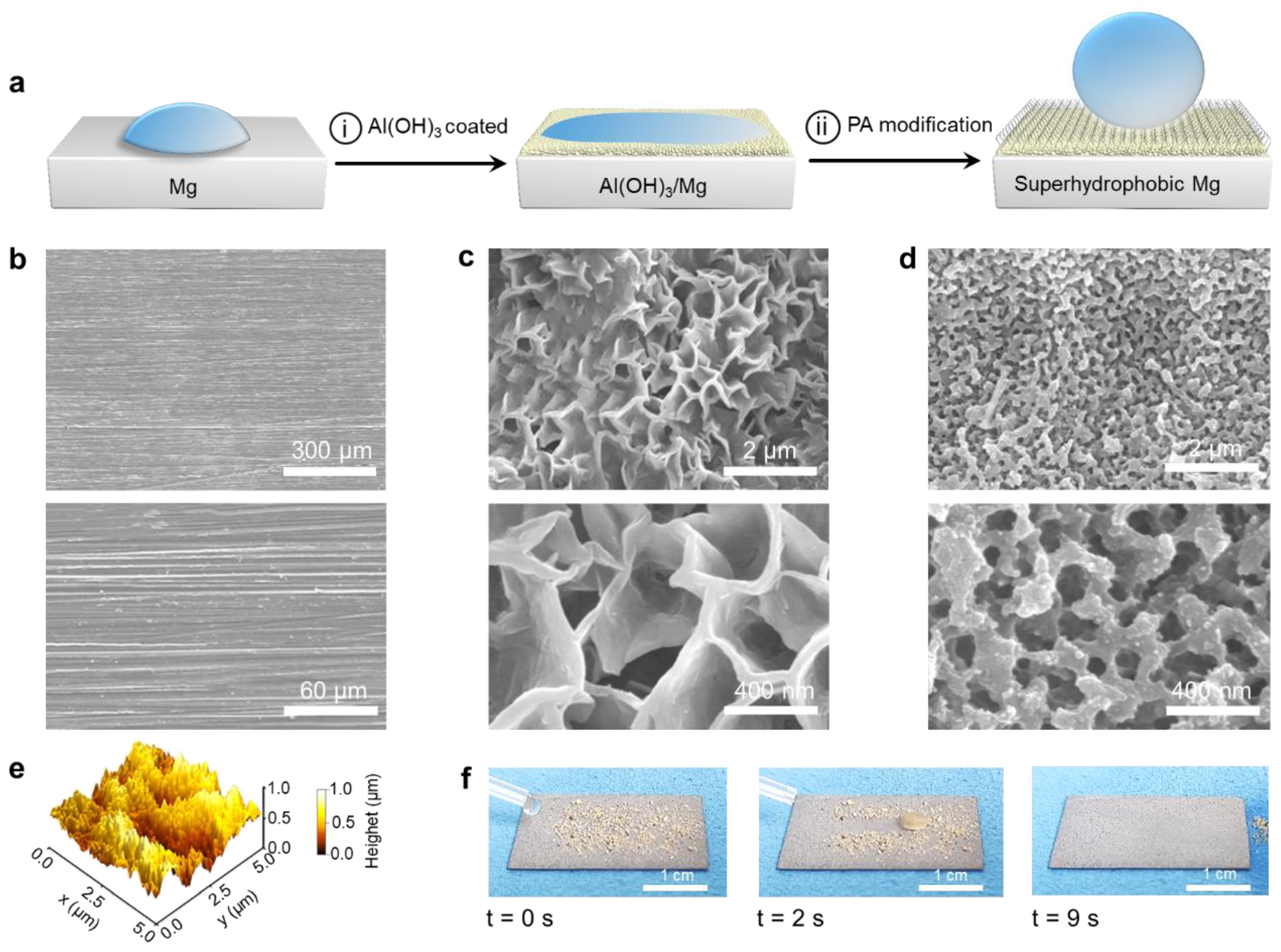
2.3. Treatment of Magnesium
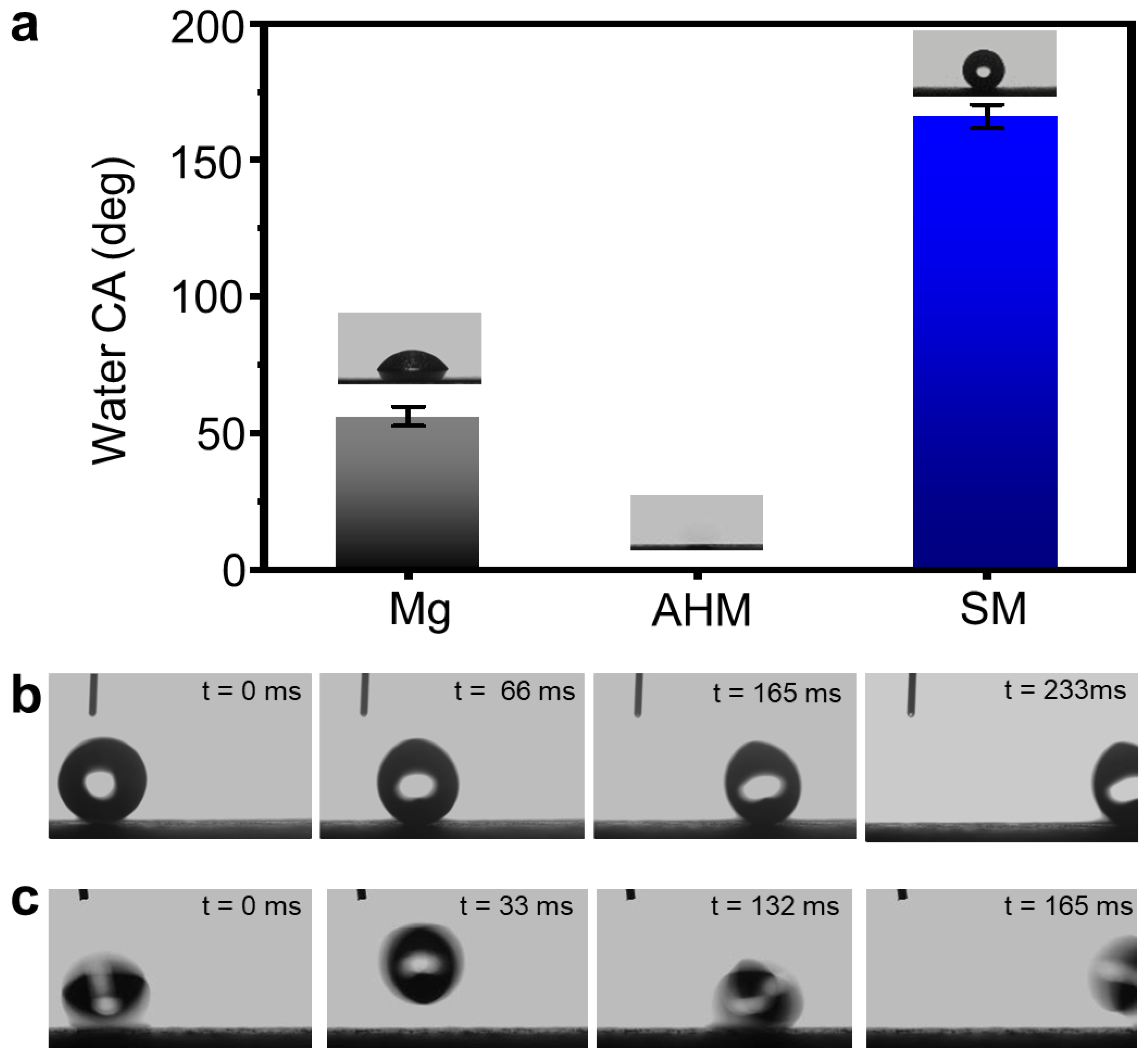
2.4. Self-Cleaning Test
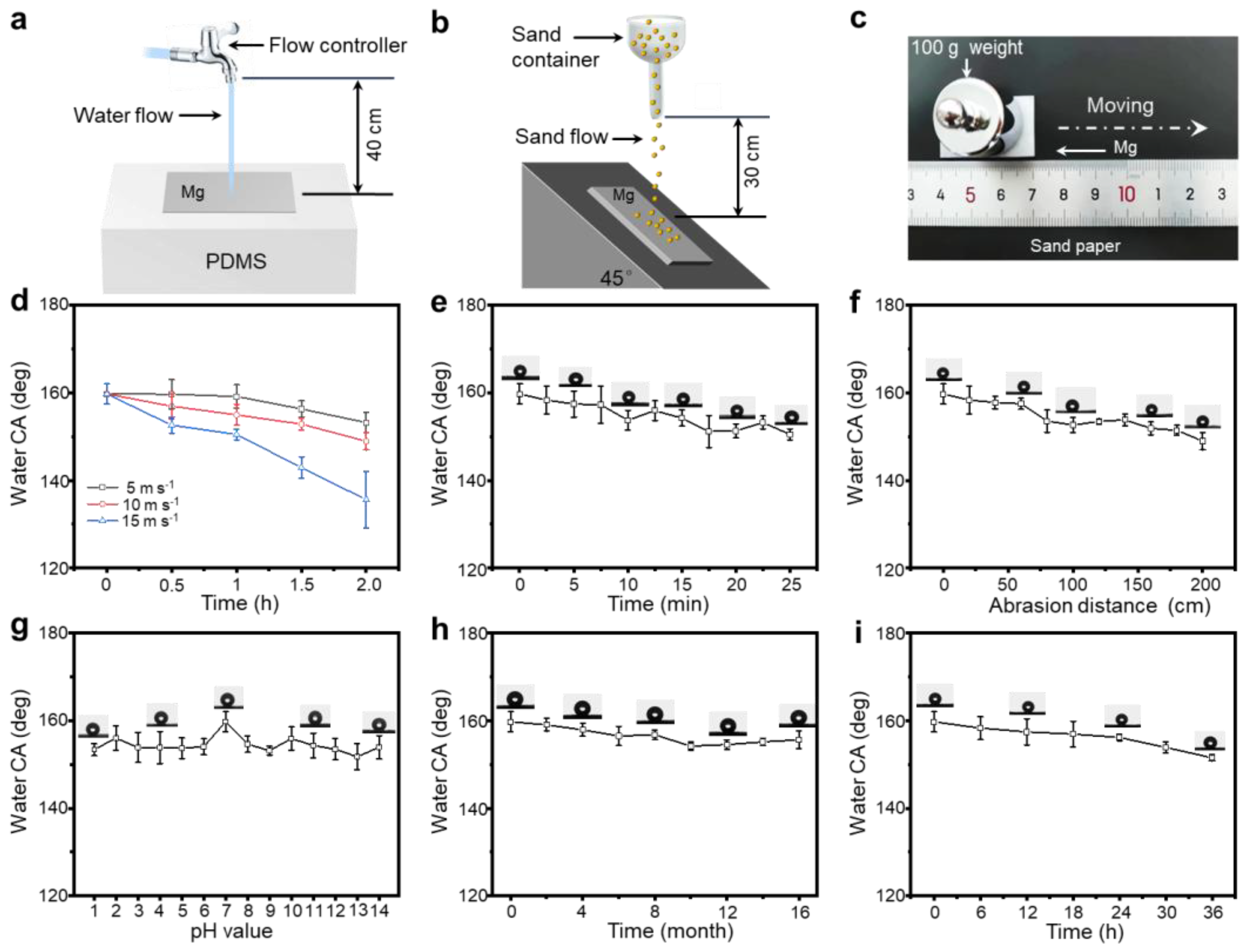
2.5. Water Flow Shear Test
2.6. Grit Impact Test
2.7. Sandpaper Friction Test
2.8. Chemical Stability Test
2.9. Durability Assessment
2.10. Heat Stability Test
3. Results and Discussion
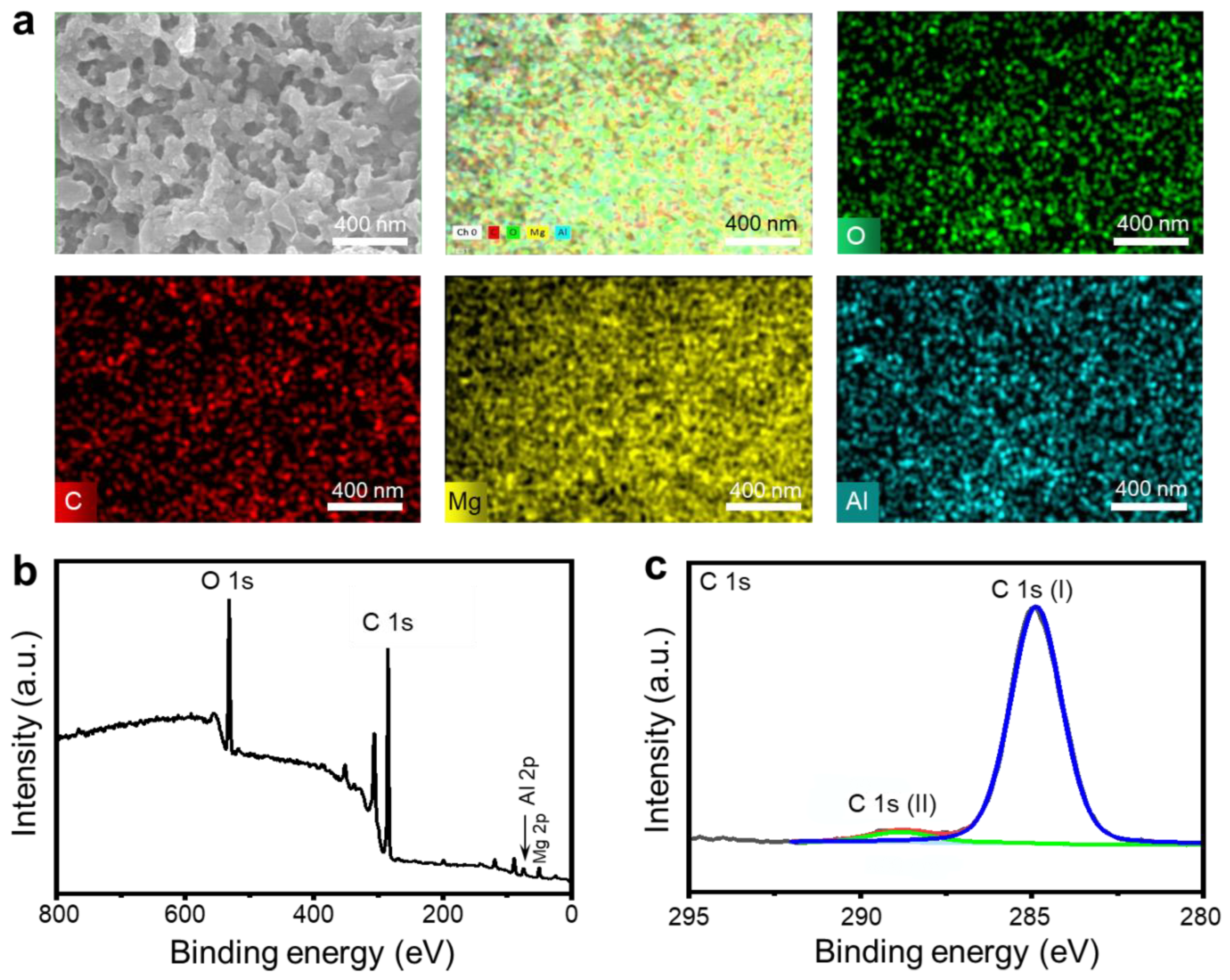
3.1. Fabrication of SM Surfaces
3.2. Mechanochemical Durability of SM Surfaces
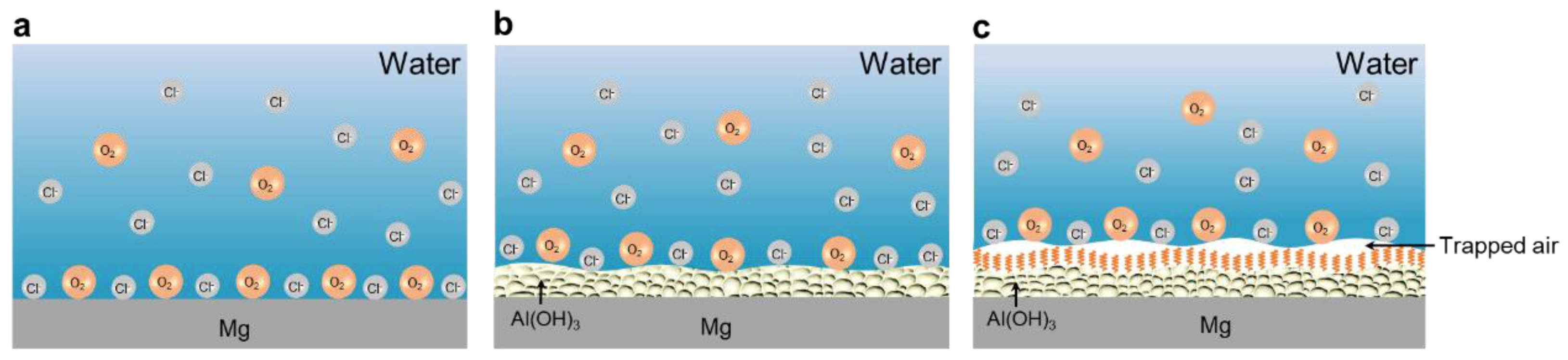
3.3. Resistance to the Corrosion of SM Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Li, X. One-Step Air Spraying of Structural Coating on Cu Alloy as Superhydrophobic Surface for Enhanced Corrosion Resistance and Anti-Icing Performance. Materials 2024, 17, 4485. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, N.; Jiang, K.; Ji, W.; Liu, Y.; Tian, F.; Zhang, X. A robust and multifunctional superhydrophobic coating based on natural palygorskite for corrosion protection of aluminum alloys. Surf. Coat. Technol. 2024, 485, 130930. [Google Scholar] [CrossRef]
- Zschach, L.G.; Baumann, R.; Soldera, F.; Méndez, C.M.; Apelt, S.; Bergmann, U.; Lasagni, A.F. On the Corrosion Properties of Aluminum 2024 Laser-Textured Surfaces with Superhydrophilic and Superhydrophobic Wettability States. Adv. Mater. Interfaces 2023, 10, 2300607. [Google Scholar] [CrossRef]
