Revealing the Effects of Methoxy Polyethylene Glycol Ether on the Performance of Polycarboxylate Superplasticizers and Their Desensitization Functions in Relation to Concrete
Highlights
- The influence mechanism of MPEG with different molecular weights on PCE-st was revealed.
- Compared with commercially available PCE-et0, PCE-st has obvious advantages in reducing the sensitivity of water variations and reducing the hydration temperature of cement.
- Compared with commercially available PCE-et0, cement paste or concrete mixed with PCE-st has good plasticity retention characteristics.
- The PCE-st mixing concrete had good workability in construction was expounded.
Abstract
1. Introduction
2. Raw Materials and Characterization
3. Synthesis and Characterization of PCE-st
3.1. Synthesis of Ester Monomers
3.2. Synthesis of PCE-st
3.3. Characterization of Polycarboxylate Superplasticizers
4. Results and Discussion
4.1. FTIR Spectroscopy
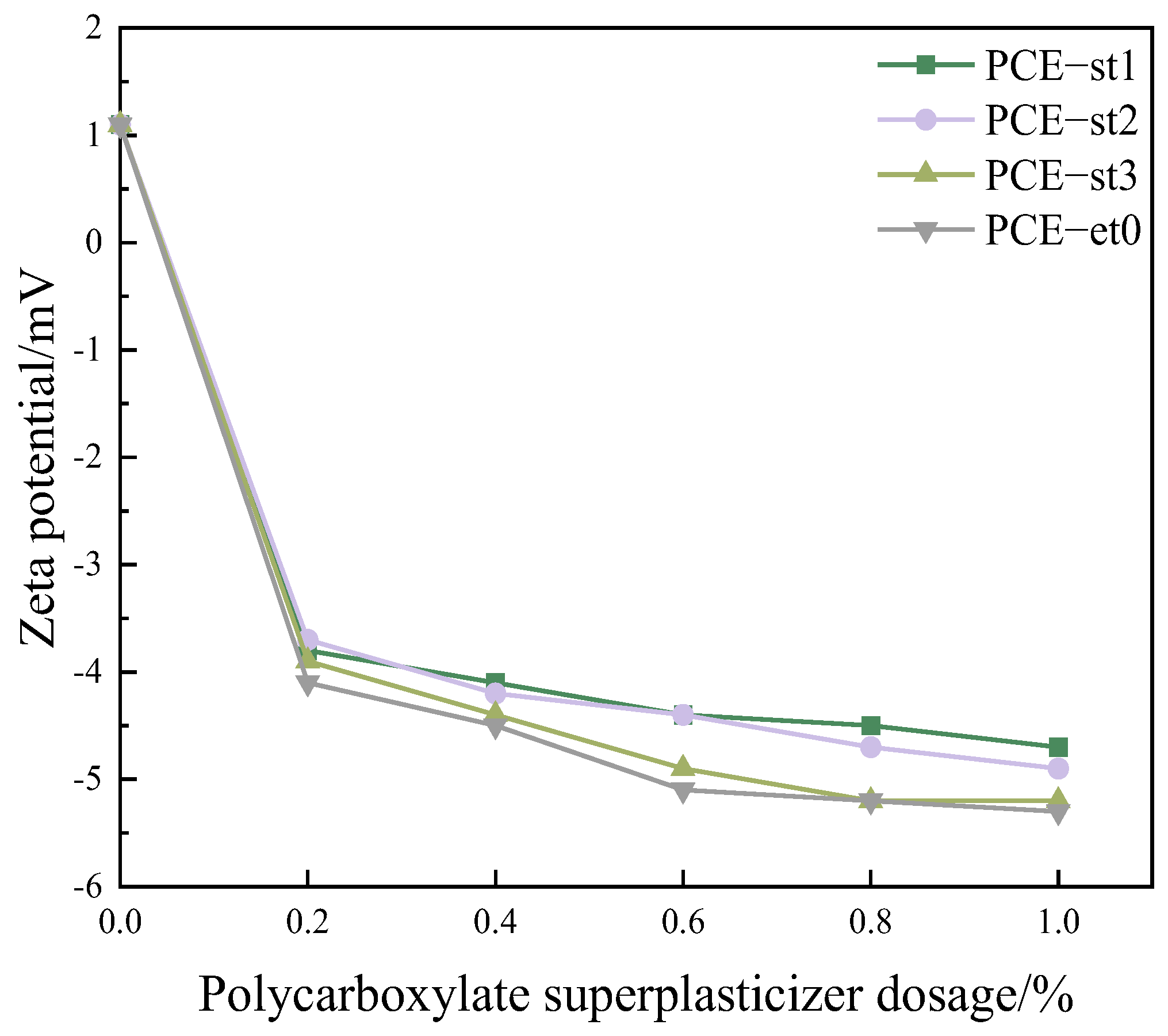
4.2. Zeta Potential Analysis
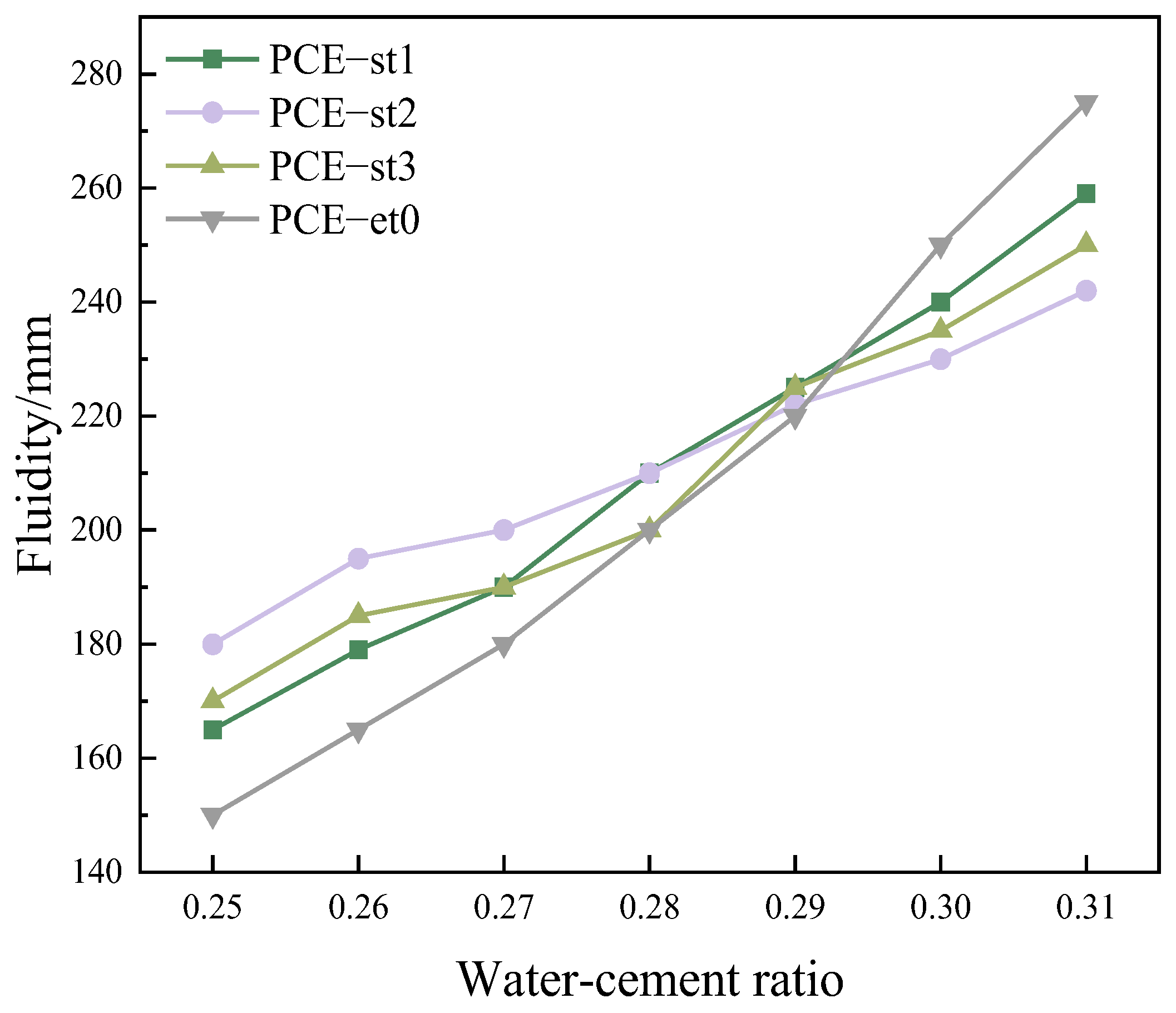
4.3. Difference in Fluidity Sensitivity of Polycarboxylate Superplasticizers with Varying Water Content
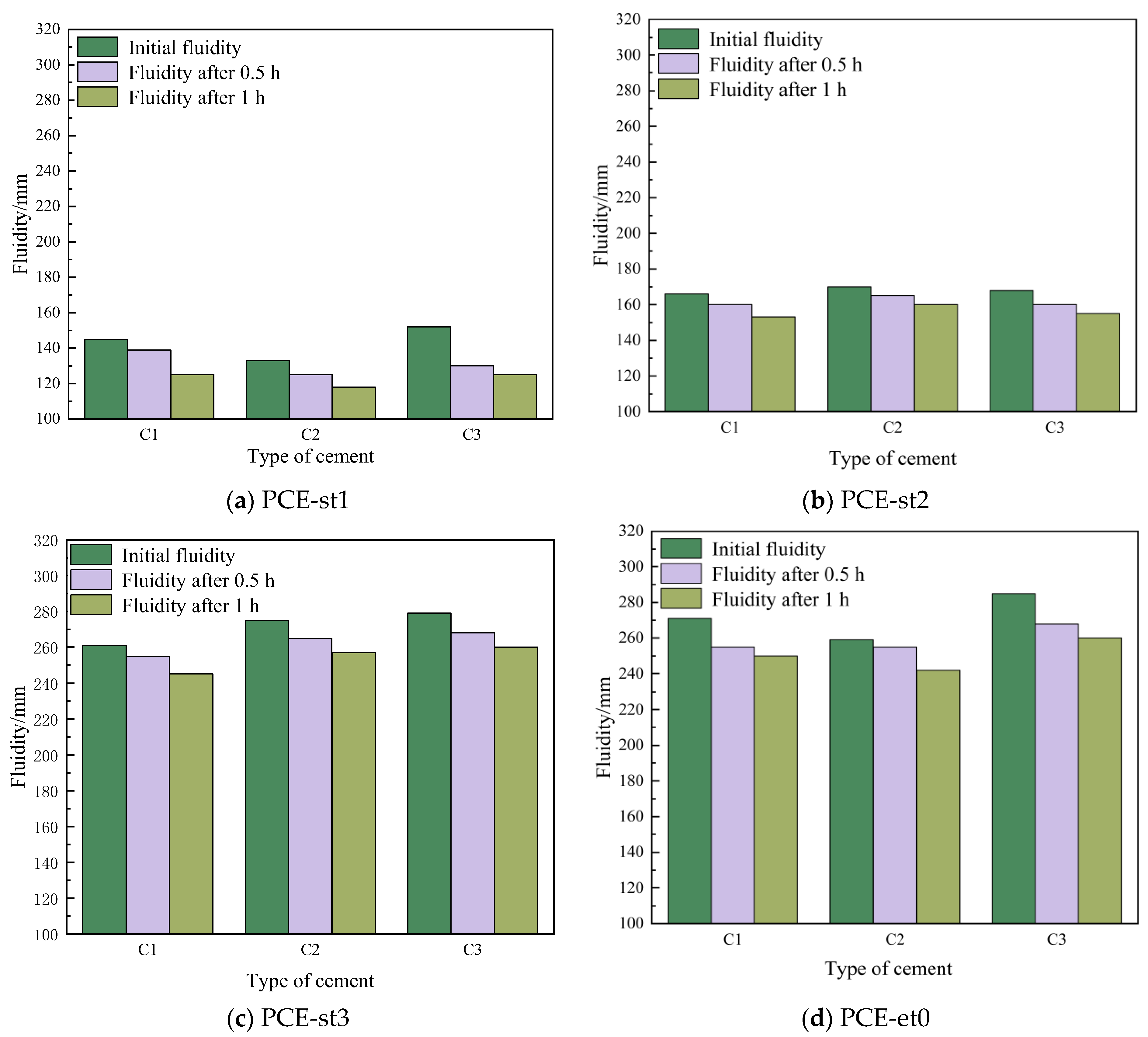
4.4. Fluidity Sensitivity of Different Polycarboxylate Superplasticizers with Different Cements
4.5. Influence of Hydration Temperature on Cement Pastes Containing Different Polycarboxylate Superplasticizers
4.6. Influence of Different Polycarboxylate Superplasticizers on the Setting Time of Cement
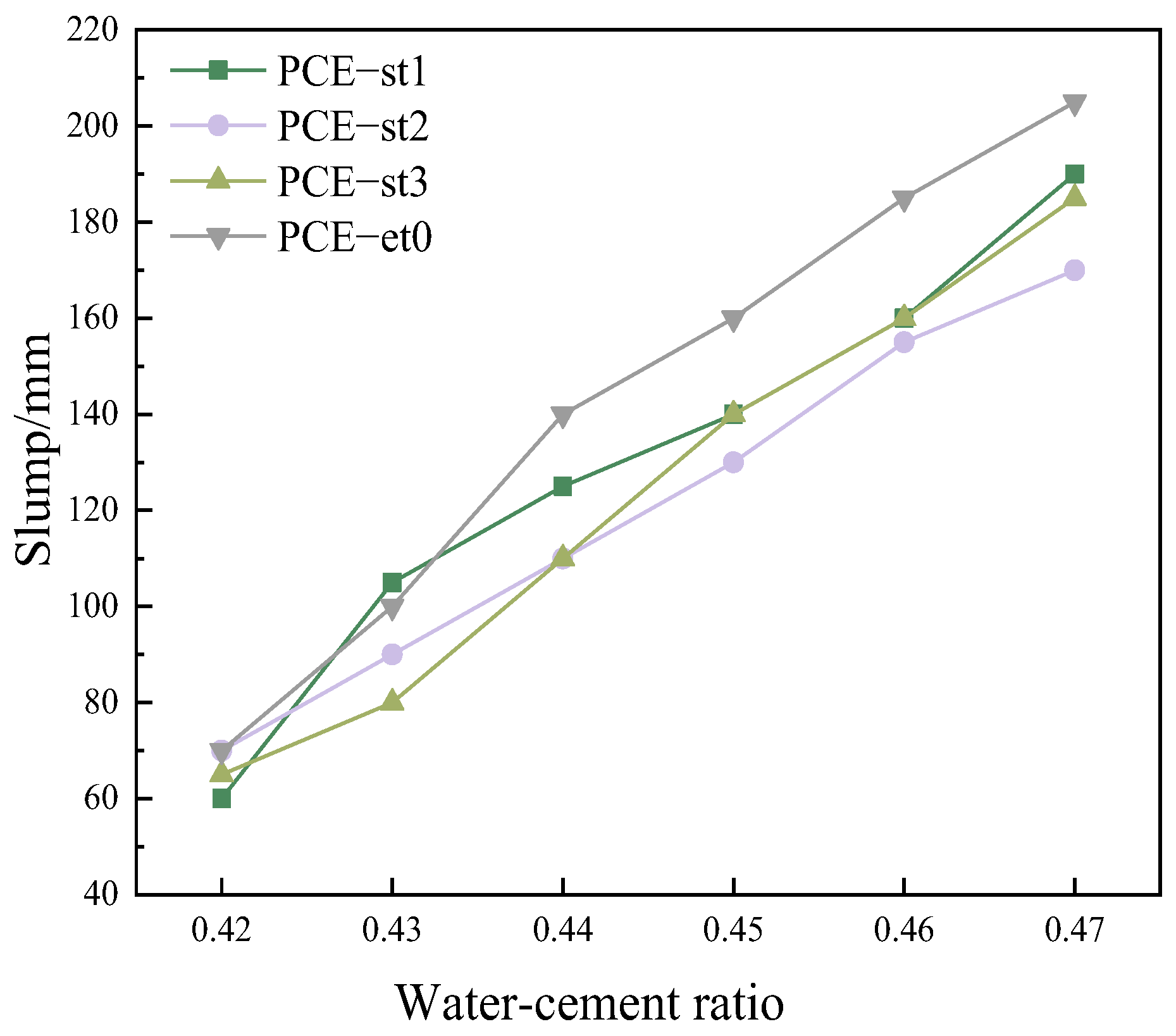
4.7. Influence of Polycarboxylate Superplasticizers on the Workability and Mechanical Properties of Concrete
5. Conclusions
- (1)
