Deterioration Effects and Microscopic Mechanisms of Solidified/Stabilized Red Mud by CGFPA Binders Under Freeze–Thaw Cycles
Abstract
1. Introduction
2. Materials and Methods
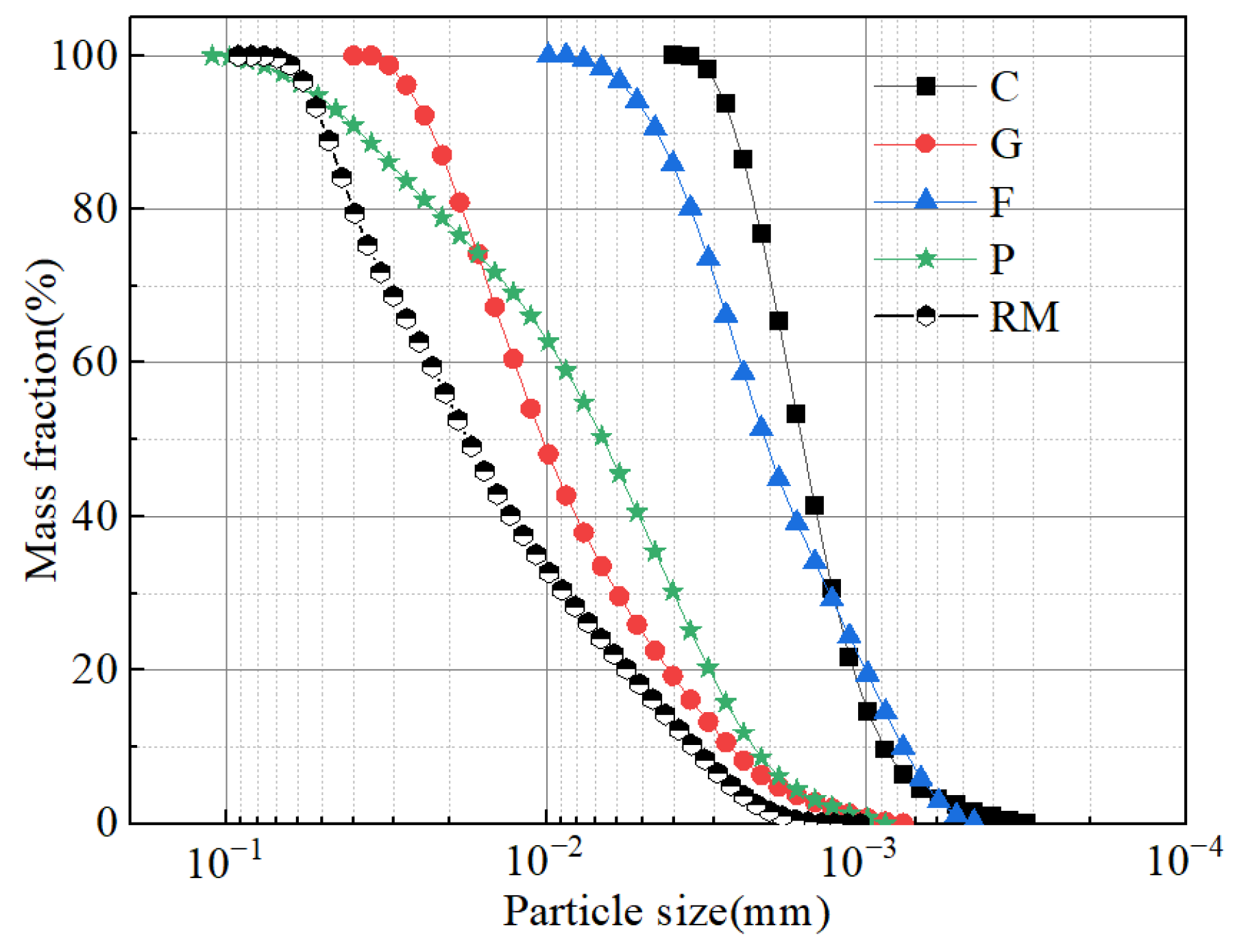
2.1. Test Materials
2.2. Test Formulas
2.3. Test Program
2.4. Test Process
2.5. Test Methods
3. Results and Discussion
3.1. Changes in Physical Indicators
3.2. Changes in Mechanical Indicators
3.2.1. Stress–Strain Curve