- Cholkar, A.; Chatterjee, S.; Richards, C.; McCarthy, É.; Perumal, G.; Regan, F.; Kinahan, D.; Brabazon, D. Biofouling and Corrosion Protection of Aluminum Alloys Through Ultrafast Laser Surface Texturing for Marine Applications. Adv. Mater. Interfaces 2024, 11, 2300835. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Liu, L.; Lei, J.; Li, L.; Zhang, J.; Shang, B.; He, J.; Li, N.; Pan, F. Robust Rare-Earth-Containing Superhydrophobic Coatings for Strong Protection of Magnesium and Aluminum Alloys. Adv. Mater. Interfaces 2018, 5, 1800213. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloys 2023, 11, 3948–3991. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Pan, F. A review on electromagnetic shielding magnesium alloys. J. Magnes. Alloys 2021, 9, 1906–1921. [Google Scholar] [CrossRef]
- Weiler, J.P. A review of magnesium die-castings for closure applications. J. Magnes. Alloys 2019, 7, 297–304. [Google Scholar] [CrossRef]
- Li, Y.; Hu, T.; Li, B.; Wei, J.; Zhang, J. Totally Waterborne and Highly Durable Superamphiphobic Coatings for Anti-Icing and Anticorrosion. Adv. Mater. Interfaces 2019, 6, 1901255. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, W.; Yin, X.; Wang, H.; Han, Z.; Ren, L. Controlling Wettability for Improved Corrosion Inhibition on Magnesium Alloy as Biomedical Implant Materials. Adv. Mater. Interfaces 2016, 3, 1500723. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Yu, X.; Lin, L.; Guo, X.; Liu, M.; Jiang, L. Corrosion-Resistant Superhydrophobic Coatings on Mg Alloy Surfaces Inspired by Lotus Seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Liu, X.; Zhang, Y. Recent progress in superhydrophobic coating on Mg alloys: A general review. J. Magnes. Alloys 2021, 9, 1471–1486. [Google Scholar] [CrossRef]
- Bi, Y.; Xiong, W.; Li, Z.; Wang, K.; Liu, L.; Yin, X.; Sun, D.; Li, H.; Song, Y.; Li, W.; et al. Preparation of Superhydrophobic Hydroxyapatite Coating on AZ31B Magnesium Alloy with Self-Cleaning Anti-Corrosion Properties and Excellent Stability. Met. Mater. Int. 2023, 30, 667–681. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Chen, J.; Liu, L.; Lei, J.; Li, N.; Liu, G.; Pan, F. One-step spraying method to construct superhydrophobic magnesium surface with extraordinary robustness and multi-functions. J. Magnes. Alloys 2021, 9, 668–675. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Zhang, Y.; Lv, Q.; Xu, T.; Liu, T. Super-hydrophobic surface treatment as corrosion protection for aluminum in seawater. Corros. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, L.; Chen, H.; Xu, S.; Evans, D.G.; Duan, X. Corrosion Resistance of Superhydrophobic Layered Double Hydroxide Films on Aluminum. Angew. Chem. Int. Ed. 2008, 47, 2466–2469. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Wu, C.; Yu, X.; Zhang, Y. Fabrication of stable superhydrophobic surface with improved anticorrosion property on magnesium alloy. Scr. Mater. 2013, 69, 614–617. [Google Scholar] [CrossRef]