- As the MPEG weight of the ester monomer increases, the sensitivity of cement to water content variations first decreases and then increases. As the water–cement ratio increases from 0.25 to 0.31, the fluidity of cement pastes mixed with PCE-st2 only increases by 62 mm, and that of cement pastes mixed with PCE-st1 and PCE-st3 increases by 94 and 80 mm, respectively.
- (2)
- The fluidity retention of cement pastes increases with increasing weight of MPEG. The flow loss of cement pastes mixed with PCE-st3 at 1 h is 6.0%, which is less than that of cement pastes mixed with PCE-st1 (14.3%).
- (3)
- Compared to the commercially available PCE-0, the synthesized PCE-st considerably delays cement hydration and reduces the peak hydration temperature. When the molecular weight of MPEG increases from 400 to 1200, the hydration temperature decreases by 10%, and the final setting time prolonged by 7.6%.
- (4)
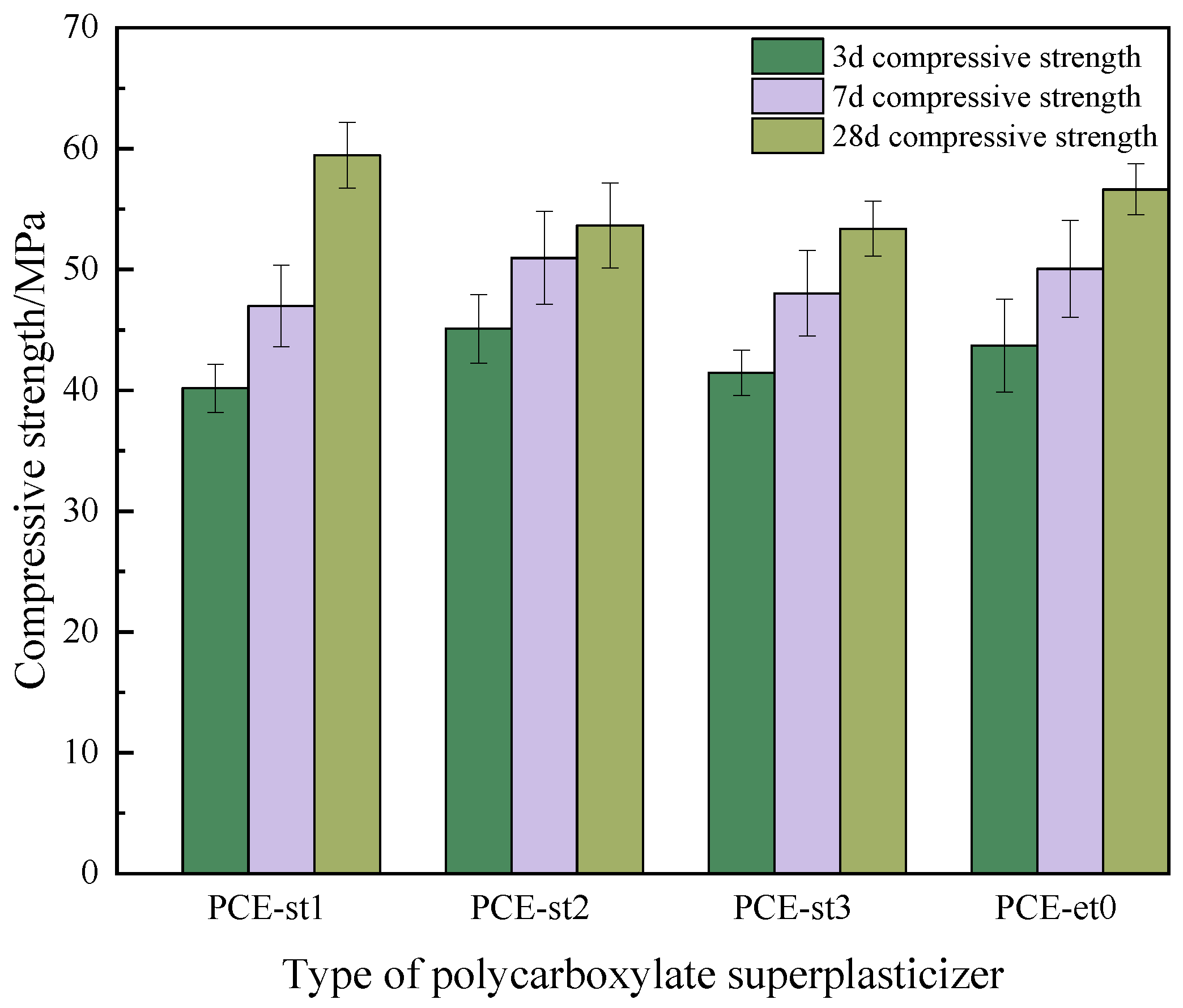
- The synthesized PCE-st has excellent construction workability, and the compressive strength of concrete can be as high as 59.4 MPa after 28 d of standard curing, which is comparable to the compressive strength (56.6 MPa) of concrete prepared by commercially available PCE-et0 under the same curing conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donaldson, L. Concrete revolutionises road construction: Composites. Mater Today 2010, 13, 10. [Google Scholar] [CrossRef]
- Takva, Ç.; Top, S.M.; Gökgöz, B.İ.; Gebel, Ş.; İlerisoy, Z.Y.; İlcan, H.; Şahmaran, M. Applicability of 3d concrete printing technology in building construction with different architectural design decisions in housing. J. Build. Eng. 2024, 98, 111257. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Wu, M. Leading-edge technologies in hydropower development in china. Glob. Energy Interconnect. 2019, 2, 244–253. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z. Experimental modeling and quantitative evaluation of mitigating cracks in early-age mass concrete by regulating heat transfer. J. Build. Eng. 2024, 96, 110641. [Google Scholar] [CrossRef]
