In Situ EBSD Study of Aluminum After Embrittlement by Gallium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Selection
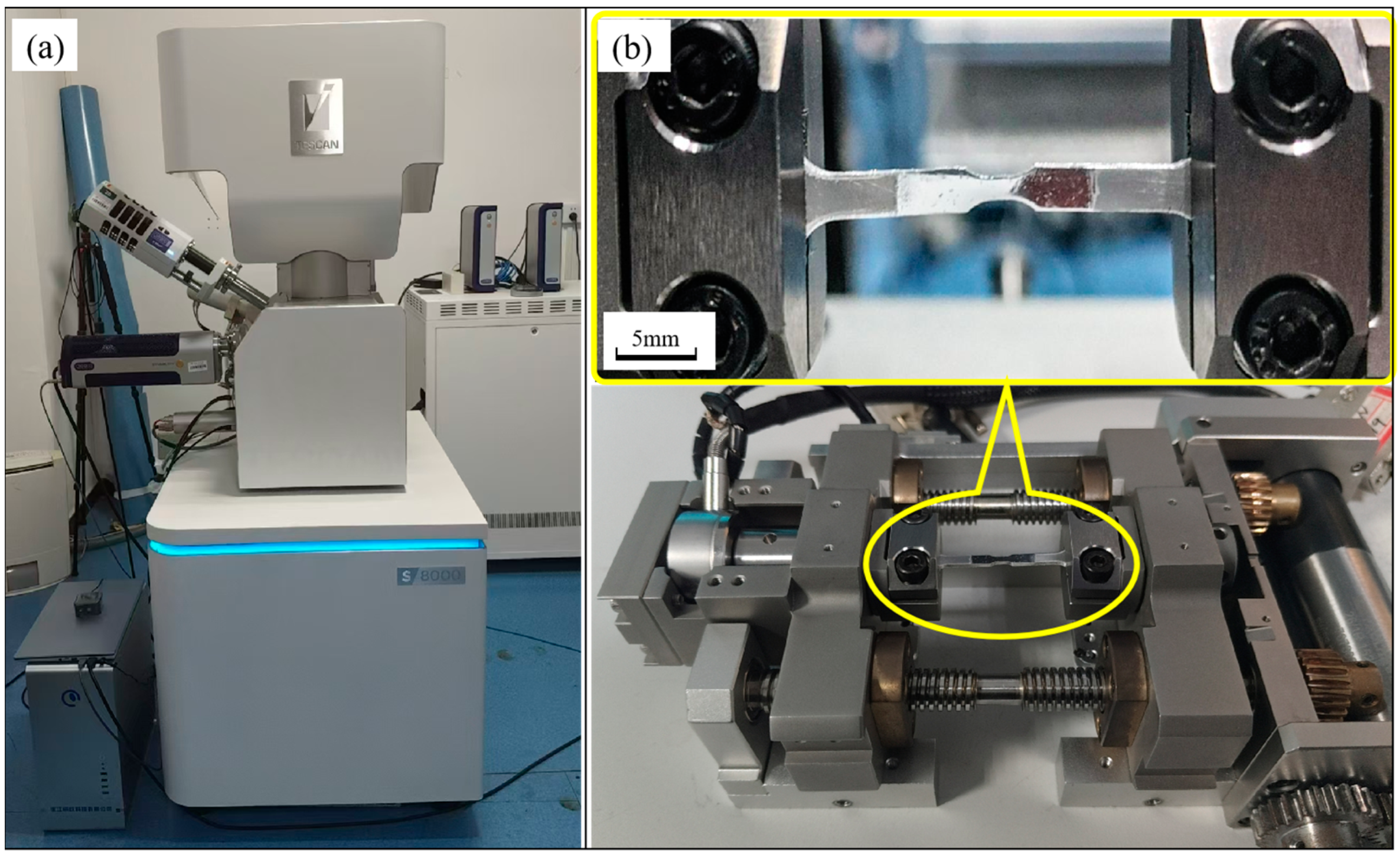
2.2. Experimental Design
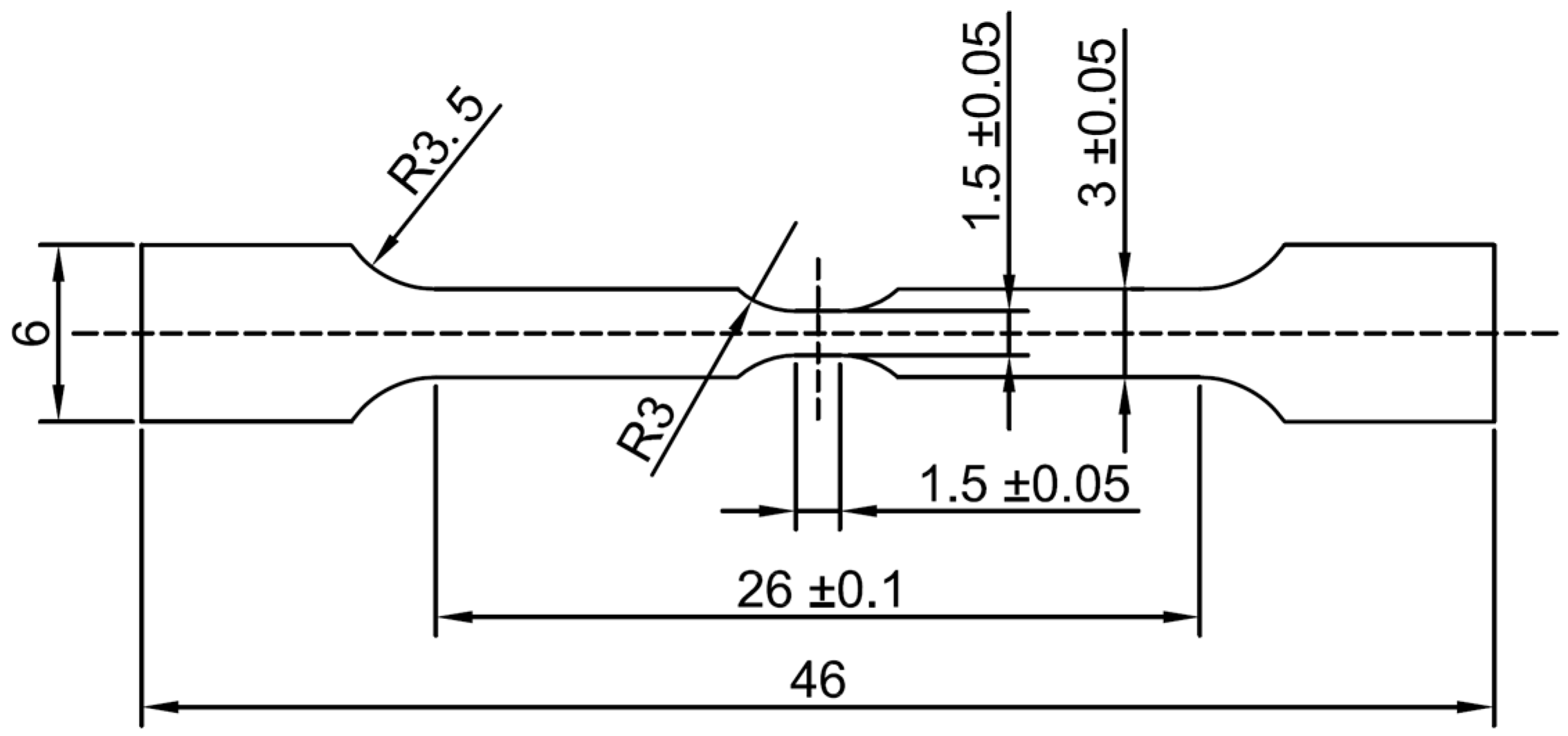
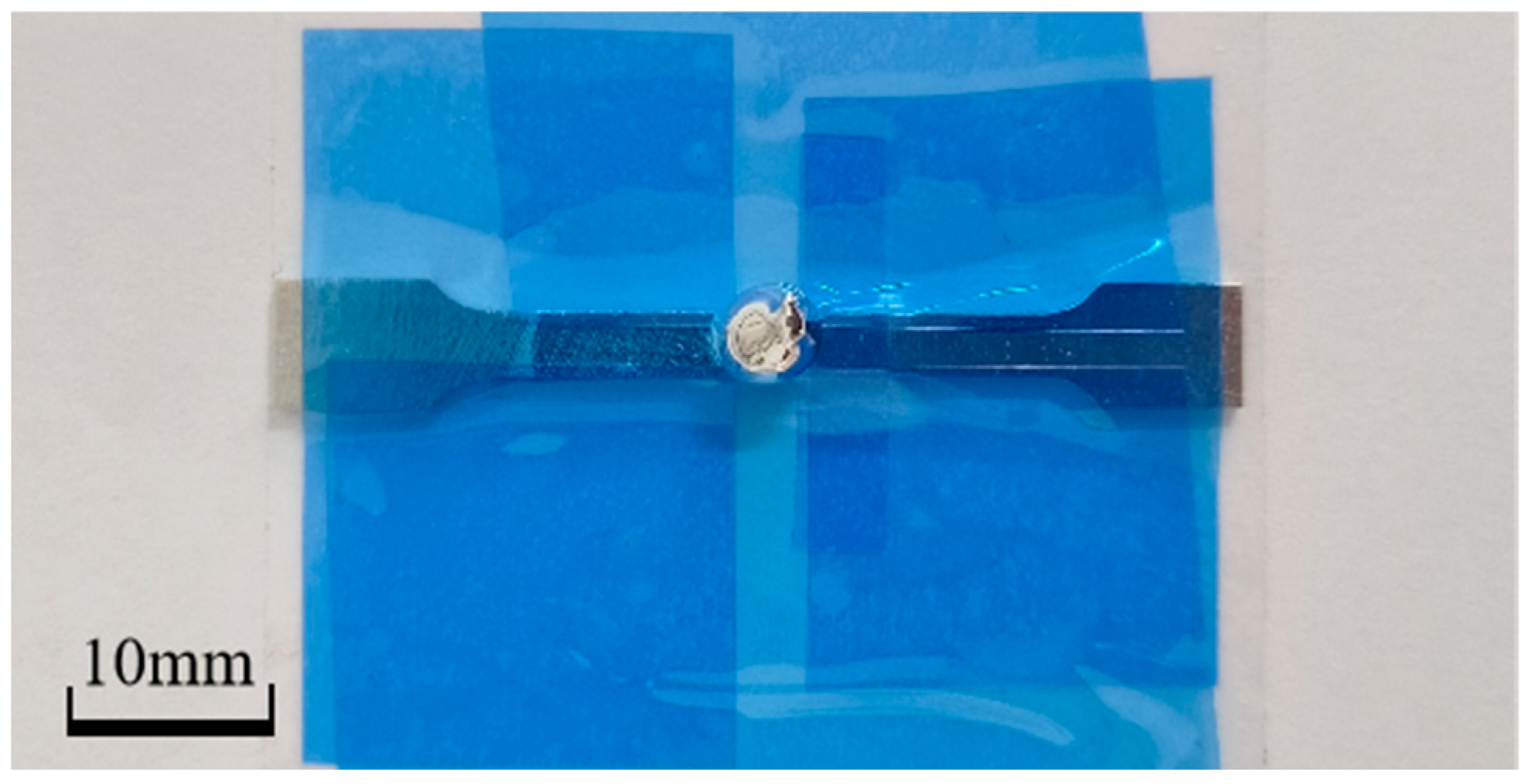