- Li, J.; Gao, R.; Wang, Y.; Zhang, T.C.; Yuan, S. Superhydrophobic palmitic acid modified Cu(OH)2/CuS nanocomposite-coated copper foam for efficient separation of oily wastewater. Colloids Surf. A 2022, 637, 128249. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Yan, Y.; Qian, T.; Wang, J.; Sun, Q.; Jin, C. Facile fabrication of a PDMS@stearic acid-Al(OH)3 coating on lignocellulose composite with superhydrophobicity and flame retardancy. Appl. Surf. Sci. 2018, 450, 387–395. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Shao, R.; Chen, J.; Zang, D. Controlled self-cleaning aluminum surfaces mediated by anodic aluminum oxide. Microsc. Res. Tech. 2023, 86, 694–700. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Akbulut, O.; Hu, J.; Suib, S.L.; Kong, J.; Stellacci, F. Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol. 2008, 3, 332–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Wu, H.; Wu, J. Facile fabrication of superhydrophobic nanostructures on aluminum foils with controlled-condensation and delayed-icing effects. Appl. Surf. Sci. 2012, 258, 8253–8257. [Google Scholar] [CrossRef]
- Frydman, E.; Cohen, H.; Maoz, R.; Sagiv, J. Monolayer Damage in XPS Measurements As Evaluated by Independent Methods. Langmuir 1997, 13, 5089–5106. [Google Scholar] [CrossRef]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Wong, K.C.; Dravid, V.P. Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett. 2004, 4, 383–386. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zang, D.; Xun, X.; Gu, Z.; Dong, J.; Pan, T.; Liu, M. Fabrication of superhydrophobic self-cleaning manganese dioxide coatings on Mg alloys inspired by lotus flower. Ceram. Int. 2020, 46, 20328–20334. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. Scale effects in friction using strain gradient plasticity and dislocation-assisted sliding (microslip). Acta Mater. 2003, 51, 4331–4345. [Google Scholar] [CrossRef]
- Hensel, R.; Neinhuis, C.; Werner, C. The springtail cuticle as a blueprint for omniphobic surfaces. Chem. Soc. Rev. 2016, 45, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Ivanovac, E.P.; Adhikari, B. Bio-inspired sustainable and durable superhydrophobic materials: From nature to market. J. Mater. Chem. 2019, 7, 16643–16670. [Google Scholar] [CrossRef]
- Zou, T.; Duan, S.; Wu, D.; Matsuda, K.; Guo, F.; Zou, Y. Stress corrosion behavior and microstructure analysis of Al-Zn-Mg-Cu alloys fabricated by CMT wire arc additive manufacturing with different post-treatments. J. Alloys Compd. 2025, 1010, 177759. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Loffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef]
- Priyantha, N.; Jayaweera, P.; Macdonald, D.D.; Sun, A. An electrochemical impedance study of Alloy 22 in NaCl brine at elevated temperature. I. Corrosion behavior. J. Electroanal. Chem. 2004, 572, 409–419. [Google Scholar] [CrossRef]