- Khan, I.; Yong, G. Role of supplementary cementiteous materials in mitigating heat of hydration in mass concrete elements. Constr. Build. Mater. 2024, 431, 136482. [Google Scholar] [CrossRef]
- Guan, J.; Liu, S.; Liu, X.; Zhang, G.; Lai, G.; Gao, R.; Zhu, H.; Huang, M.; Wang, Z.; Cui, S. Effect of side chain ionizability of polycarboxylate superplasticizer on cement hydration. Constr. Build. Mater. 2024, 436, 136940. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, G.; Zhang, W.; Cui, H.; Xin, T. Comparison of ester-based slow-release polycarboxylate superplasticizers with their polycarboxylate counterparts. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127878. [Google Scholar] [CrossRef]
- Lin, Z. Synthesis and performance study of a highly dispersed HPEG-type polycarboxylate superplasticizer. In Proceedings of the International Conference on Advanced Materials and Chemical Engineering, Harbin, China, 3–5 December 2022; p. 12028. [Google Scholar]
- Palma-Lemus, K.; Hamzehlou, S.; Froidevaux, V.; Boustingorry, P.; Leiza, J.R. Acidic aqueous-phase copolymerization of aa and hpeg macromonomer: Influence of monomer concentration on reactivity ratios. Ind. Eng. Chem. Res. 2023, 62, 18427–18437. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 years of pce superplasticizers—History, current state-of-the-art and an outlook. Cem. Concr. Res. 2022, 157, 106826. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiong, Q.; Chen, X.; Fang, Y. Synthesis and properties of a new clay-resistant polycarboxylic acid water reducer research. In Proceedings of the 2022 3rd International Conference on Material Chemistry and Composite Materials, Zhuhai, China, 16–18 December 2023; p. 12061. [Google Scholar]