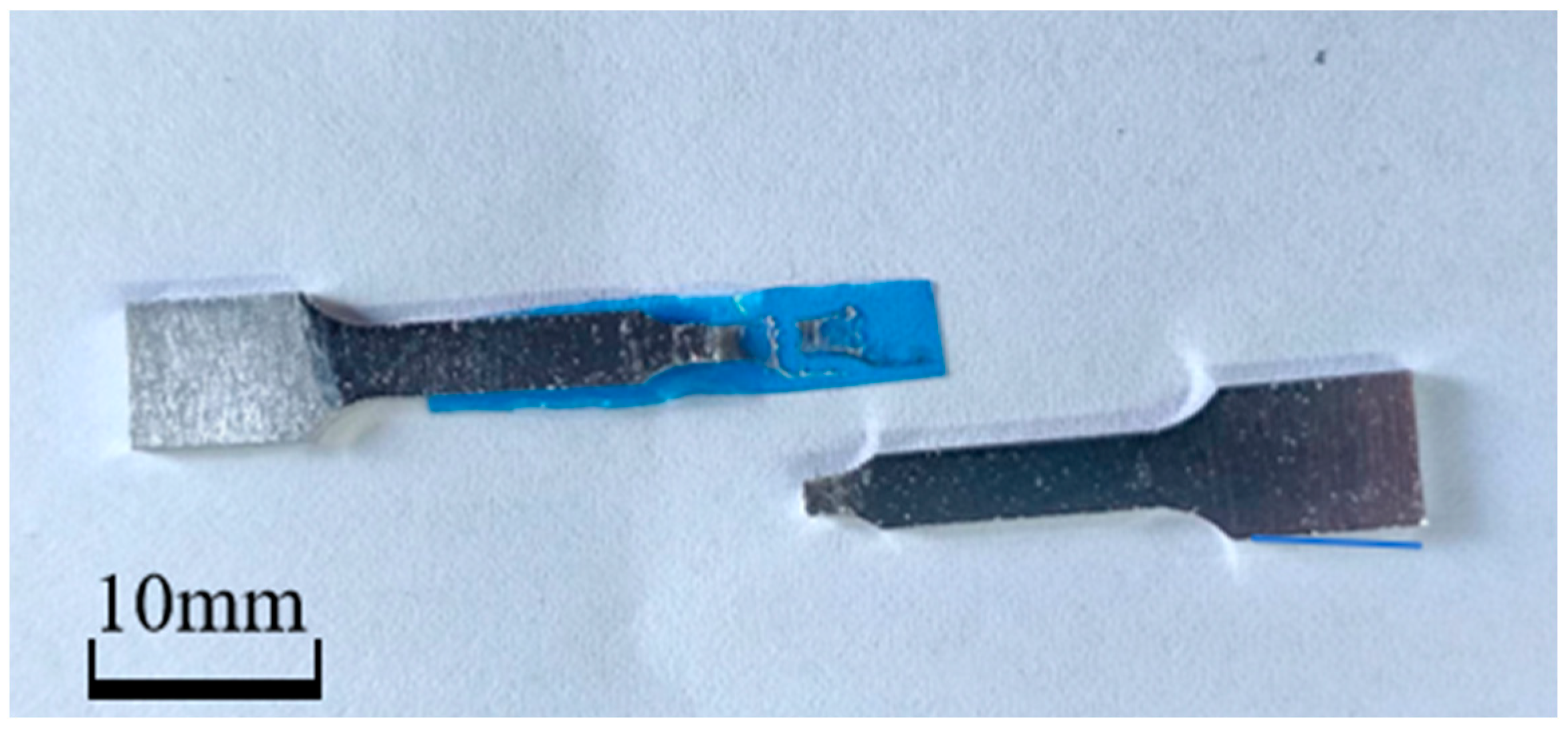
2.3. Sample Preparation
3. Results
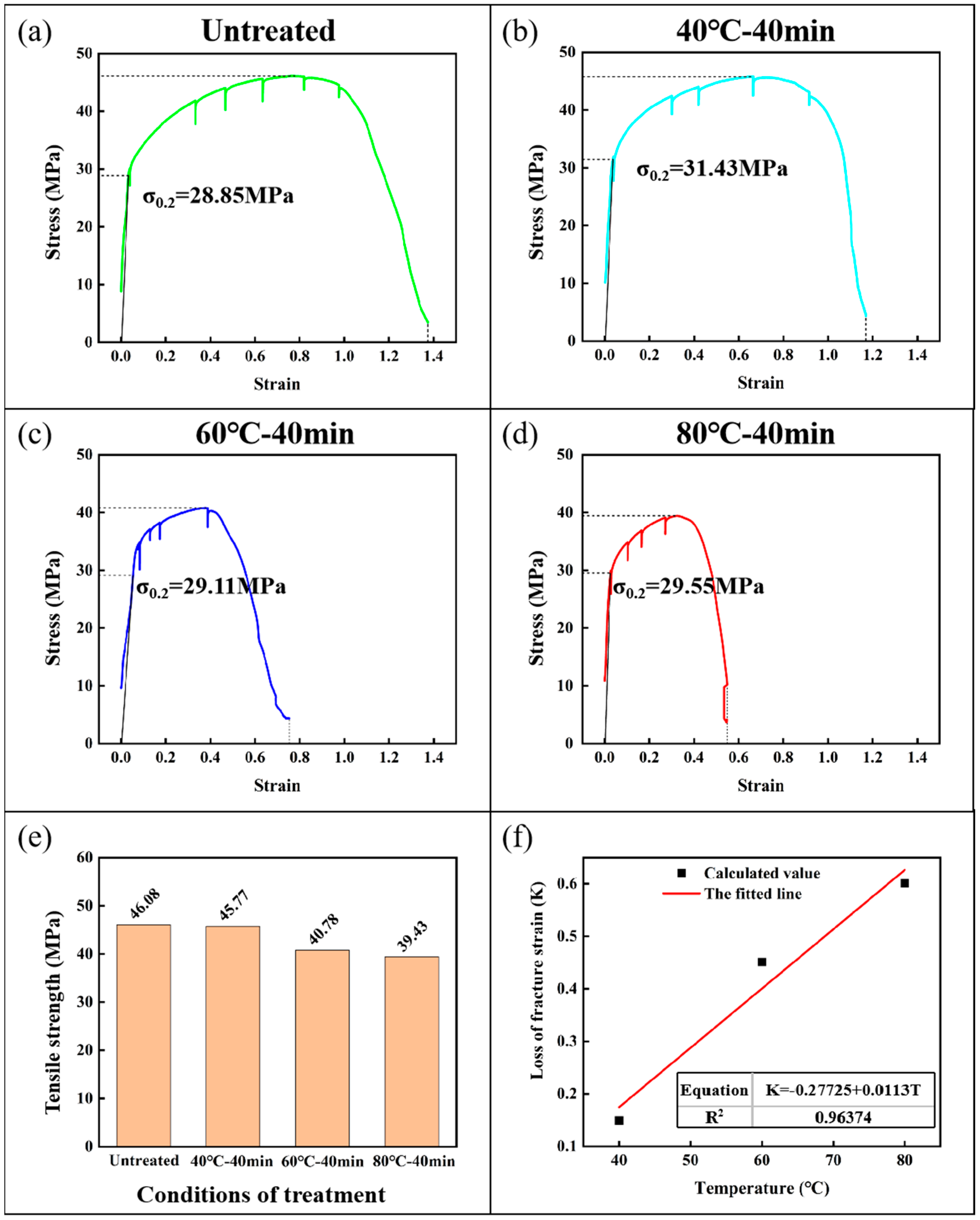
3.1. Mechanical Test Results
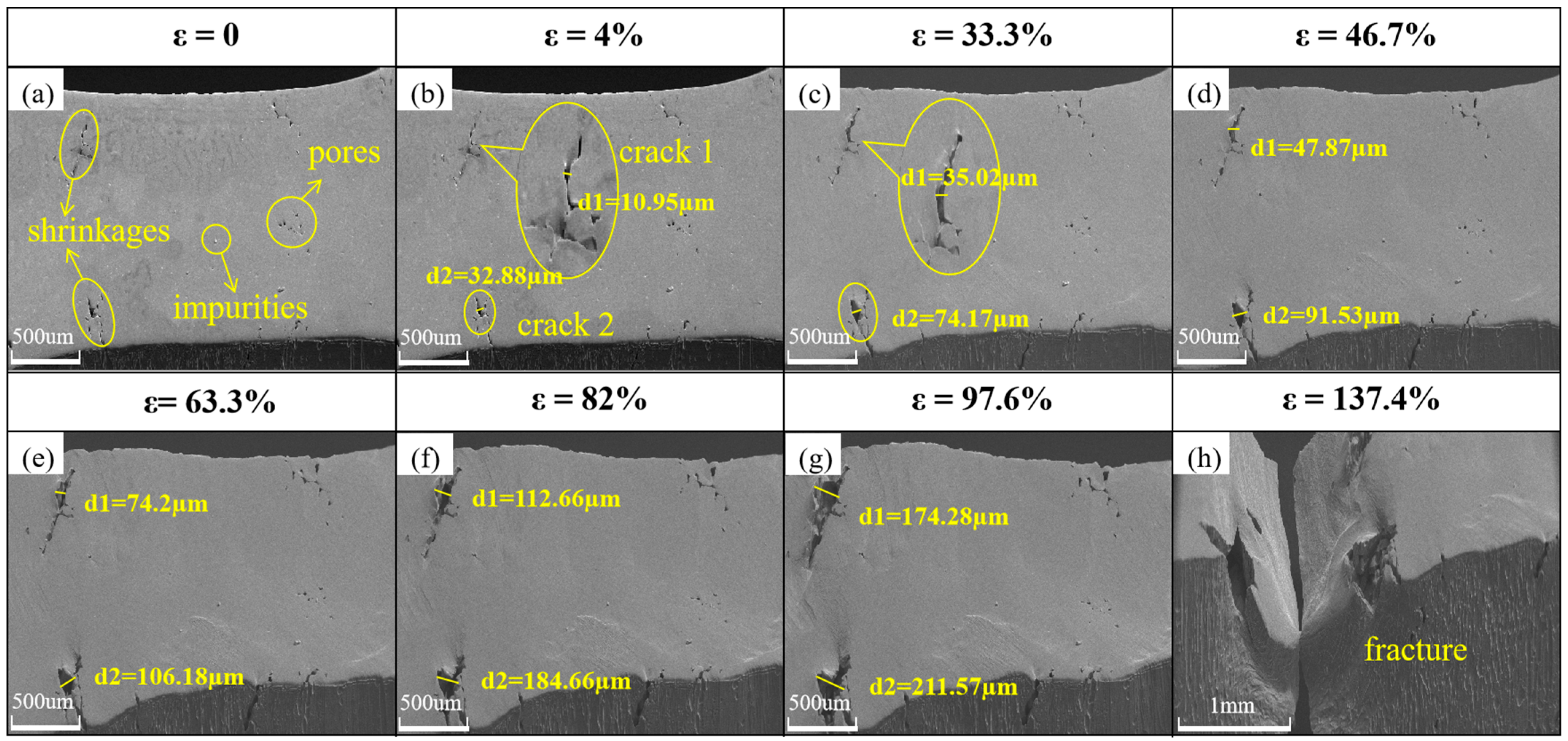
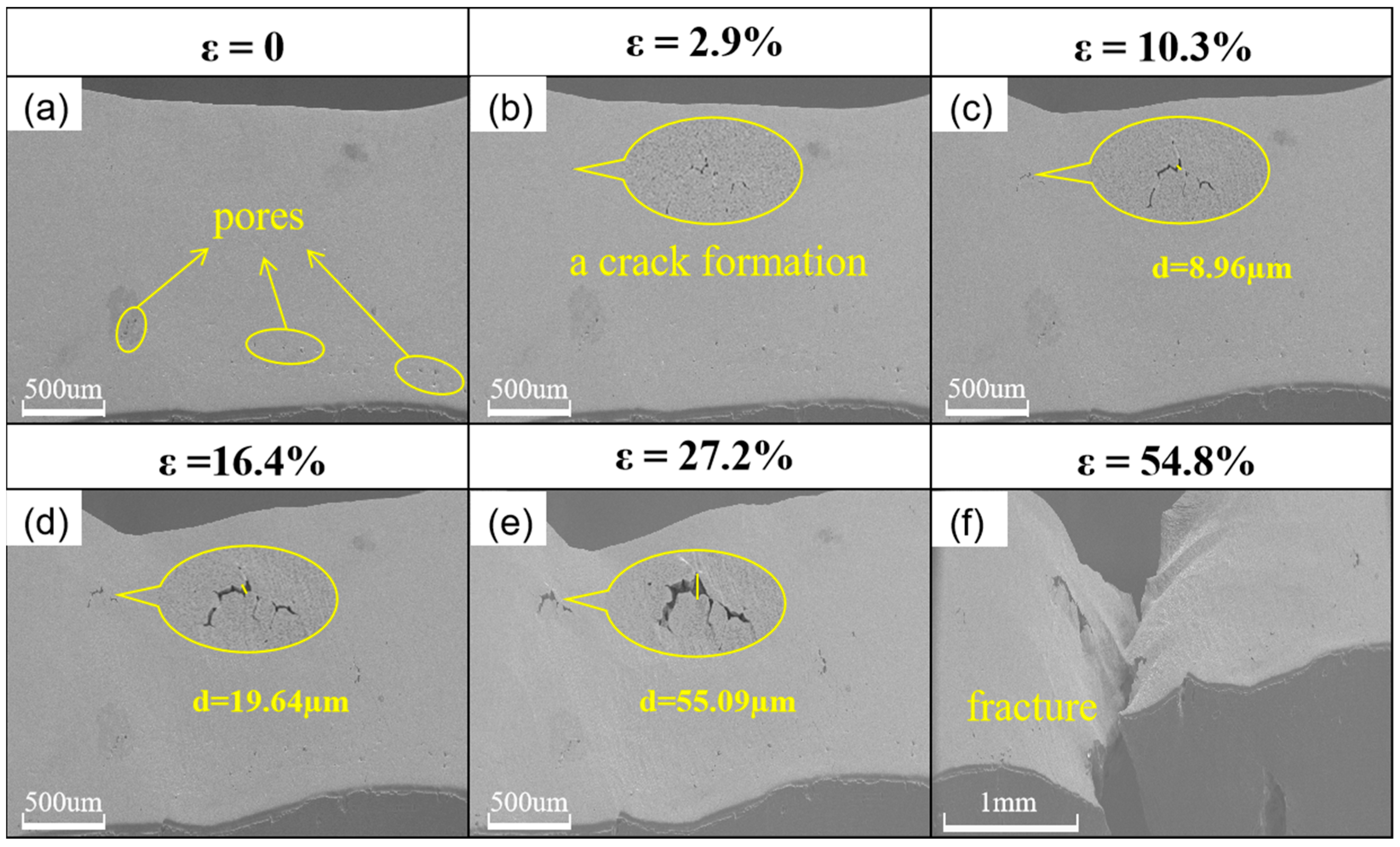
3.2. Microcosmic Observation
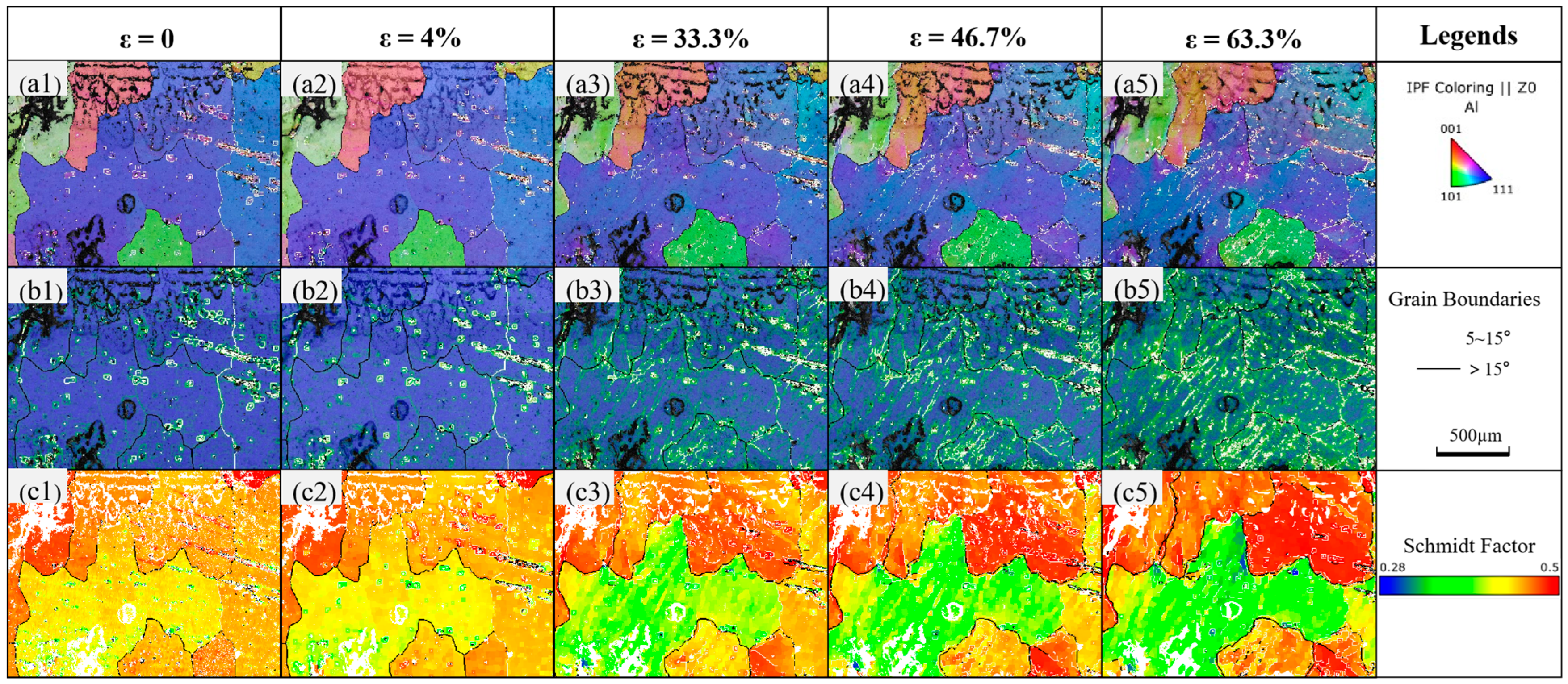