- Memon, H.; Wang, J.; Hou, X.H. Interdependence of Surface Roughness on Icephobic Performance: A Review. Materials 2023, 16, 4607. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wang, D.; Ma, X.; Zhu, H.; Qiao, Y.; Liu, X. “Petal effect”-inspired superhydrophobic and highly adhesive coating on magnesium with enhanced corrosion resistance and biocompatibility. Sci. China Mater. 2017, 4, 629–642. [Google Scholar] [CrossRef]
- Ishizaki, T.; Shimada, Y.; Tsunakawa, M.; Lee, H.; Yokomizo, T.; Hisada, S.; Nakamura, K. Rapid fabrication of a crystalline myristic acid-based superhydrophobic film with corrosion resistance on magnesium alloys by the facile one-step immersion process. ACS Omega 2017, 2, 7904–7915. [Google Scholar] [CrossRef]
- Kuang, J.; Ba, Z.; Li, Z.; Jia, Y.; Wang, Z. Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surf. Coat. Technol. 2019, 361, 75–82. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Song, L.; Zeng, R.; Li, S.; Han, E. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Fang, R.; Liu, R.; Xie, Z.H.; Wu, L.; Ouyang, Y.; Li, M. Corrosion-resistant and superhydrophobic nickel-phosphorus/nickel/PFDTMS triple-layer coating on magnesium alloy. Surf. Coat. Technol. 2022, 432, 128054. [Google Scholar] [CrossRef]
- Xue, M.; Wang, J.; Zhang, J.; Niu, B.; Gao, X.; Hong, Y. Electroplated super-hydrophobic Zn-Fe coating for corrosion protection on magnesium alloy. Trans. Nonferrous Met. Soc. China 2022, 32, 3250–3258. [Google Scholar]