- Erzengin, S.G.; Kaya, K.; Özkorucuklu, S.P.; Özdemir, V.; Yıldırım, G. The properties of cement systems superplasticized with methacrylic ester-based polycarboxylates. Constr. Build. Mater. 2018, 166, 96–109. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X. Research on the effect of two different polycarboxylic acid water-reducing agent on the sensitivity of concrete. In Proceedings of the 2022 3rd International Conference on Material Chemistry and Composite Materials, Zhuhai, China, 16–18 December 2023; p. 012019. [Google Scholar]
- Xu, Y.; Liu, X.; Jiang, M.; Lai, G.; Li, S.; Wang, Z.; Cui, S. Effect of competitive hydrolysis of diester in polycarboxylate superplasticizer on the fluidity of cement paste. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131691. [Google Scholar] [CrossRef]
- Jiang, Z.; You, R.; Guo, X. Study on preparation of low sensitive and stable type polycarboxylate superplasticizer small monomers. In Proceedings of the 2019 5th International Conference on Applied Materials and Manufacturing Technology, ICAMMT 2019, Singapore, 21–23 June 2019; p. 22030. [Google Scholar]
- Tingshu, H.; Renhe, Y.; Yilun, X. Synthesis and properties of ester ether copolymerization polycarboxylate superplasticizer. J. Chin. Ceram. Soc. 2018, 46, 218–223. [Google Scholar]
- GB/T 17671-2021; Test method of cement mortars strength (ISO Method). Standards Press of China: Beijing, China, 2017.
- GB/T 8077-2023; Methods for testing uniformity of concrete admixtures. Standards Press of China: Beijing, China, 2017.
- GB/T1346-2011; Test methods for water requirement of normal consistency, setting time and soundness of the portland cement. Standards Press of China: Beijing, China, 2017.
- GB/T 50080-2016; Standard for test method of performance on ordinary fresh concrete. Standards Press of China: Beijing, China, 2017.