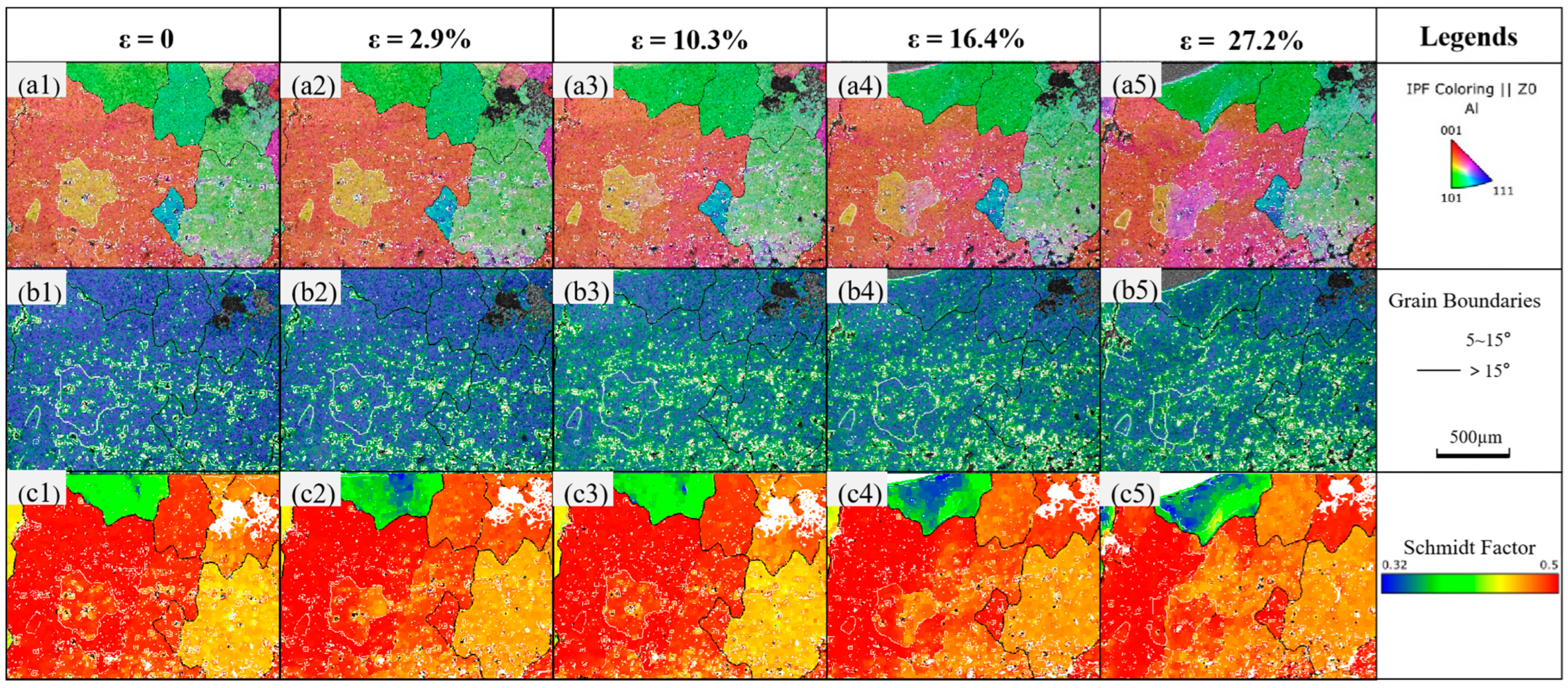
3.3. Substructure and Dislocation Motion
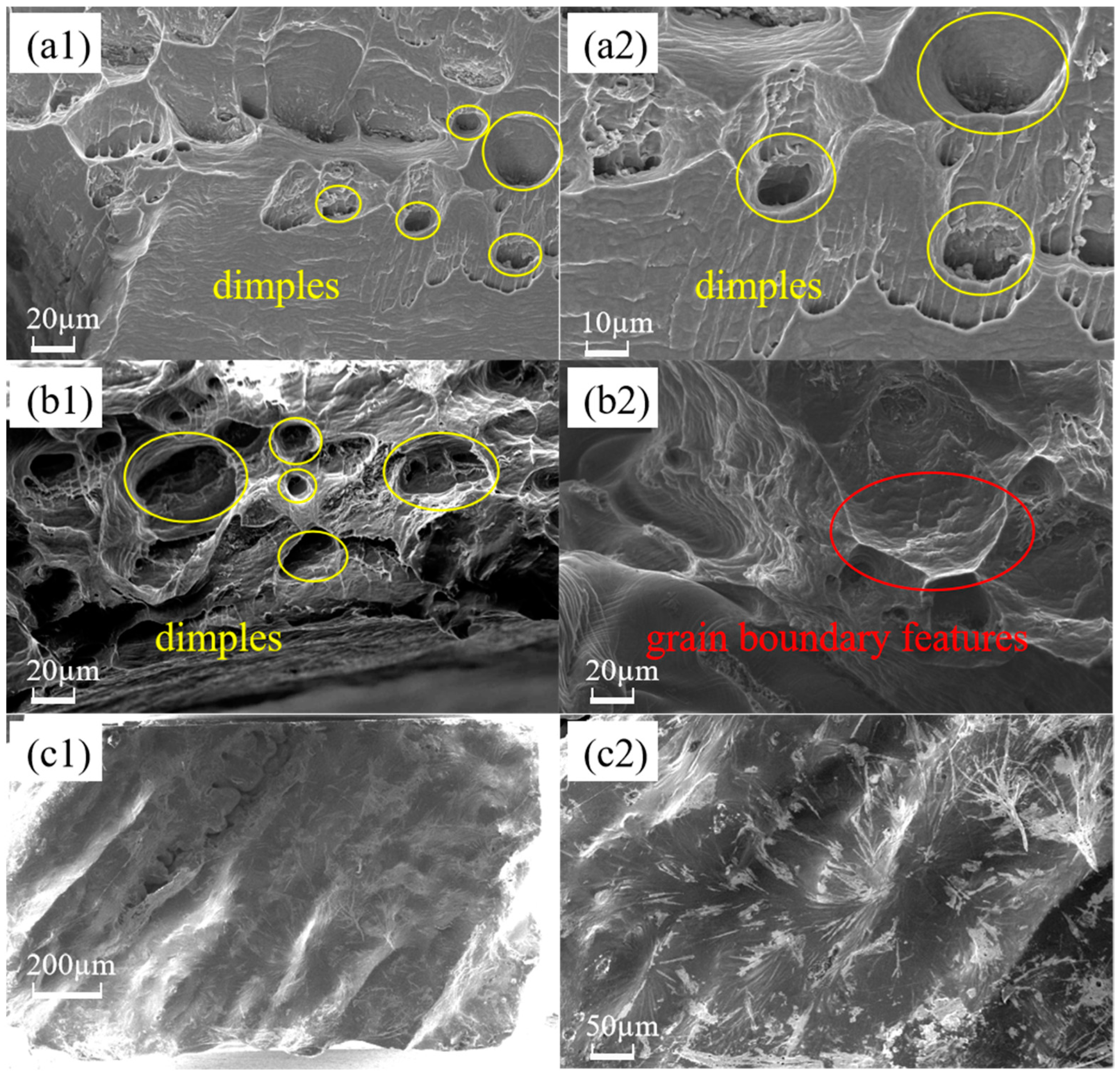
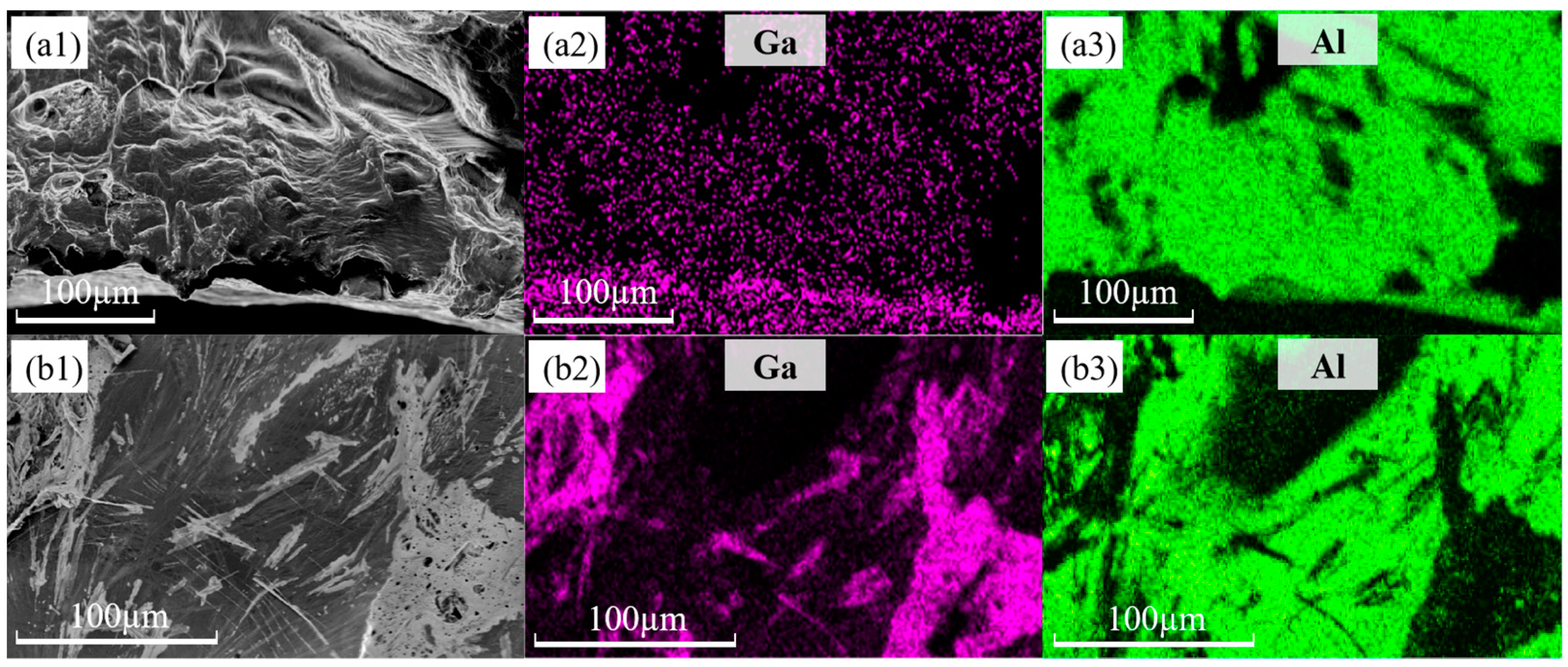
3.4. Micro-Morphologic Observation of the Fracture
4. Discussion
5. Conclusions
- Liquid gallium metal will cause the serious LME of aluminum, mainly manifested in the reduction in elongation at the break. In a certain temperature range, when the embrittlement time of the specimens is the same, the higher the embrittlement temperature, the more the embrittlement effect is obvious. In aluminum specimens at 80 °C and 40 min of liquid gallium embrittlement, the fracture strain fell to 40% of the untreated specimens.
- After embrittlement, the plastic deformation ability of the specimen is greatly reduced, and cracks are easily generated at grain boundaries and grow along the grain boundaries under the action of external forces, and the crack expansion rate of the embrittled specimen is significantly higher than that of the untreated specimen.
- Under stress, the penetration of liquid metal gallium atoms can promote dislocation emission and substructure formation in aluminum.