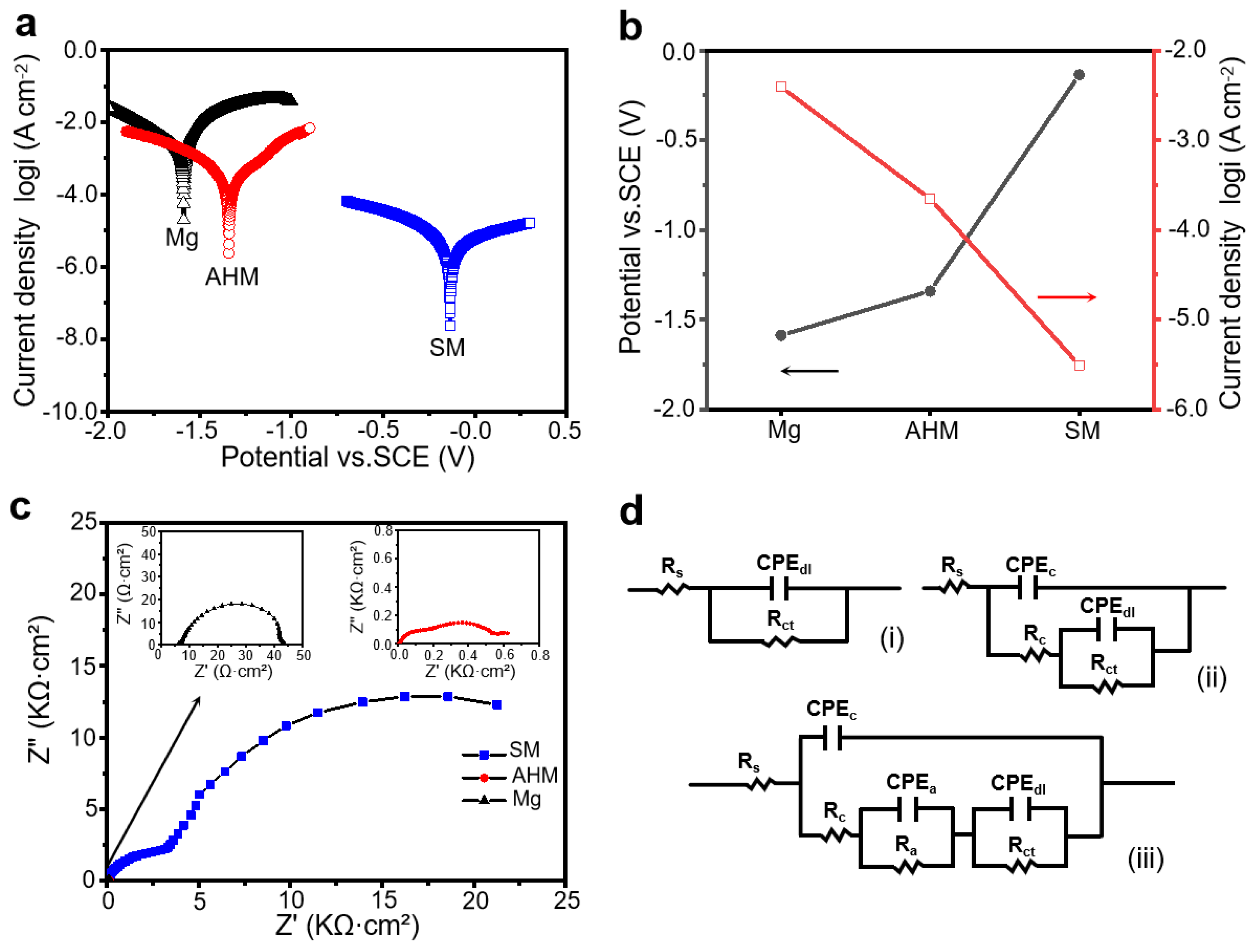
| Sample | Rs/(Ω·cm2) | CPEdl | n | Rct/(Ω·cm2) | CPEc | n | Rc/(Ω·cm2) | CPEa | n | Ra/(Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Y0/(Ω−1 cm−2 sn) | Y0/(Ω−1·cm−2 sn) | Y0/(Ω−1·cm−2 sn) | ||||||||
| Mg | 6.839 | 5.193 × 10−5 | 0.9161 | 3.761 × 101 | — | — | — | — | — | — |
| AHM | 8.230 | 5.975 × 10−4 | 0.4926 | 5.353 × 102 | 2.671 × 10−5 | 0.8126 | 1.901 × 102 | — | — | — |
| SM | 1.461 | 3.904 × 10−5 | 0.9713 | 2.435 × 104 | 2.008 × 10−6 | 0.9999 | 1.287 × 103 | 5.388 × 10−6 | 0.9945 | 2.502 × 103 |
| Substrate | Surface Coating | Water CA | Mechanical Durability | Icorr Decreased by Orders of Magnitude | Application | Ref. |
|---|---|---|---|---|---|---|
| Mg | LDH/Sodium oleate | 151.2 ± 2.4° | The length of abrasion = 50 cm (2.45 kPa, 1000 grit sandpaper) | 2 | Anti-corrosion Biomedicine | [36] |
| AZ31B | Crystalline solid myristic | 156.2 ± 2° | / | 2 | Anti-corrosion | [37] |
| Mg | LDH/Myristic acid | 152.2° | / | 1 | Anti-corrosion | [38] |
| Mg-Li-Ca alloy | MAO/Stearic acid | 155.5° | / | 3 | Anti-corrosion | [39] |
| AZ31B | Ni-phosphorus/Ni/PFDTMS | 153.0 ± 4.6° | / | 2 | Anti-corrosion | [40] |
| AZ31B | Zn-Fe/Myristic acid | 153.0° | The length of abrasion = 1 m (2.45 Pa, 2000 grit sandpaper) | 1 | Anti-corrosion Self-cleaning | [41] |