- Feng, H.; Feng, Z.; Wang, W.; Deng, Z.; Zheng, B. Impact of polycarboxylate superplasticizers (pces) with novel molecular structures on fluidity, rheological behavior and adsorption properties of cement mortar. Constr. Build. Mater. 2021, 292, 123285. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Kong, X.-M.; Lu, Z.-B.; Lu, Z.-C.; Hou, S.-S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xie, Y.; Liu, Y.; Tao, J.; Liu, R.; Li, Z.; Liu, F.; Li, M. The effect of alkyl acrylate ester side chain length of polycarboxylate superplasticizer on the flow behaviour of concrete. Constr. Build. Mater. 2024, 443, 137691. [Google Scholar] [CrossRef]
- Ma, Y.; Jiao, D.; Sha, S.; Zhou, B.; Liu, Y.; Shi, C. Effect of anchoring groups of polycarboxylate ether superplasticizer on the adsorption and dispersion of cement paste containing montmorillonite. Cem. Concr. Compos. 2022, 134, 104737. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Xu, Y.; Lai, G.; Ding, Y.; Chen, Y.; Xia, C.; Wang, Z.; Cui, S. Synthesis and performances of shrinkage-reducing polycarboxylate superplasticizer in cement-based materials. Materials 2022, 15, 7002. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Zhu, J.; Zheng, Y.; Cui, S.; Lan, M.; Li, H. Synthesis, characterization and performance of a polycarboxylate superplasticizer with amide structure. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 119–129. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Fu, X.; Xie, X.; Wang, T.; Rong, Z.; Nong, Y.; Lu, Z. Enhanced adsorption and properties of TPEG-type superplasticizers modified by maleic anhydride: A perspective from molecular architecture and conformation. Mater. Struct. 2024, 57, 4. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, L. Synthesis and performance of a non-air entraining polycarboxylate superplasticizer. Cem. Concr. Res. 2022, 159, 106853. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, Y.; Sha, S.; Wang, Y.; Liu, Y.; Xiao, Y.; Shi, C. Synthesis, performance and mechanism of shrinkage-reducing agents with water-reducing function for cement-based materials. Constr. Build. Mater. 2024, 425, 135994. [Google Scholar] [CrossRef]
- Lu, F.; Xiong, W. Effect of shrinkage-reducing polycarboxylate superplasticizer on cement-based materials shrinkage performance. J. Concrete. 2014, 485, 91–96. [Google Scholar]