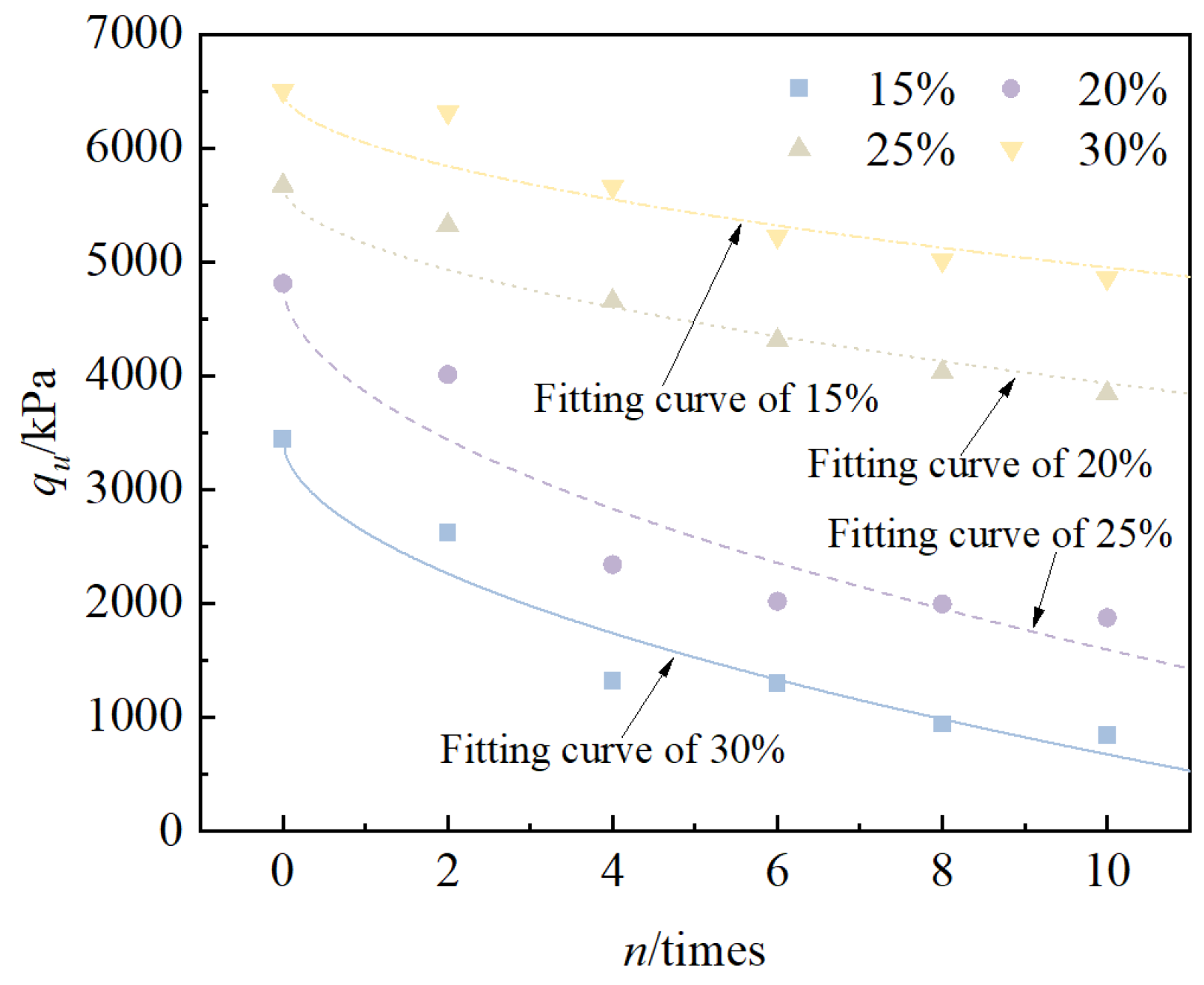
3.2.2. Unconfined Compressive Strength Test
3.3. Changes in Chemical Indicators
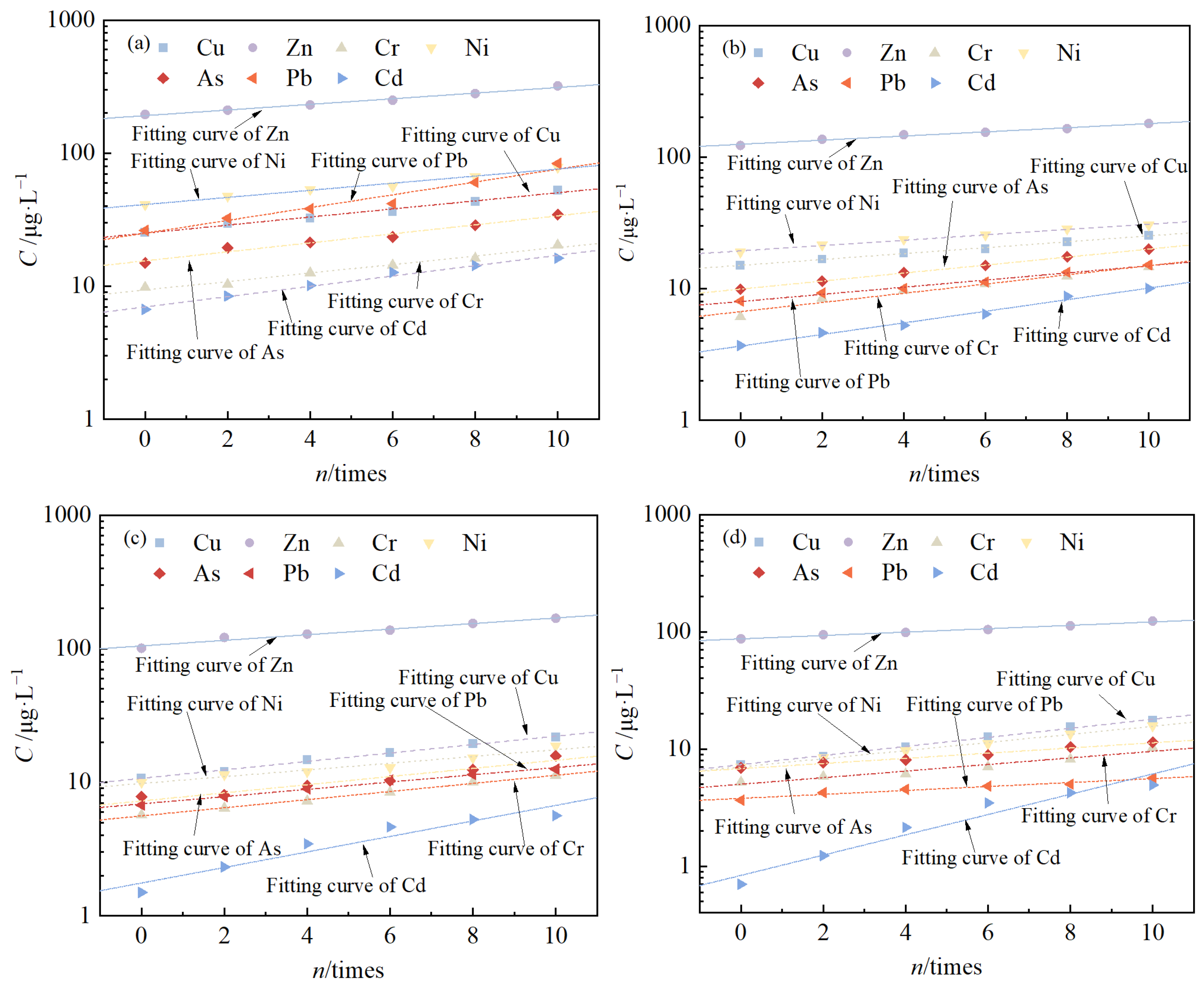
3.4. Changes in Leaching Toxicity
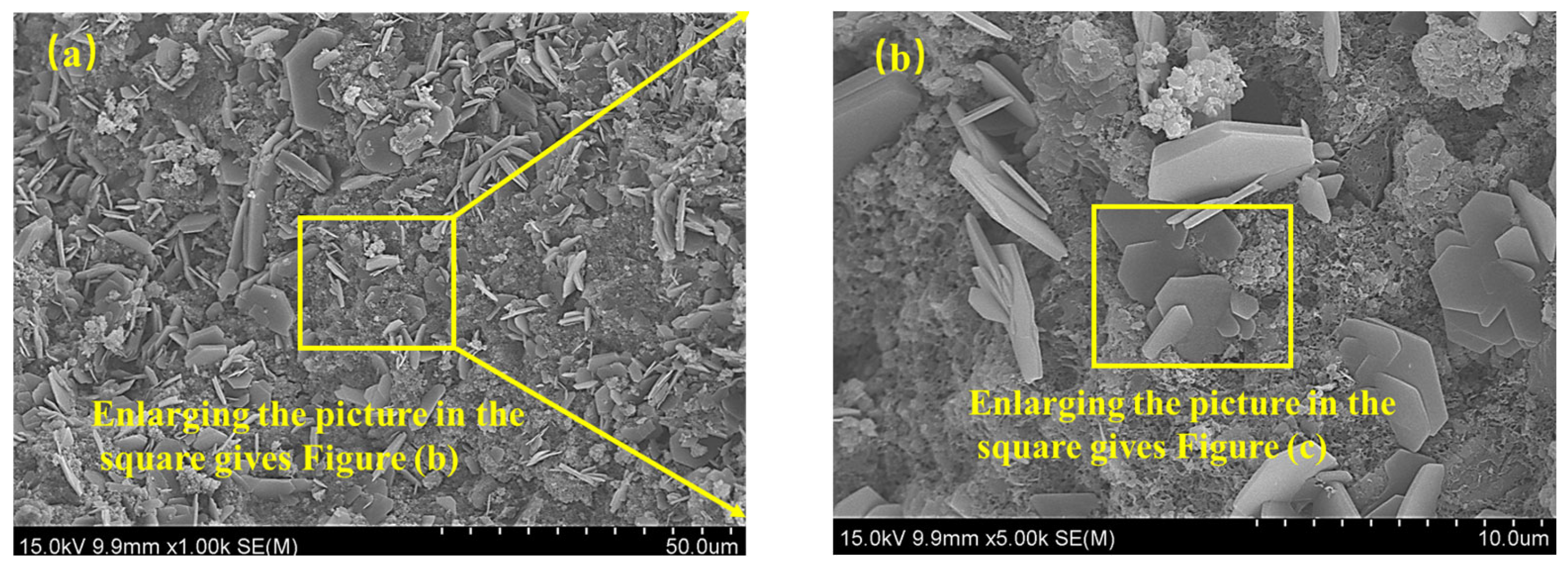
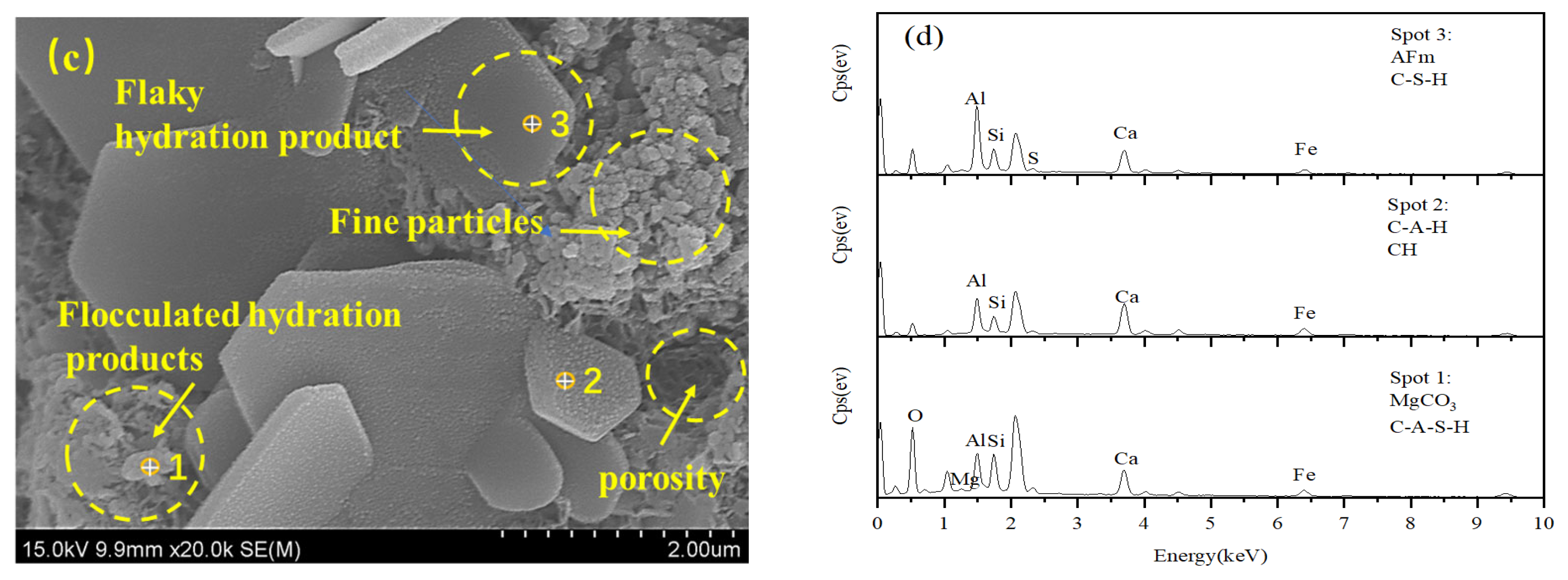
3.5. Microstructure Analysis and Degradation Mechanism
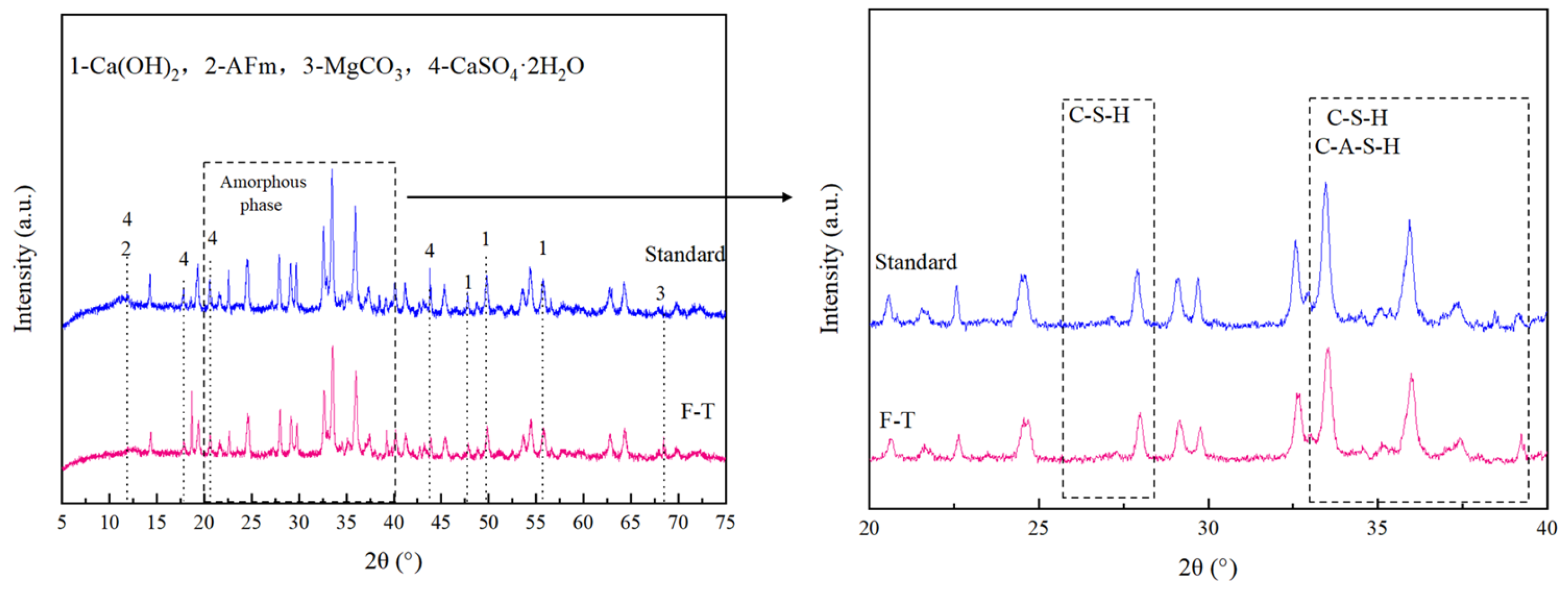
3.5.1. XRD Results and Analysis
3.5.2. SEM-EDS Results and Analysis