| Mg | Al(OH)3/Palmitic acid | 159.0 ± 2.3° | (1) The length of abrasion = 200 cm (100 g weight, 1000 grit sandpaper). (2) The time of water flow shear = 2.0 h (speed = 10 cm/s). (3) The time of grit impact = 25 min (drop height = 30 cm). | 3 | Anti-corrosion Self-cleaning | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Xu, B.; Zhao, Y.; Zhou, K.; Shao, R.; Xun, X.; Zhang, F.; Zang, D. An Acid–Base Proton Transfer Approach to Robust Superhydrophobic Self-Cleaning Surfaces for the Corrosion Protection of Magnesium. Materials 2025, 18, 1028. https://doi.org/10.3390/ma18051028
Chen J, Xu B, Zhao Y, Zhou K, Shao R, Xun X, Zhang F, Zang D. An Acid–Base Proton Transfer Approach to Robust Superhydrophobic Self-Cleaning Surfaces for the Corrosion Protection of Magnesium. Materials. 2025; 18(5):1028. https://doi.org/10.3390/ma18051028
Chicago/Turabian StyleChen, Junjie, Baoshan Xu, Yunhao Zhao, Ke Zhou, Ruijuan Shao, Xiaowei Xun, Fan Zhang, and Dongmian Zang. 2025. "An Acid–Base Proton Transfer Approach to Robust Superhydrophobic Self-Cleaning Surfaces for the Corrosion Protection of Magnesium" Materials 18, no. 5: 1028. https://doi.org/10.3390/ma18051028
APA StyleChen, J., Xu, B., Zhao, Y., Zhou, K., Shao, R., Xun, X., Zhang, F., & Zang, D. (2025). An Acid–Base Proton Transfer Approach to Robust Superhydrophobic Self-Cleaning Surfaces for the Corrosion Protection of Magnesium. Materials, 18(5), 1028. https://doi.org/10.3390/ma18051028