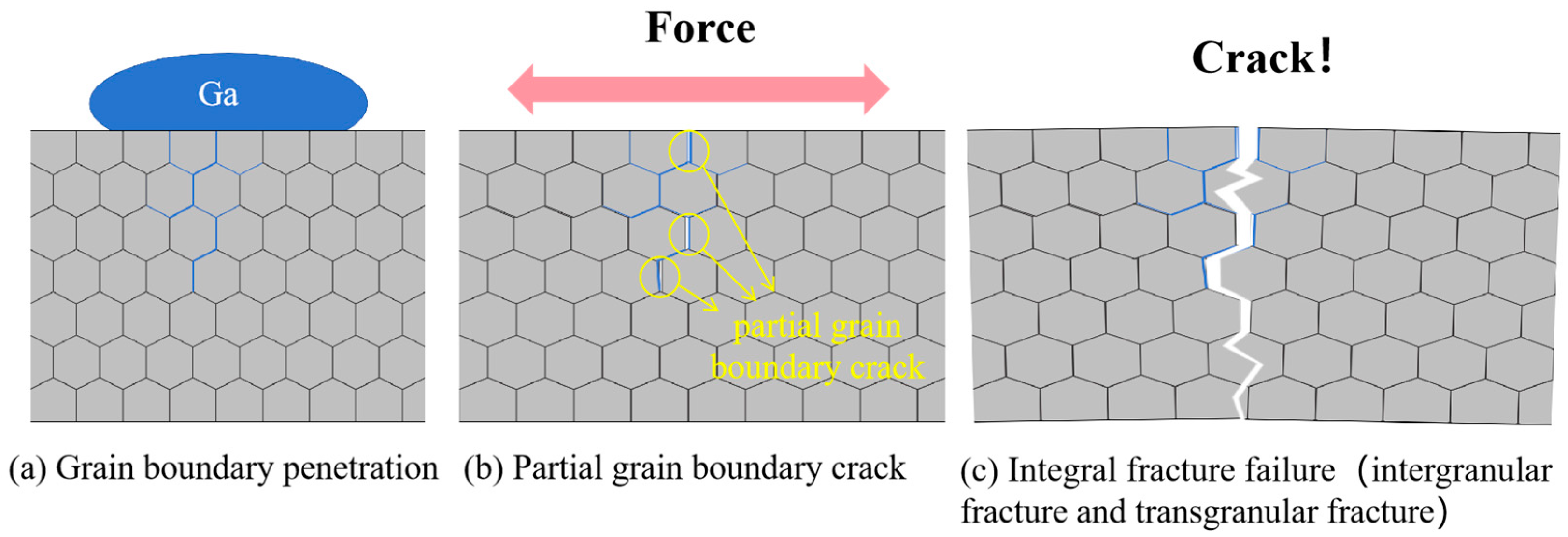
- Aluminum specimens under the action of LME can be divided into three stages of tensile fracture, which are as follows: gallium atoms in the penetration of aluminum grain boundaries, local intergranular cracking under the action of external forces, and the integral failure of the specimen, and, ultimately, the fracture presents a complex mixture of fracture characteristics. However, excessive gallium atoms in the aluminum matrix will lead to the specimen exhibiting the second stage of the direct occurrence of cleavage fracture.
- The microscopic mechanisms and macroscopic regulations of LME have essentially been clarified after almost a century of research. The LME phenomenon for common binary metal systems is also more clearly recognized. However, there is a general difficulty in studying the LME phenomenon in poly-alloy systems and conducting quantitative studies of LME because of the excessive number and coupling of factors affecting liquid metal embrittlement. A more comprehensive examination of LME from the standpoint of atomic energy can be carried out in the future by applying the first nature principle. On the other hand, a large number of experiments can be carried out under different conditions for certain typical combinations of metal materials and embrittlement agents with mature preparation processes (e.g., 7075 aluminum alloy and gallium) to obtain relatively accurate empirical formulas for LME under specific conditions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westwood, A.R.C.; Kamdar, M.H. Concerning liquid metal embrittlement, particularly of zinc monocrystals by mercury. Philos. Mag. A 1963, 8, 787–804. [Google Scholar] [CrossRef]
- English, J.J.; Duquette, D.J. Mercury liquid embrittlement failure of 5083-o aluminum alloy piping. J. Fail. Anal. Prev. 2022, 22, 2095–2103. [Google Scholar] [CrossRef]
- Dinda, S.; Warke, W.R. The effect of grain boundary segregation on liquid metal induced embrittlement of steel. Mater. Sci. Eng. 1976, 24, 199–208. [Google Scholar] [CrossRef]
- Sun, Z.; Li, P.; Zhang, T.; Wang, J.; Xu, D.; Chen, H.; Liu, D.; Pei, Y. Effect of stress and Si on liquid metal embrittlement of 316l(N)-copper. Mater. Sci. Technol. 2021, 37, 552–559. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, K.; Zheng, J.; Feng, H.; Song, P.; Hu, H.; Mao, Z. Experimental study on liquid metal embrittlement of Al-Zn-Mg aluminum alloy (7075): From macromechanical property experiment to microscopic characterization. Materials 2024, 17, 628. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.G.; Old, C.F. Liquid metal embrittlement. J. Mater. Sci. 1979, 14, 1–18. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tsuru, T.; Itakura, M.; Abe, E. Atomistic weak interaction criterion for the specificity of liquid metal embrittlement. Sci. Rep. 2022, 12, 10886. [Google Scholar] [CrossRef]
- Bingham, M.D. Field detection and implications of mercury in natural gas. SPE Prod. Eng. 1990, 5, 120–124. [Google Scholar] [CrossRef]
- Xia, J.S.; Wang, Y.D.; Wang, L.C. Natural gas mercury removal technology in Hainan Fushan oilfield. Nat. Gas Ind. 2007, 7, 127–128. [Google Scholar]
- Legris, A.; Nicaise, G.; Vogt, J.-B.; Foct, J.; Gorse, D.; Vançon, D. Embrittlement of a martensitic steel by liquid lead. Scr. Mater. 2000, 43, 997–1001. [Google Scholar] [CrossRef]
- Nicaise, G.; Legris, A.; Vogt, J.B.; Foct, J. Embrittlement of the martensitic steel 91 tested in liquid lead. J. Nucl. Mater. 2001, 296, 256–264. [Google Scholar] [CrossRef]
- Van Den Bosch, J.; Bosch, R.W.; Sapundjiev, D.; Almazouzi, A. Liquid metal embrittlement susceptibility of ferritic–martensitic steel in liquid lead alloys. J. Nucl. Mater. 2008, 376, 322–329. [Google Scholar] [CrossRef]
- Gorse, D.; Auger, T.; Vogt, J.-B.; Serre, I.; Weisenburger, A.; Gessi, A.; Agostini, P.; Fazio, C.; Hojna, A.; Di Gabriele, F.; et al. Influence of liquid lead and lead-bismuth eutectic on tensile, fatigue and creep properties of ferritic/martensitic and austenitic steels for transmutation systems. J. Nucl. Mater. 2011, 415, 284–292. [Google Scholar] [CrossRef]
- DiGiovanni, C.; Shojaee, M.; Biro, E.; Zhou, N.Y. Effect of external loading on liquid metal embrittlement severity during resistance spot welding. Manuf. Lett. 2022, 33, 11–14. [Google Scholar] [CrossRef]
- Akbari, E.; Kürnsteiner, P.; Steineder, K.; Gruber, M.; Arndt, M.; Letofsky-Papst, I.; Sierlinger, R.; Groiss, H. Insight into grain boundaries with reduced liquid metal embrittlement susceptibility in a boron-added 3rd generation advanced high strength steel. Mater. Des. 2024, 237, 112584. [Google Scholar] [CrossRef]
- Dong, W.; Pan, H.; Lei, M.; Wang, S.; Ding, K.; Gao, Y. Three-dimension characterization of the liquid metal embrittlement crack in the resistance spot welded joint of the advanced high strength steel. Mater. Today Commun. 2023, 34, 105322. [Google Scholar] [CrossRef]
- Han, J.-H.; Jee, K.-K.; Oh, K.H. Orientation rotation behavior during in situ tensile deformation of polycrystalline 1050 aluminum alloy. Int. J. Mech. Sci. 2003, 45, 1613–1623. [Google Scholar] [CrossRef]
- Kahl, S.; Peng, R.L.; Johansson, S. Tensile failure of thin aluminum sheet observed by in-situ EBSD. IOP Conf. Ser. Mater. Sci. Eng. 2015, 82, 012095. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y. A quasi-in-situ EBSD study on the effect of abnormal grain growth on mechanical properties of the friction-stir welds of 2219 aluminum alloy. Mater. Today Commun. 2025, 42, 111212. [Google Scholar] [CrossRef]