3.5.3. FT-IR Results and Analysis
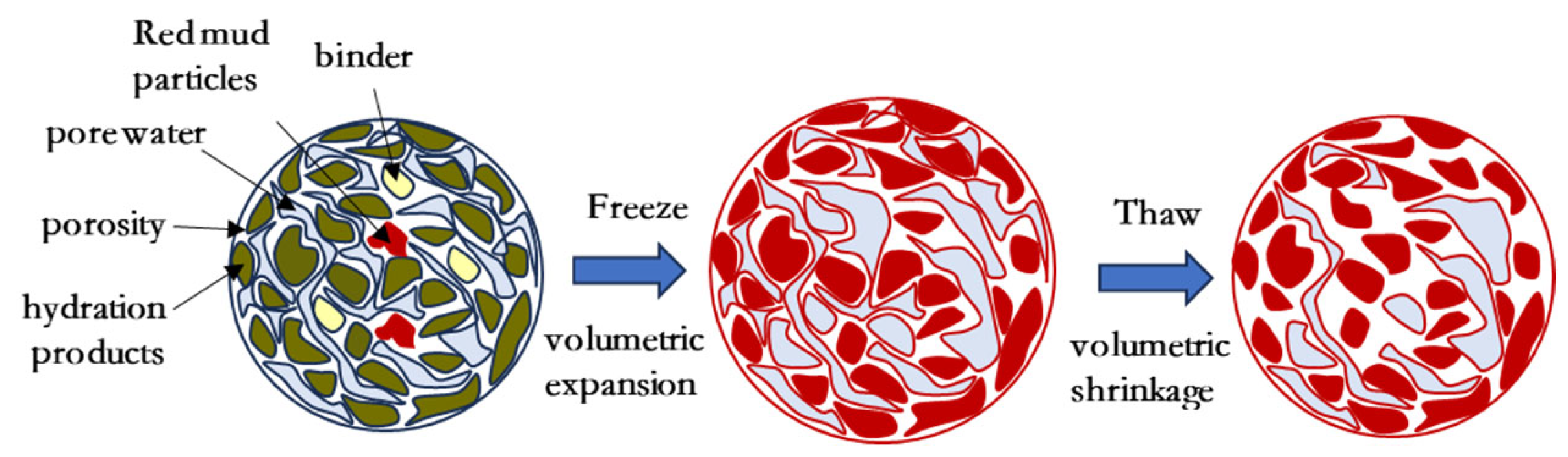
3.5.4. Structural Modeling of Solidified/Stabilized Red Mud
4. Conclusions
- (1)
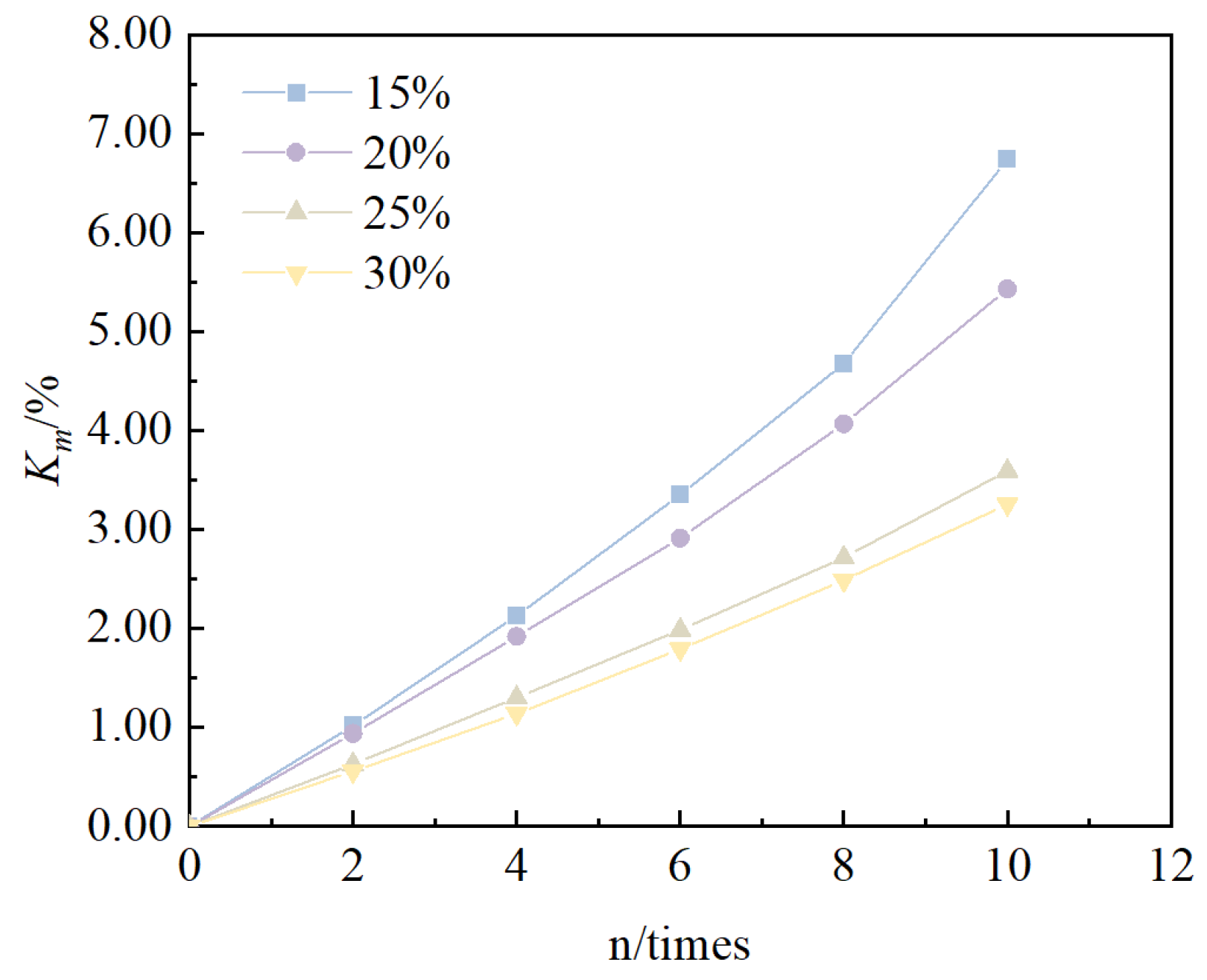
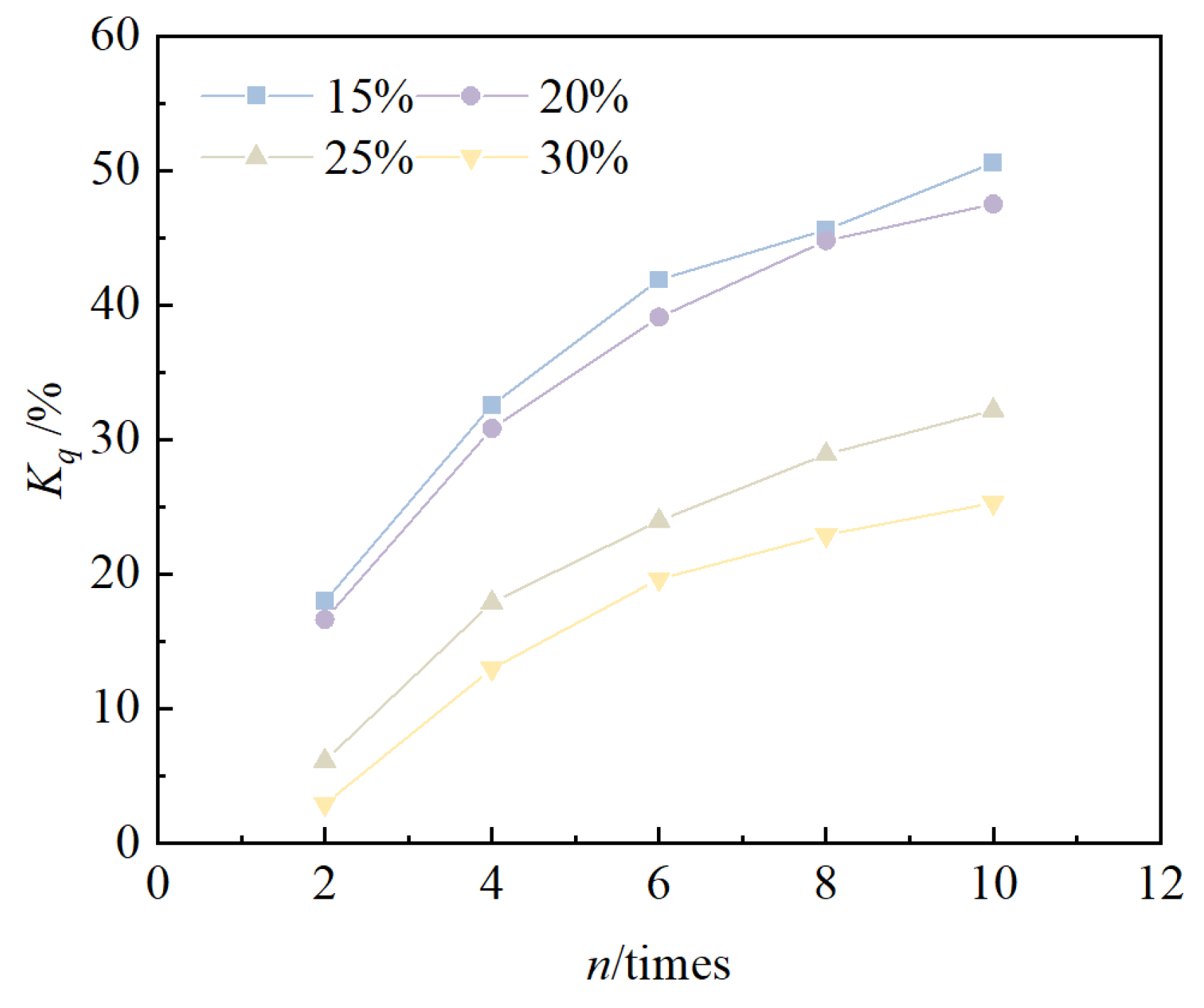
- Under the action of freeze–thaw cycles, the unconfined compressive strength of solidified/stabilized red mud decreases with an increase in the number of cycles, and the pattern is consistent with an exponential function. When the mixing ratio was 15%, 20%, 25%, and 30%, the cumulative loss rate of the solidified/stabilized red mud’s cumulative rate of loss of unconfined compressive strength was 50.6%, 47.5%, 32.2%, and 25.3%, respectively. Based on the functional failure evaluation criteria and the derived equations, it can be deduced that the number of freeze–thaw cycles for the failure of solidified/stabilized red mud is 2, 3, 16, and 26, respectively.