- Ma, R.; Wang, Y.; Li, H.; Bai, Y. Progress in the polycarboxylate superplasticizer and their structure-activity relationship—A review. Mater. Today Commun. 2023, 35, 105838. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Wang, J.; Gao, N.; Hu, Z.; Liu, J.; Wang, K. A novel shrinkage-reducing polycarboxylate superplasticizer for cement-based materials: Synthesis, performance and mechanisms. Constr. Build. Mater. 2022, 321, 126342. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Zhao, H.; Sun, H.; Liu, J. Mitigating autogenous shrinkage of cement paste with novel shrinkage-reducing polycarboxylate superplasticizer. Mater. Struct. 2022, 55, 231. [Google Scholar] [CrossRef]
- Ran, Q.; Somasundaran, P.; Miao, C.; Liu, J.; Wu, S.; Shen, J. Adsorption mechanism of comb polymer dispersants at the cement/water interface. J. Dispers. Sci. Technol. 2010, 31, 790–798. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Zhong, L. Development and properties research of different structural EPEG-type polycarboxylate superplasticizers in UHPC. In Proceedings of the International Conference on Advanced Materials and Mechatronics, Xi’an, China, 10–12 May 2024; p. 012006. [Google Scholar]
- Zhang, L.; Miao, X.; Kong, X.; Zhou, S. Retardation effect of pce superplasticizers with different architectures and their impacts on early strength of cement mortar. Cem. Concr. Compos. 2019, 104, 103369. [Google Scholar] [CrossRef]
- Yoshioka, K.; Sakai, E.; Daimon, M.; Kitahara, A. Role of steric hindrance in the performance of superplasticizers for concrete. J. Am. Ceram. Soc. 1997, 80, 2667–2671. [Google Scholar] [CrossRef]
- Ma, B.; Li, C.; Lv, Y.; Tan, H.; Wang, H.; Qi, H.; Liu, X.; Yang, Q.; Chen, P. Preparation for polyacrylic acid modified by ester group in side chain and its application as viscosity enhancing agent in polycarboxylate superplasticizer system. Constr. Build. Mater. 2020, 233, 117272. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Miao, Y.; Ju, S.; Sui, S.; Wang, F.; Liu, Z.; Jiang, J. Temperature damage assessment of mass concrete based on the coupling mechanism of hydration-temperature-humidity-constraint factors. J. Build. Eng. 2024, 90, 109211. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem. Concr. Res. 2009, 39, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y.; Nian, F.; Pang, H.; Huang, J.; Zhao, H.; Wang, K.; Liao, B. Regulating the arm structure of star-shaped polycarboxylate superplasticizers as a means to enhance cement paste workability. J. Appl. Polym. Sci. 2018, 135, 46312. [Google Scholar] [CrossRef]