- Lupo, M.; Ajabshir, S.Z.; Sofia, D.; Barletta, D.; Poletto, M. Experimental metrics of the powder layer quality in the selective laser sintering process. Powder Technol. 2023, 419, 118346. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Liquid metal material genome: Initiation of a new research track towards discovery of advanced energy materials. Front. Energy 2013, 7, 317–332. [Google Scholar] [CrossRef]
- Zuraiqi, K.; Zavabeti, A.; Allioux, F.-M.; Tang, J.; Nguyen, C.K.; Tafazolymotie, P.; Mayyas, M.; Ramarao, A.V.; Spencer, M.; Shah, K.; et al. Liquid metals in catalysis for energy applications. Joule 2020, 4, 2290–2321. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.; Liu, J. Liquid metal technology in solar power generation—Basics and applications. Sol. Energy Mater. Sol. Cells 2021, 222, 110925. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J. Recent advancements in liquid metal flexible printed electronics: Properties, technologies, and applications. Micromachines 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Norkett, J.E.; Dickey, M.D.; Miller, V.M. A review of liquid metal embrittlement: Cracking open the disparate mechanisms. Metall. Mater. Trans. A 2021, 52, 2158–2172. [Google Scholar] [CrossRef]
- Rostoker, W.; McCaughey, J.M.; Markus, H. Embrittlement by liquid metals. Reinhold Publishing Corporation, New York. 1960. 162 Pp. Illustrated. 64s. J. R. Aeronaut. Soc. 1961, 65, 443. [Google Scholar] [CrossRef]
- Stoloff, N.S.; Johnston, T.L. Crack propagation in a liquid metal environment. Acta Metall. 1963, 11, 251–256. [Google Scholar] [CrossRef]
- Lynch, S.P. A fractographic study of gaseous hydrogen embrittlement and liquid-metal embrittlement in a tempered-martensitic steel. Acta Metall. 1984, 32, 79–90. [Google Scholar] [CrossRef]
- Fernandes, P.J.L.; Jones, D.R.H. Mechanisms of liquid metal induced embrittlement. Int. Mater. Rev. 1997, 42, 251–261. [Google Scholar] [CrossRef]
- Gordon, P.; An, H.H. The mechanisms of crack initiation and crack propagation in metal-induced embrittlement of metals. Metall. Trans. A 1982, 13, 457–472. [Google Scholar] [CrossRef]
- Tanaka, R.; Choi, P.-K.; Koizumi, H.; Hyodo, S. Fast penetration of liquid gallium in polycrystalline aluminum films. Mater. Trans. 2001, 42, 138–140. [Google Scholar] [CrossRef]
- Lee, C.W.; Fan, D.W.; Sohn, I.R.; Lee, S.-J.; De Cooman, B.C. Liquid-metal-induced embrittlement of Zn-coated hot stamping steel. Metall. Mater. Trans. A 2012, 43, 5122–5127. [Google Scholar] [CrossRef]
- Cox, B.; Wong, Y.-M. Liquid metal embrittlement of Zr-2.5% Nb alloy. J. Nucl. Mater. 1997, 245, 34–43. [Google Scholar] [CrossRef]
- Senel, E.; Walmsley, J.C.; Diplas, S.; Nisancioglu, K. Liquid metal embrittlement of aluminum by segregation of trace element gallium. Corros. Sci. 2014, 85, 167–173. [Google Scholar] [CrossRef]
- Liu, Y. A Study of Liquid Metal Embrittlement of Aluminum by Mercury and Gallium via Crack Growth Analysis and Atomistic Modeling. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 1992. [Google Scholar]
- Van Den Bosch, J.; Hosemann, P.; Almazouzi, A.; Maloy, S.A. Liquid metal embrittlement of silicon enriched steel for nuclear applications. J. Nucl. Mater. 2010, 398, 116–121. [Google Scholar] [CrossRef]
- Old, C.F.; Trevena, P. Embrittlement of zinc by liquid metals. Met. Sci. 1979, 13, 487–495. [Google Scholar] [CrossRef]
- Sun, M.C. Mechanical Properties of Metals, 1st ed.; Harbin Institute of Technology Press: Harbin, China, 2003; pp. 37–54. [Google Scholar]
- Tan, H.; Zhong, S.; Deng, Y.; Liu, Z.; Zhong, Y.; Tian, W.; Li, S.; Wang, L. In-situ EBSD study on deformation mechanism of Ti60 titanium alloy equiaxed α phase. Ordnance Mater. Sci. Eng. 2024, 47, 107–111. [Google Scholar]
- Zhen, L.; Shao, W.; Yang, D. Strength of Crystalline Materials and Microscopic Theory of Fracture, 1st ed.; Science Press: Beijing, China, 2018; pp. 73–75. [Google Scholar]
- Herbsleb, G.; Schwenk, W. Investigation of liquid metal embrittlement of high alloy steels. Werkst. Korros. 1977, 28, 145–153. [Google Scholar] [CrossRef]
- Pereiro-López, E.; Ludwig, W.; Bellet, D. Discontinuous penetration of liquid Ga into grain boundaries of Al polycrystals. Acta Mater. 2004, 52, 321–332. [Google Scholar] [CrossRef]
- Kelley, M.J.; Stoloff, N.S. Analysis of liquid metal embrittlement from a bond energy viewpoint. Metall. Trans. A 1975, 6, 159–166. [Google Scholar] [CrossRef]
- Nam, H.-S.; Srolovitz, D.J. Molecular dynamics simulation of Ga penetration along grain boundaries in Al: A dislocation climb mechanism. Phys. Rev. Lett. 2007, 99, 025501. [Google Scholar] [CrossRef] [PubMed]
| Elements | Fe | Si | V | Zn | Al |
|---|---|---|---|---|---|
| wt% | 0.11% | 0.04% | 0.02% | 0.01% | ≥99.8% |
| Specimen Number | Embrittlement Liquid | Embrittlement Temperature/°C | Embrittlement Time/min |
|---|---|---|---|
| 1 | / | / | / |
| 2 | Ga | 40 | 40 |
| 3 | Ga | 60 | 40 |
| 4 | Ga | 80 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Wang, S.; Zhang, D.; Feng, H.; Song, P.; Hu, H. In Situ EBSD Study of Aluminum After Embrittlement by Gallium. Materials 2025, 18, 1026. https://doi.org/10.3390/ma18051026
Cai K, Wang S, Zhang D, Feng H, Song P, Hu H. In Situ EBSD Study of Aluminum After Embrittlement by Gallium. Materials. 2025; 18(5):1026. https://doi.org/10.3390/ma18051026
Chicago/Turabian StyleCai, Kaikai, Shuo Wang, Daixin Zhang, Haiyun Feng, Pu Song, and Hongwei Hu. 2025. "In Situ EBSD Study of Aluminum After Embrittlement by Gallium" Materials 18, no. 5: 1026. https://doi.org/10.3390/ma18051026
APA StyleCai, K., Wang, S., Zhang, D., Feng, H., Song, P., & Hu, H. (2025). In Situ EBSD Study of Aluminum After Embrittlement by Gallium. Materials, 18(5), 1026. https://doi.org/10.3390/ma18051026