- (2)
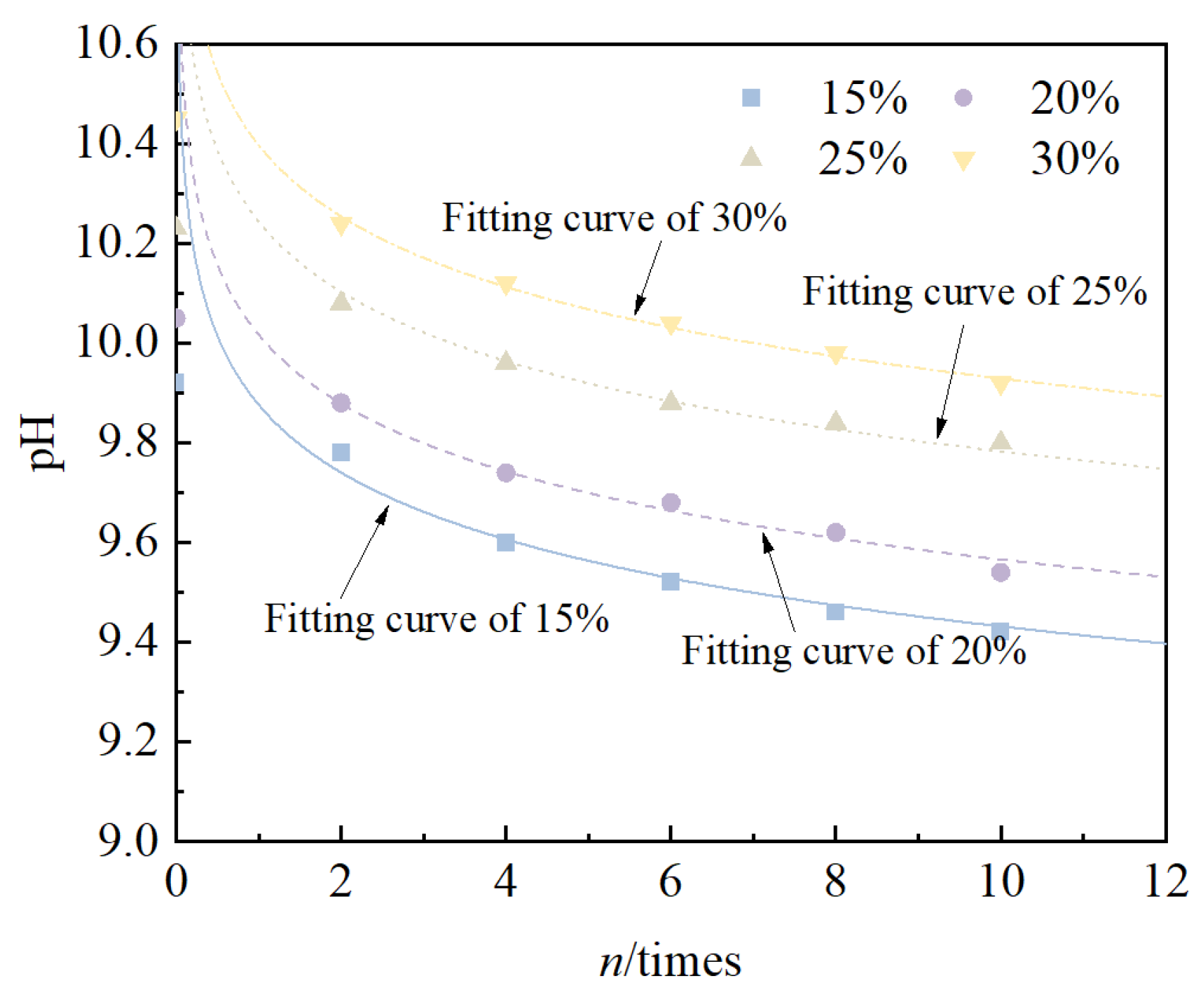
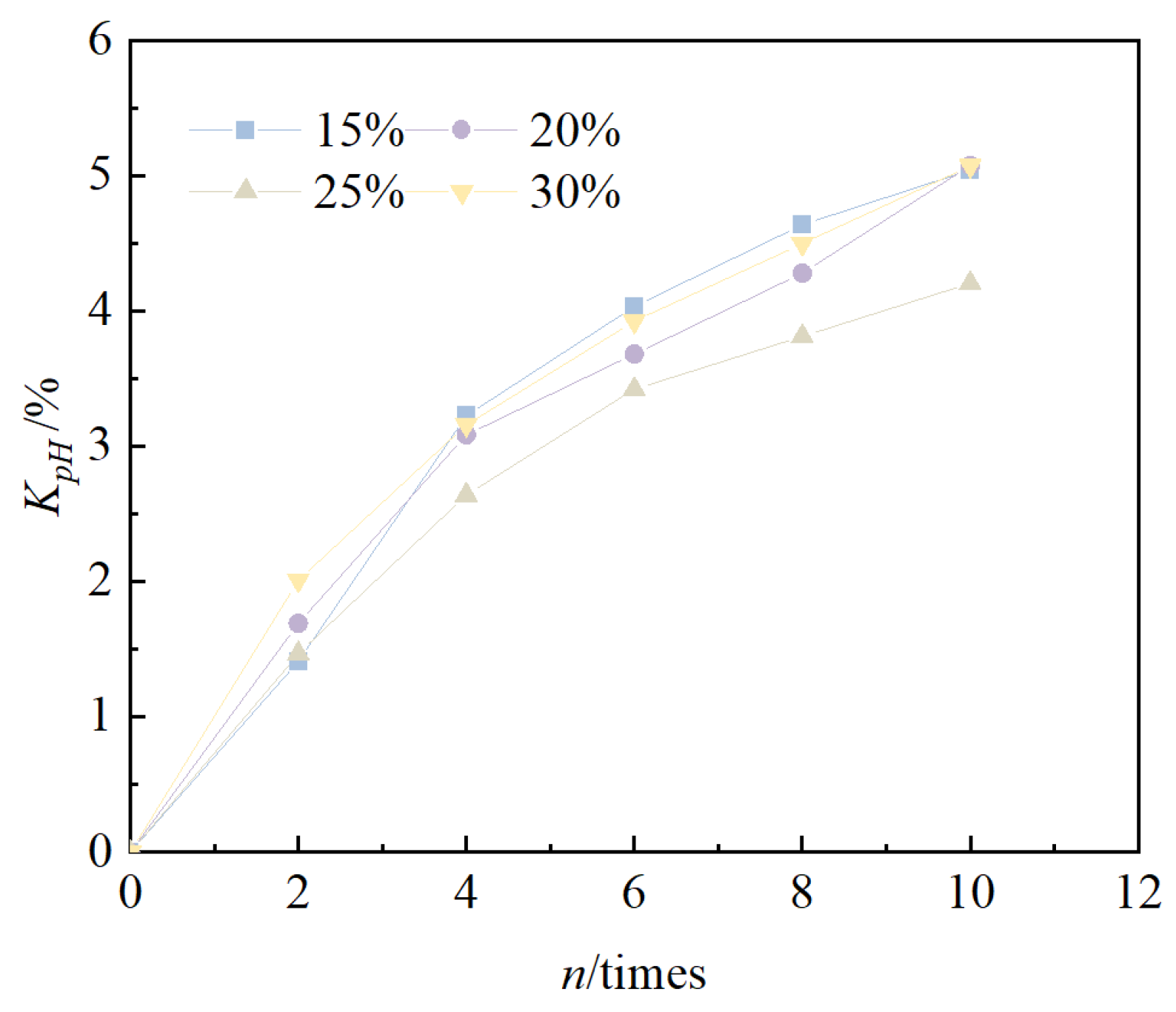
- The pH value of solidified/stabilized red mud under different mixing ratios gradually decreased with an increase in the number of cycles, the decreasing tendency gradually became slower, and the pattern satisfied an exponential function relationship.