- Qi, H.; Ma, B.; Tan, H.; Su, Y.; Jin, Z.; Li, C.; Liu, X.; Yang, Q.; Luo, Z. Polycarboxylate superplasticizer modified by phosphate ester in side chain and its basic properties in gypsum plaster. Constr. Build. Mater. 2021, 271, 121566. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Xun, W.; Wu, C.; Li, J.; Yang, C.; Leng, X.; Xin, D.; Li, Y. Effect of functional polycarboxylic acid superplasticizers on mechanical and rheological properties of cement paste and mortar. Appl. Sci. 2020, 10, 5418. [Google Scholar] [CrossRef]

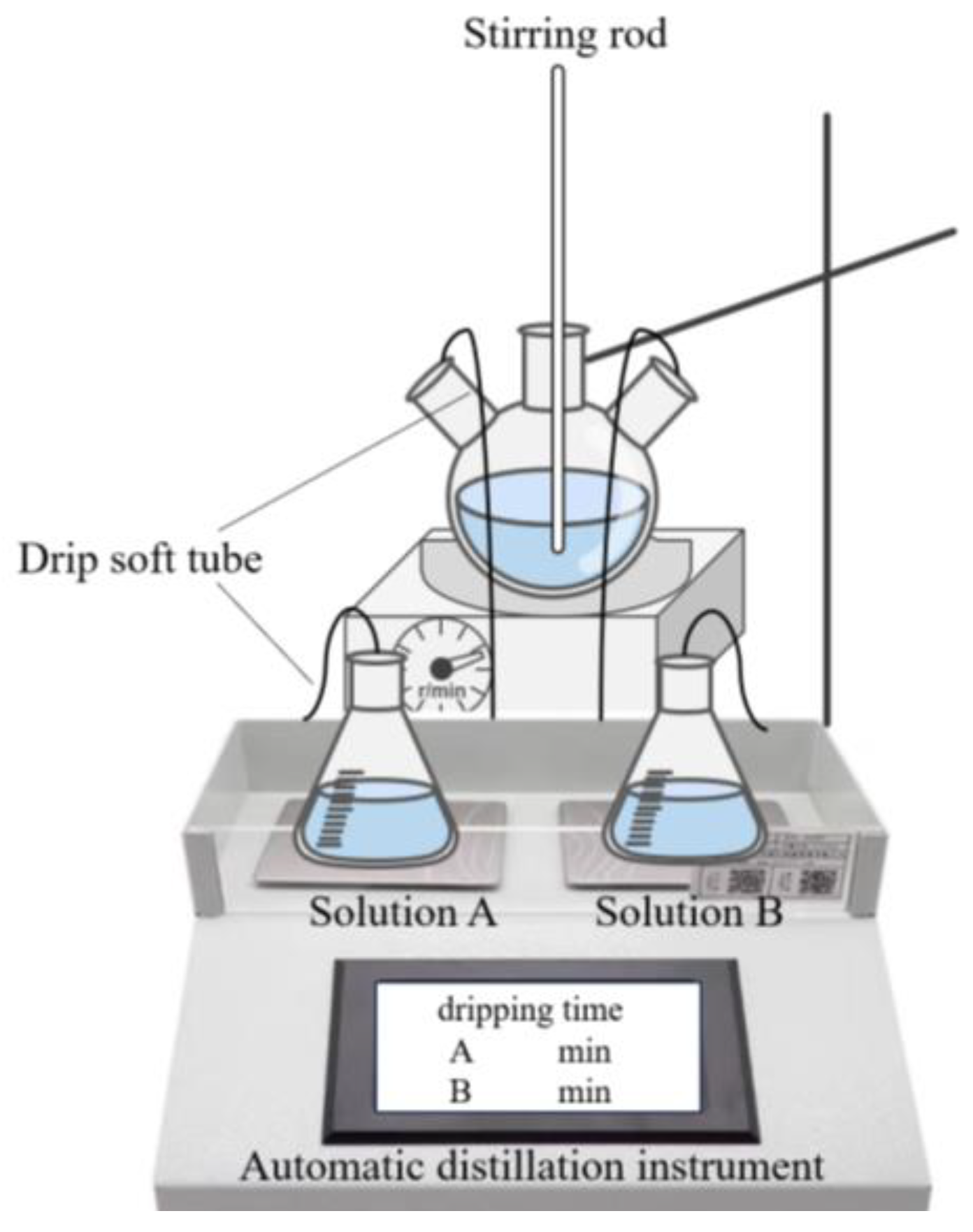
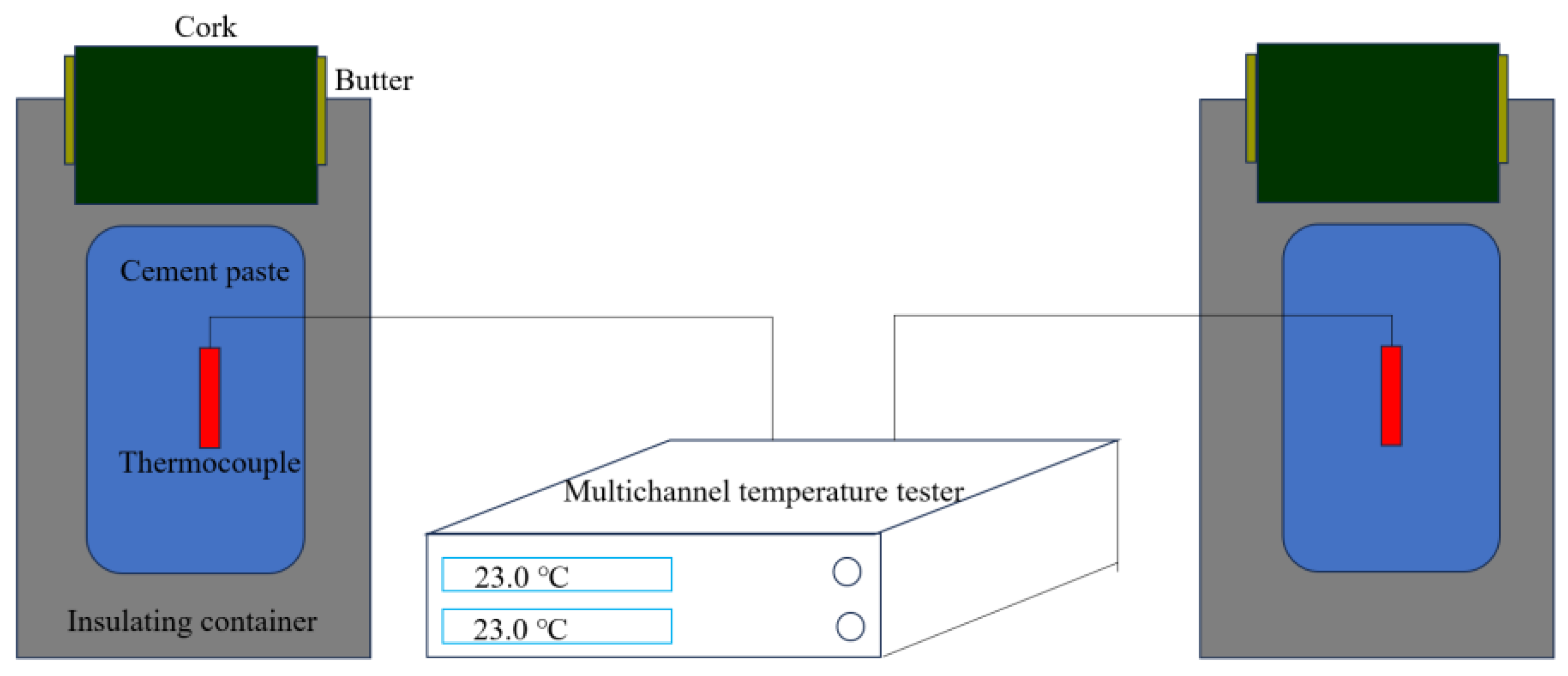
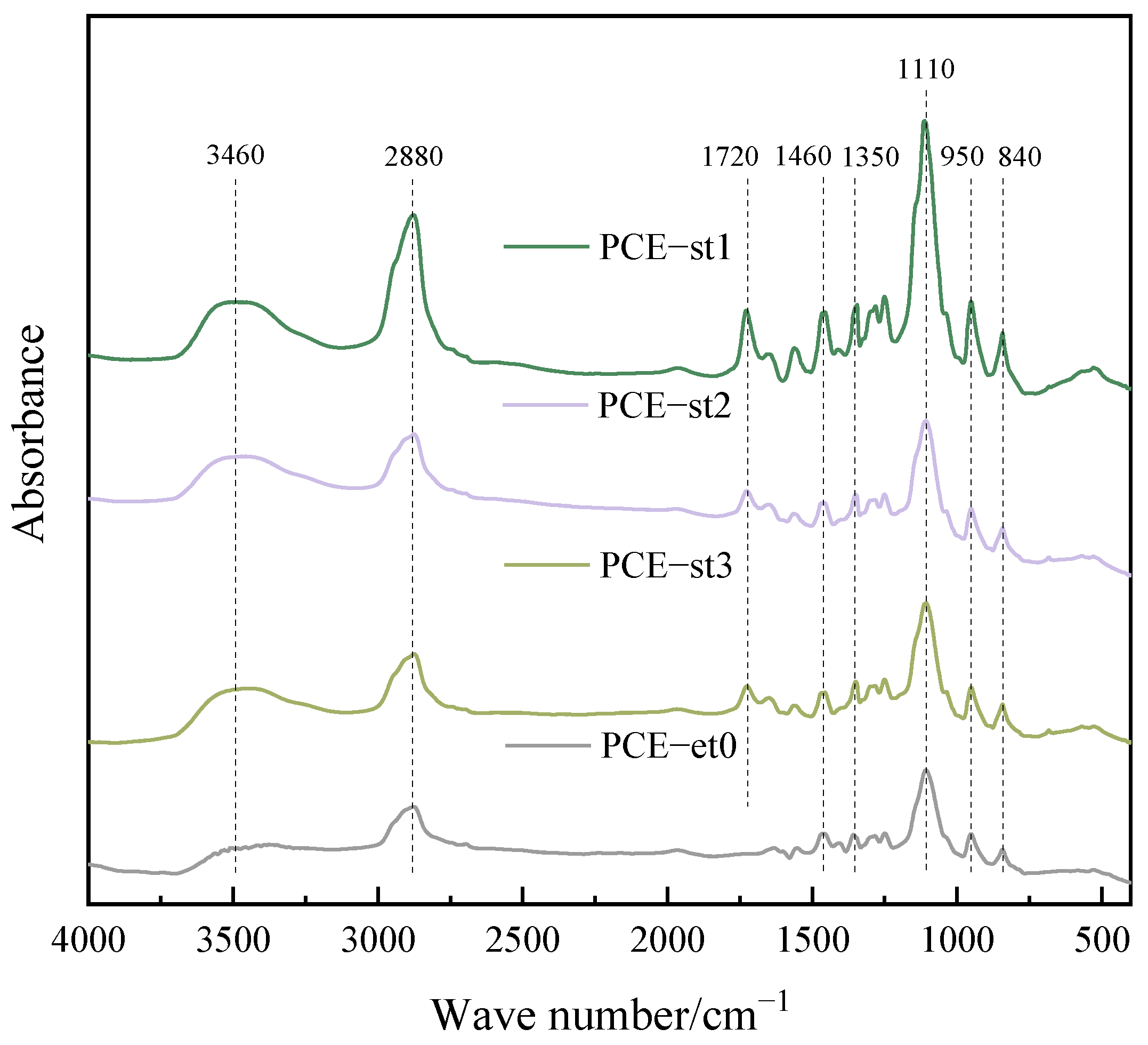




| Chemical Raw Materials and Reagents | Specification | Manufacturer |
|---|---|---|
| Ethylene glycol polyethylene glycol ether (EPEG = 3000) | Industrial grade | Liaoning Xindun Pharmaceutical Chemical Co., Ltd. (Liaoning, China) |
| Methoxy polyethylene glycol ether (MPEG1 = 400, MPEG2 = 600, and MPEG3 = 1200) | Industrial grade | Shandong Yousuo Chemical Technology Co., Ltd. (Shandong, China) |
| Methacrylic acid (MAA) | Analytical reagent | Jinan Haokun Chemical Co., Ltd. (Shandong, China) |
| P-toluene sulfonic acid (PTSA) | Analytical reagent | Guangzhou Shanghe Chemical Technology Co., Ltd. (Guangdong, China) |
| Hydroquinone (HQ) | Analytical reagent | Guangzhou Shanghe Chemical Technology Co., Ltd. (Guangdong, China) |
| Cyclohexane (CH) | Analytical reagent | Guangzhou Shanghe Chemical Technology Co., Ltd. (Guangdong, China) |
| Acrylic acid (AA) | Analytical reagent | Jinan Haokun Chemical Co., Ltd. (Shandong, China) |
| Ascorbic acid (VC) | Analytical reagent | Jiangsu Aofu Biotechnology Co., Ltd. (Jiangsu, China) |
| Hydrogen peroxide solution (H2O2) | 28% concentration | Guangdong Shangshan Chemical Co., Ltd. (Guangdong, China) |
| Mercaptopropionic acid (MPA) | Analytical reagent | Guangzhou Shanghe Chemical Technology Co., Ltd. (Guangdong, China) |
| Sodium hydroxide (NaOH) | Analytical reagent | Guangzhou Shanghe Chemical Technology Co., Ltd. (Guangdong, China) |
| Polycarboxylate superplasticizer (PCE-et0) | Industrial grade | Shaanxi Kezhijie New Material Co., Ltd. (Shaanxi, China) |
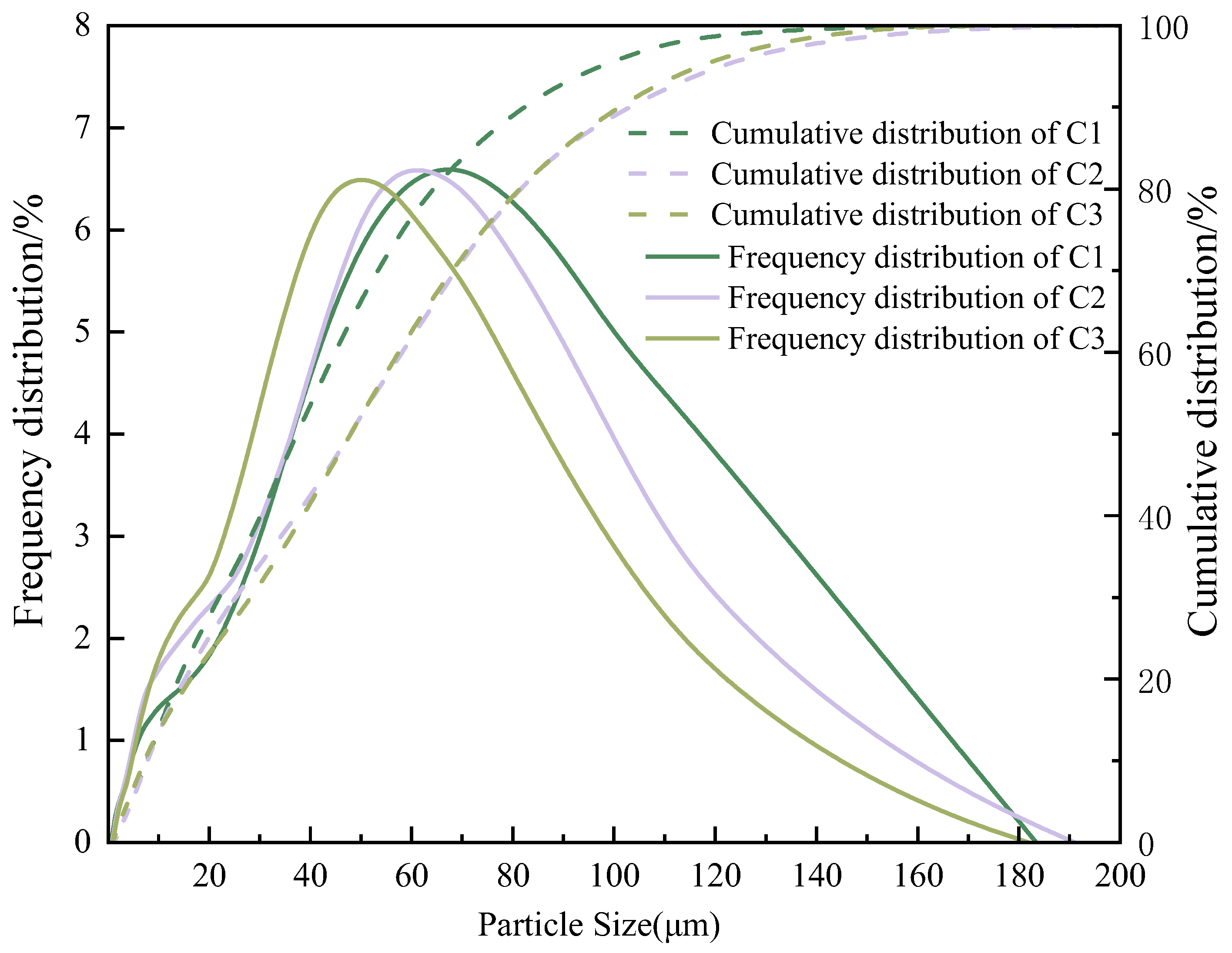
| Cement | CaO | Al2O3 | SiO2 | Fe2O3 | SO3 | Na2O | K2O | Loss | Others |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 60.20 | 4.50 | 23.81 | 3.33 | 2.33 | 0.56 | 0.20 | 1.58 | 3.49 |
| C2 | 59.70 | 4.40 | 22.40 | 3.27 | 2.28 | 0.39 | 0.31 | 1.48 | 5.77 |