- (3)
- With an increase in the number of cycles, the leaching concentration of all seven pollutants increased continuously, and the logarithm of the leaching concentration and the number of cycles satisfied a linear relationship. If the leaching toxicity of the pollutants in red mud is considered, the number of freeze–thaw cycles for the failure of solidified/stabilized red mud at the mixing ratios of 15%, 20%, 25%, and 30% are 0, 2, 6, and 8 times, respectively, when the groundwater quality of Class III is used as the failure criterion.
- (4)
- The hydration reaction of the CGFPA binder generates products such as C-S-H, C-A-S-H, C-A-H, AFm, etc. These products make the structure of solidified/stabilized red mud stronger by cementing the red mud particles and filling the internal pores and forming a grid-like interconnected structure. Under the action of freeze–thaw cycling, the lattice structure of solidified/stabilized red mud is damaged, which leads to a decrease in its strength and an increase in pollutant leaching concentration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GB50986-2014; Code for Design of Dry Red Mud Stack; Ministry of Housing and Urban-Rural Development of the People’s Republic of China. China Construction Industry Press: Beijing, China, 2014. (In Chinese)
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1, 100007. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.M.; Zhang, Z.Q.; Liu, Y. Synergistic utilization, critical mechanisms, and environmental suitability of bauxite residue (red mud) based multi-solid wastes cementitious materials and special concrete. J. Environ. Manag. 2024, 361, 121255. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wasewar, K.L.; Mukhopadhyay, J.; Yoo, C.K.; Hslu, H. Neutralization and utilization of red mud for its better waste management. Arch. Environ. Sci. 2012, 6, 13–33. [Google Scholar]
- He, J.; Jie, Y.X.; Zhang, J.H.; Yu, Y.Z.; Zhang, G.P. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Tuazon, D.; Corder, G.D. Life cycle assessment of seawater neutralised red mud for treatment of acid mine drainage. Resour. Conserv. Recy. 2008, 52, 1307–1314. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, D.Y. Mineral phase and physical properties of red mud calcined at different temperatures. J. Nanomater. 2012, 1, 628592. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.L.; Liu, X.M.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Akinci, A.; Artir, R. Characterization of trace elements and radionuclides and their risk assessment in red mud. Mater. Charact. 2008, 59, 417–421. [Google Scholar] [CrossRef]
- Somlai, J.; Jobbagy, V.; Kovacs, J.; Tarján, S.; Kovács, T. Radiological aspects of the usability of red mud as building material additive. J. Hazard. Mater. 2008, 150, 541–545. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation—United Nations Scientific Committe on the Effects of Atomic Radiation; UNSCEAR 1993 Report to the General Assembly with Scientific Annexes; United Nations: New York, NY, USA, 1993. [Google Scholar]
- Novais, R.M.; Carvalheiras, J.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Innovative application for bauxite residue: Red mud-based inorganic polymer spheres as pH regulators. J. Hazard. Mater. 2018, 358, 69–81. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Sun, X. The Research on Environmental Risk Assessment System of the Typical Staple Industrial Solid Wastes. Ph.D. Thesis, Kunming University of Science and Technology, Kunming, China, 2016. (In Chinese). [Google Scholar]
- Zhou, Y.; Li, Z.F. Composition Design and Engineering Practice of Red Mud-Based Polymer Cementitious Materials; Science Press: Beijing, China, 2023. (In Chinese) [Google Scholar]
- Li, X.Y.; Yang, Z.P.; Yang, S.; Zhang, K.S.; Chang, J.Z. Synthesis process-based mechanical property optimization of alkali-activated materials from red mud: A review. J. Environ. Manag. 2023, 344, 118616. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.L.; Du, Y.J.; You, X.Y.; Yang, Y.L.; Jiang, Z.Y. Influences of red mud leachates on hydraulic performance of a modified geosynthetic clay liner. Chin. J. Geotech. Eng. 2021, 43, 706–714. (In Chinese) [Google Scholar] [CrossRef]
- Balomenos, E.; Gianopoulou, I.; Panias, D.; Paspaliaris, I.; Perry, K.; Boufounos, D. Efficient and complete exploitation of the bauxite residue (red mud) produced in the Bayer process. Proc. EMC 2011, 3, 745–758. [Google Scholar]
- Deelwal, K.; Dharavath, K.; Kulshreshtha, M. Evaluation of characteristic properties of red mud for possible use as a geotechnical material in civil construction. Int. J. Adv. Eng. Technol. 2014, 7, 1053. [Google Scholar]
- Hua, Y.M.; Heal, K.V.; Friesl-hanl, W. The use of red mud as an immobilizer for metal/metalloid-contaminated soil: A review. J. Hazard. Mater. 2017, 325, 17–30. [Google Scholar] [CrossRef]
- DB 37/T 3559-2019; Technical Regulations for the Application of Red Mud (Bayer Method) Roadbed in Highway Engineering; Shandong Province Market Supervision Administration. People’s Transportation Press: Beijing, China, 2019. (In Chinese)
- Sun, Z.Y.; Wu, H.; Hou, J.L. Study on construction and quality evaluation for subgrade filling of red mud in modified Bayer process. Subgrade Eng. 2018, 03, 69–72. (In Chinese) [Google Scholar] [CrossRef]
- Jin, S.Y.; Lou, X.Y.; Hu, J.; Zhu, J.; Chen, H. Preparation of high-strength permeable bricks with red mud: Influencing factors and structure–performance mechanism. J. Chin. Ceram. Soc. 2024, 52, 3159–3169. (In Chinese) [Google Scholar] [CrossRef]
- Qiao, J. Study on the Preparation of Environmentally Friendly Fire-Free Bricks from Red Mud of Industrial Solid Waste. Master’s Thesis, University of Jinan, Jinan, China, 2022. (In Chinese). [Google Scholar]
- Li, Y.; Liu, X.M.; Li, Z.P.; Ren, Y.Y.; Wang, Y.G.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Xu, Y.T.; Yang, B.; Liu, X.M.; Gao, S.; Li, D.S.; Mukiza, E.; Li, H.J. Investigation of the medium calcium based non-burnt brick made by red mud and fly ash: Durability and hydration characteristics. Int. J. Miner. Metal. Mater. 2019, 26, 983–991. [Google Scholar] [CrossRef]
- Wang, Z.K.; Wang, Y.M.; Wu, L.B.; Wu, A.X.; Ruan, Z.E.; Zhang, M.Z.; Zhao, R.K. Effective reuse of red mud as supplementary material in cemented paste backfill: Durability and environmental impact. Constr. Build. Mater. 2022, 328, 127002. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilization of heavy metal in cement-based solidification/stabilization: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Jiang, N.J. Stabilization/Solidification of Contaminated Soils: A Case Study; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–92. [Google Scholar]
- Chang, R.Q.; Yang, J.J.; Wu, Y.L.; Lu, R.F. Strength and leaching characteristics of CGF solidification/stabilization heavy metal contaminated soil. China Environ. Sci. 2024, 44, 2580–2594. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, J.J.; Chang, R.Q. The design of ternary all-solid-waste binder for solidified soil and the mechanical properties, mechanism and environmental benefits of CGF solidified soil. J. Clean. Prod. 2023, 429, 139439. [Google Scholar] [CrossRef]
- Yi, Y.L.; Gu, L.Y.; Liu, S.Y.; Puppala, A.J. Carbide slag–activated ground granulated blast furnace slag for soft clay stabilization. Can. Geotech. J. 2014, 52, 656–663. [Google Scholar] [CrossRef]
- Siddiqua, S.; Barreto, P.N.M. Chemical stabilization of rammed earth using calcium carbide residue and fly ash. Constr. Build. Mater. 2018, 2, 169. [Google Scholar] [CrossRef]
- Li, W.T.; Yi, Y.L.; Anand, J.P. Comparing carbide sludge-ground granulated blastfurnace slag and ordinary Portland cement: Different findings from binder paste and stabilized clay slurry. Constr. Build. Mater. 2022, 321, 126382. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, J.J.; Chang, R.Q.; Li, S.C.; Kou, H.L. Strength, leaching characteristics and microstructure of CGF+P all-solid-waste binder solidification/stabilization Cu(II) contaminated soil. Constr. Build. Mater. 2024, 411, 134431. [Google Scholar] [CrossRef]
- Sun, Y.J.; Ma, J.; Chen, Y.G.; Tan, B.H.; Chen, W.J. Mechanical behavior of copper contaminated soil solidified/stabilized with carbide slag and metakaolin. Environ. Earth Sci. 2020, 79, 423. [Google Scholar] [CrossRef]
- Feng, Y.S.; Du, Y.J.; Zhou, A.; Zhang, M.; Li, J.S.; Zhou, S.J.; Xia, W.Y. Geoenvironmental properties of industrially contaminated site soil solidified/stabilized with a sustainable by-product-based binder. Sci. Total Environ. 2021, 765, 142778. [Google Scholar] [CrossRef]
- Chen, Y.G.; Zhu, S.Y.; Tan, B.H.; Pan, K. Leaching characteristic of solidification/stabilization for cu2+ contaminated soils with carbide slag and metakaolin. J. Tongji Univ. (Nat. Sci.) 2018, 46, 182–187. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y.G.; Pan, K.; Tan, B.H.; Ye, W.M.; Chen, B. Soaking experimental study on solidification/stabilization of Cu2+ contaminated soils with carbide slag and metakaolin. J. Cent. South Univ. (Sci. Technol.) 2018, 49, 678–683. (In Chinese) [Google Scholar] [CrossRef]
- Guo, X.L.; Hu, W.P.; Shi, H.S. Microstructure and self-solidification/stabilization(S/S) of heavy metals of nano-modified CFA–MSWIFA composite geopolymers. Constr. Build. Mater. 2014, 56, 81–86. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Sun, W.; Chen, Q.L.; Chen, L. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef]
- Wang, Y.G.; Han, F.L.; Mu, J.Q. Solidification/stabilization mechanism of Pb(II), Cd(II), Mn(II) and Cr(III) in fly ash based geopolymers. Constr. Build. Mater. 2018, 160, 818–827. [Google Scholar] [CrossRef]
- Chao, L.; Zhao, F.Q. Application of fly ash/granulated blast-furnace slag cementing material for immobilization of Pb2+. MATEC Web Conf. 2018, 175, 01020. [Google Scholar] [CrossRef][Green Version]
- Li, L.H.; Li, X.; Li, W.T.; Huang, S.P. Strength and leaching characteristics of copper contaminated soil solidified with solid waste based material. Nonferrous Met. Eng. 2023, 13, 136–142. (In Chinese) [Google Scholar] [CrossRef]
- Golewski, G.L. Determination of fracture mechanic parameters of concretes based on cement matrix enhanced by fly ash and nano-silica. Materials 2024, 17, 4230. [Google Scholar] [CrossRef]
- Wang, F.; Du, H.H.; Zheng, Z.; Xu, D.; Wang, Y.; Li, N.; Ni, W.; Ren, C. The Impact of Fly Ash on the Properties of Cementitious Materials Based on Slag-Steel Slag-Gypsum Solid Waste. Materials 2024, 17, 4696. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Wang, H.; Ma, Y.X.; Lu, X.Y. Stress-Strain Behavior and Strength Development of High-Amount Phosphogypsum-Based Sustainable Cementitious Materials. Materials 2024, 17, 4927. [Google Scholar] [CrossRef]
- Wang, H.W.; Ding, Y.B.; Kong, Y.; Sun, D.Y.; Shi, Y.; Cai, X. Predicting the Compressive Strength of Sustainable Portland Cement–Fly Ash Mortar Using Explainable Boosting Machine Learning Techniques. Materials 2024, 17, 4744. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Bai, B. Availability and interfacial energy characteristics of heavy metal ions in soils solidified by graphene oxide. China Environ. Sci. 2023, 43, 1277–1287. (In Chinese) [Google Scholar] [CrossRef]
- Kang, D.; Seo, K.S.; Lee, H.Y.; Chung, W. Experimental study on mechanical strength of GO-cement composites. Constr. Build. Mater. 2017, 131, 303–308. [Google Scholar] [CrossRef]
- Yan, L.J.; Yang, J.J.; Wu, Y.L.; Li, F.M. Experimental study of multifaceted solid waste synergistic treatment based on harmlessness and resource utilization of red mud. J. Civ. Environ. Eng. 2025; accepted. (In Chinese) [Google Scholar]
- Cui, W.; Dong, X.; Yang, F.; He, G.; Zhao, R. Damage degradation pattern and life time prediction of solidified red mud under coupled environment of corrosive salt and freeze-thaw cycles. Constr. Build. Mater. 2024, 440, 137455. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.L.; Liu, X.M. Durability and microstructure analysis of the road base material prepared from red mud and flue gas desulfurization fly ash. Int. J. Min. Metal. and Mater. 2020, 27, 555–568. [Google Scholar] [CrossRef]
- Wen, H.; Suo, C.; Hao, Y.; Fan, P.; Dong, X. Effect of Freezing-Thawing Cycle on the Mechanical Properties and Micromechanism of Red Mud-Calcium-Based Composite Cemented Soil. Adv. Civ. Eng. 2020, 1, 8825576. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C. Experimental study on lime and fly ash–stabilized sintered red mud in road base. J. Test. Eval. 2018, 46, 1539–1547. [Google Scholar] [CrossRef]
- Liu, X.M.; Tang, B.W.; Yin, H.F.; Mukiza, E. Durability and environmental performance of Bayer red mud-coal gangue-based road base material. Chin. J. Eng. 2018, 40, 438–445. [Google Scholar] [CrossRef]
- Shi, M.L.; Du, X.Y.; Yu, C.Y.; Zhang, R.K.; Yang, Z.H.; Tian, X.T. Experimental research on feasibility and durability of stable red mud in road use. J. Eng. Geol. 2022, 6, 1974–1985. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.J.; Shi, M.L.; Tian, X.T.; Yu, C.Y.; Du, X.Y. Experimental Study on Phosphogypsum-Amended Red Mud as Road Base Material. Sustainability 2023, 15, 1719. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, P.; Zhang, T.T.; Li, Z.Z.; Xue, Q. Effect of freeze-thaw cycle on engineering properties and microstructure of stabilized/solidified lead contaminated soil treated by cement. Chin. J. Geotech. Eng. 2016, 38, 2043–2050. (In Chinese) [Google Scholar] [CrossRef]
- GBT14848-2017; Groundwater Quality Standards. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China. China Standard Press: Beijing, China, 2017. (In Chinese)
- Feng, Y.S. Solidification/Stabilization of Clay Soil Contaminated by Nickel and Zinc: Sustainable Binder Development and Performance Evaluation. Ph.D. Thesis, Southeast University, Nanjing, China, 2021. (In Chinese). [Google Scholar]
- ASTM-D560-03; Standard Test Methods for Freezing and Thawing Compacted Soil-Cement Mixtures. ASTM International: West Conshohocken, PA, USA, 2003.