| C3 | 59.71 | 4.59 | 22.56 | 3.11 | 2.01 | 0.23 | 0.51 | 1.60 | 5.68 |
| Strength | Flexural Strength (MPa) | Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|---|---|
| Cement | 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | |
| C1 | 5.8 | 6.5 | 7.3 | 28.2 | 36.5 | 45.0 | |
| C2 | 5.9 | 6.1 | 7.4 | 27.3 | 35.8 | 45.2 | |
| C3 | 6.2 | 6.1 | 7.5 | 30.3 | 37.4 | 48.3 | |
| Aggregate | Particle Size (mm) |
|---|---|
| River sand | 0.25–0.35 |
| Machine-made sand 1 | 0.35–0.65 |
| Machine-made sand 2 | 1.20–2.20 |
| Small stone | 4.80–9.50 |
| Large stone | 9.50–19.0 |
| Cement (kg/m3) | Water–Cement Ratio | River Sand (kg/m3) | Machine-Made Sand 1 (kg/m3) | Machine-Made Sand 2 (kg/m3) | Small Stone (kg/m3) | Large Stone (kg/m3) |
|---|---|---|---|---|---|---|
| 360 | 0.42 | 400 | 66 | 200 | 800 | 430 |
| n (EPEG and MPEG1-MAA):nAA | (EPEG and MPEG2-MAA):nAA | n(EPEG and MPEG3-MAA):nAA | H2O2 | VC | MPA | |
|---|---|---|---|---|---|---|
| PCE-st1 | 3.5:1 | 0 | 0 | 1.0% | 0.4% | 0.4% |
| PCE-st1 | 0 | 3.5:1 | 0 | 1.0% | 0.4% | 0.4% |
| PCE-st1 | 0 | 0 | 3.5:1 | 1.0% | 0.4% | 0.4% |
| Cohesiveness | Slump (mm) | ||
|---|---|---|---|
| 0 h | 1 h | ||
| PCE-st1 | Normal | 75 | 55 |
| PCE-st2 | Excellent | 70 | 55 |
| PCE-st3 | Excellent | 65 | 55 |
| PCE-et0 | Normal | 70 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da, Y.; Yu, L.; He, T.; Zheng, Z. Revealing the Effects of Methoxy Polyethylene Glycol Ether on the Performance of Polycarboxylate Superplasticizers and Their Desensitization Functions in Relation to Concrete. Materials 2025, 18, 772. https://doi.org/10.3390/ma18040772
Da Y, Yu L, He T, Zheng Z. Revealing the Effects of Methoxy Polyethylene Glycol Ether on the Performance of Polycarboxylate Superplasticizers and Their Desensitization Functions in Relation to Concrete. Materials. 2025; 18(4):772. https://doi.org/10.3390/ma18040772
Chicago/Turabian StyleDa, Yongqi, Longgang Yu, Tingshu He, and Zihan Zheng. 2025. "Revealing the Effects of Methoxy Polyethylene Glycol Ether on the Performance of Polycarboxylate Superplasticizers and Their Desensitization Functions in Relation to Concrete" Materials 18, no. 4: 772. https://doi.org/10.3390/ma18040772
APA StyleDa, Y., Yu, L., He, T., & Zheng, Z. (2025). Revealing the Effects of Methoxy Polyethylene Glycol Ether on the Performance of Polycarboxylate Superplasticizers and Their Desensitization Functions in Relation to Concrete. Materials, 18(4), 772. https://doi.org/10.3390/ma18040772