- Yang, A.W.; Yang, S.K.; Wang, Z.; Zhang, X.W.; Wang, Y.C. Long-term mechanical properties of solidified municipal sludge under freeze-thaw cycles. J. Basic Sci. Eng. 2023, 31, 65–80. (In Chinese) [Google Scholar] [CrossRef]
- JTG 3430-2020; Test Methods of Soils for Highway Engineering; Ministry of Housing and Urban-Rural Development of the People’s Republic of China. China Construction Industry Press: Beijing, China, 2020. (In Chinese)
- USEPA (United States Environmental Protection Agency). Toxicity Characteristic Leaching Procedure: SW-846, Method 1311; USEPA: Washington, DC, USA, 1992. [Google Scholar]
- Liu, T.Y.; Cui, M.Y.; Zhou, C.Y.Z.K.P.; Shi, W.C.; Cao, P. Nuclear magnetic resonance analysis of the failure and damage model of rock masses during freeze–thaw cycles. Bull. Eng. Geol. Environ. 2022, 81, 445. [Google Scholar] [CrossRef]
- Li, J.L.; Tan, S.J.; Yang, C.; Chen, H.; Lin, Y. Analysis of Damage Characteristics for Skarn Subjected to Freeze-Thaw Cycles Based on Fractal Theory. Fractal Fract. 2023, 7, 354. [Google Scholar] [CrossRef]
- Liao, J.; Wang, J.; Kong, X.; Xu, Z.; Zhou, P. Insights into the effect of aluminum sulfate and sodium aluminateon the compressive strength development of cement mortars. J. Build. Mater. 2024, 27, 1071–1080. (In Chinese) [Google Scholar]
- Li, C.M.; Zhang, T.T.; Wang, L.J. Mechanical properties and microstructure of alkali activated Pisha sandstone geopolymer composites. Constr. Build. Mater. 2014, 68, 233–239. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Reactivity and reaction products of alkali-activated, fly ash/slag paste. Constr. Build. Mater. 2015, 81, 303–312. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.F. Investigation the synergistic effects in quaternary binder containing red mud, blast furnace slag, steel slag and flue gas desulfurization gypsum based on artificial neural networks. J. Clean. Prod. 2020, 273, 122972. [Google Scholar] [CrossRef]
- Wu, Y.L. Development of All Solid Waste Binders & Stabilization/Solidification Mechanism of Heavy Metal and Application in Contaminated Site Soil. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2022. (In Chinese). [Google Scholar]








| Natural Moisture Content (%) | Specific Gravity | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index |
|---|---|---|---|---|
| 31.9 | 2.72 | 37.8 | 25.2 | 12.6 |
| Chemical Composition | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | Na2O | TiO2 | ZrO2 | Other |
|---|---|---|---|---|---|---|---|---|---|
| Percentage | 12.66 | 15.79 | 36.41 | 14.98 | 0.86 | 9.61 | 7.34 | 0.61 | 1.74 |
| Pollutants | Cu | Zn | Cr | Ni | As | Pb | Cd |
|---|---|---|---|---|---|---|---|
| Red mud | 63.7 | 441.2 | 30.6 | 140 | 314 | 418 | 20.6 |
| Water quality standard of groundwater class III | 1000 | 1000 | 50 | 20 | 10 | 10 | 5 |
| Test Soil | Type of Binder | Mixing Ratio of Binder (%) a | Total Water Content Ratio | Curing Age (d) | Number of Cycles | Test Content | Curing Environment |
|---|---|---|---|---|---|---|---|
| Red mud | CGFPA | 15 | 1 | 28 | 0, 2, 4, 6, 8, 10 | Unconfined compressive strength | Freeze–thaw cycle |
| Acidity and | |||||||
| alkalinity | |||||||
| 20 | 1.2 | Toxicity leaching | |||||
| 25 | 1.3 | XRD, SEM-EDS | |||||
| 30 | 1.4 | FT-IR b |
| Mixing Ratio (%) | a | R2 |
|---|---|---|
| 15 | 816.4 | 0.939 |
| 20 | 948.6 | 0.903 |
| 25 | 512.9 | 0.934 |
| 30 | 457.7 | 0.900 |
| Mixing Ratio (%) | b | R2 |
|---|---|---|
| 15 | 9.93 | 0.995 |
| 20 | 10.03 | 0.985 |
| 25 | 10.20 | 0.999 |
| 30 | 10.39 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Yang, J.; Wu, Y.; Li, F. Deterioration Effects and Microscopic Mechanisms of Solidified/Stabilized Red Mud by CGFPA Binders Under Freeze–Thaw Cycles. Materials 2025, 18, 592. https://doi.org/10.3390/ma18030592
Yan L, Yang J, Wu Y, Li F. Deterioration Effects and Microscopic Mechanisms of Solidified/Stabilized Red Mud by CGFPA Binders Under Freeze–Thaw Cycles. Materials. 2025; 18(3):592. https://doi.org/10.3390/ma18030592
Chicago/Turabian StyleYan, Lijun, Junjie Yang, Yalei Wu, and Fengmin Li. 2025. "Deterioration Effects and Microscopic Mechanisms of Solidified/Stabilized Red Mud by CGFPA Binders Under Freeze–Thaw Cycles" Materials 18, no. 3: 592. https://doi.org/10.3390/ma18030592
APA StyleYan, L., Yang, J., Wu, Y., & Li, F. (2025). Deterioration Effects and Microscopic Mechanisms of Solidified/Stabilized Red Mud by CGFPA Binders Under Freeze–Thaw Cycles. Materials, 18(3), 592. https://doi.org/10.3390/ma18030592






