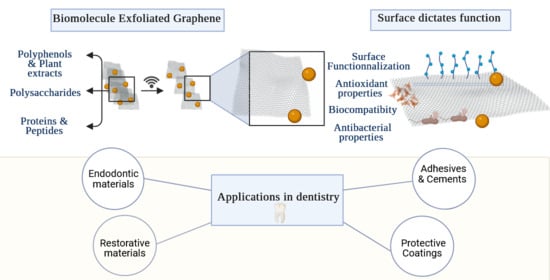
Graphene and Related Materials: Properties and Applications in Dentistry
Abstract
1. Introduction
- Provide a framework of the GRM family and their synthesis routes;
- Review biomolecule-mediated graphene synthesis and its influence on physicochemical properties relevant to dental applications;
- Review current and emerging dental uses of GRM, identify key challenges, and present a roadmap to guide the selection of appropriate GRM forms depending on end use.
2. Framework of Graphene and Related Materials: A Focus on Liquid-Phase Exfoliation
2.1. Overview of Biomolecule-Mediated Green Synthesis of Graphene Related Materials
2.1.1. Polyphenols and Plant Extract-Assisted Synthesis
2.1.2. Polysaccharides
2.1.3. Peptides and Proteins
2.1.4. Biocompatible Polymers
- Anionic polysaccharides offer strong negative zeta potentials and long-term dispersion stability [58];
3. Physicochemical Properties Dictates Function
3.1. Biocompatibility
3.2. Bioactivity and Tissue Remineralization
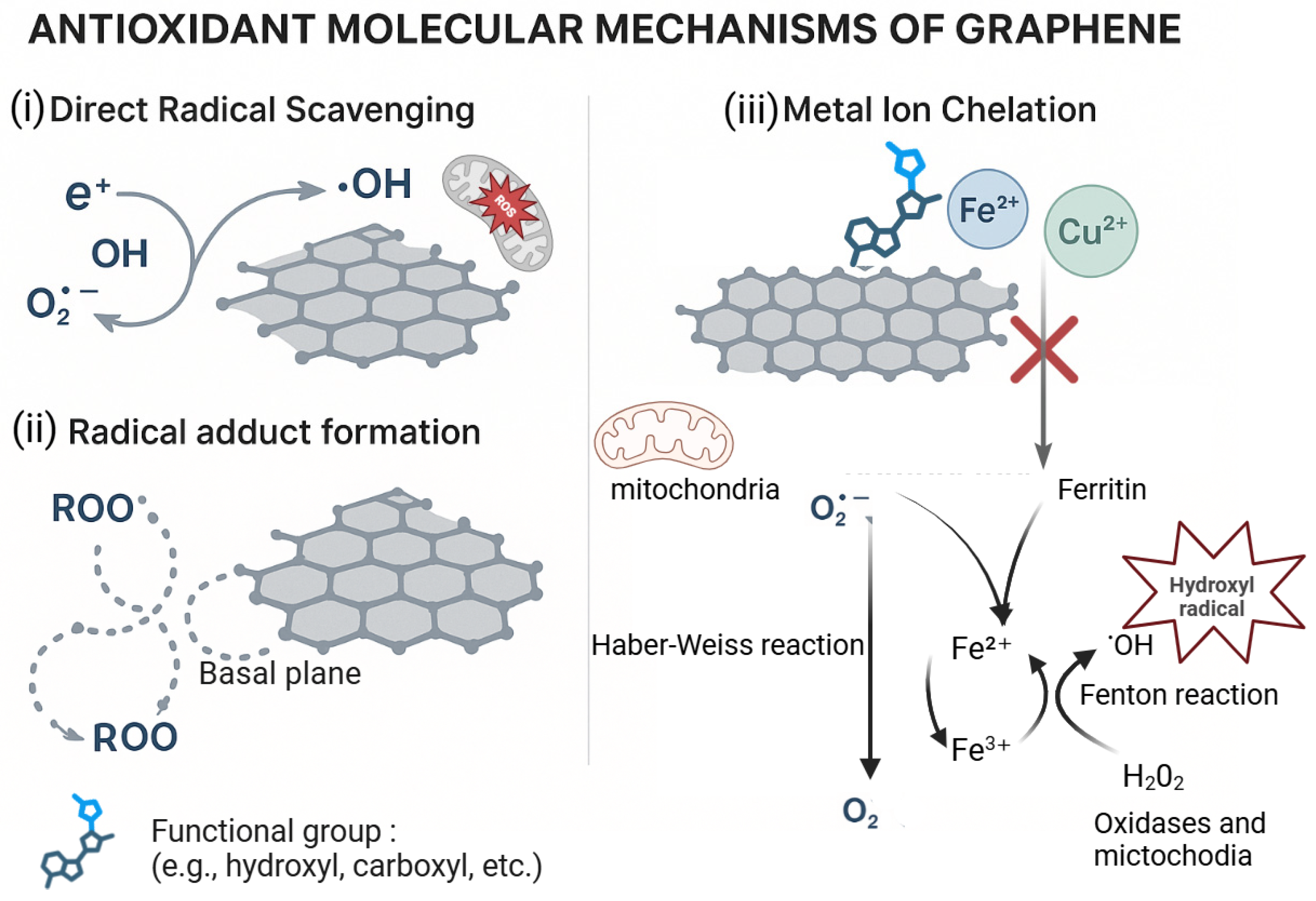
3.3. Antioxidant Properties
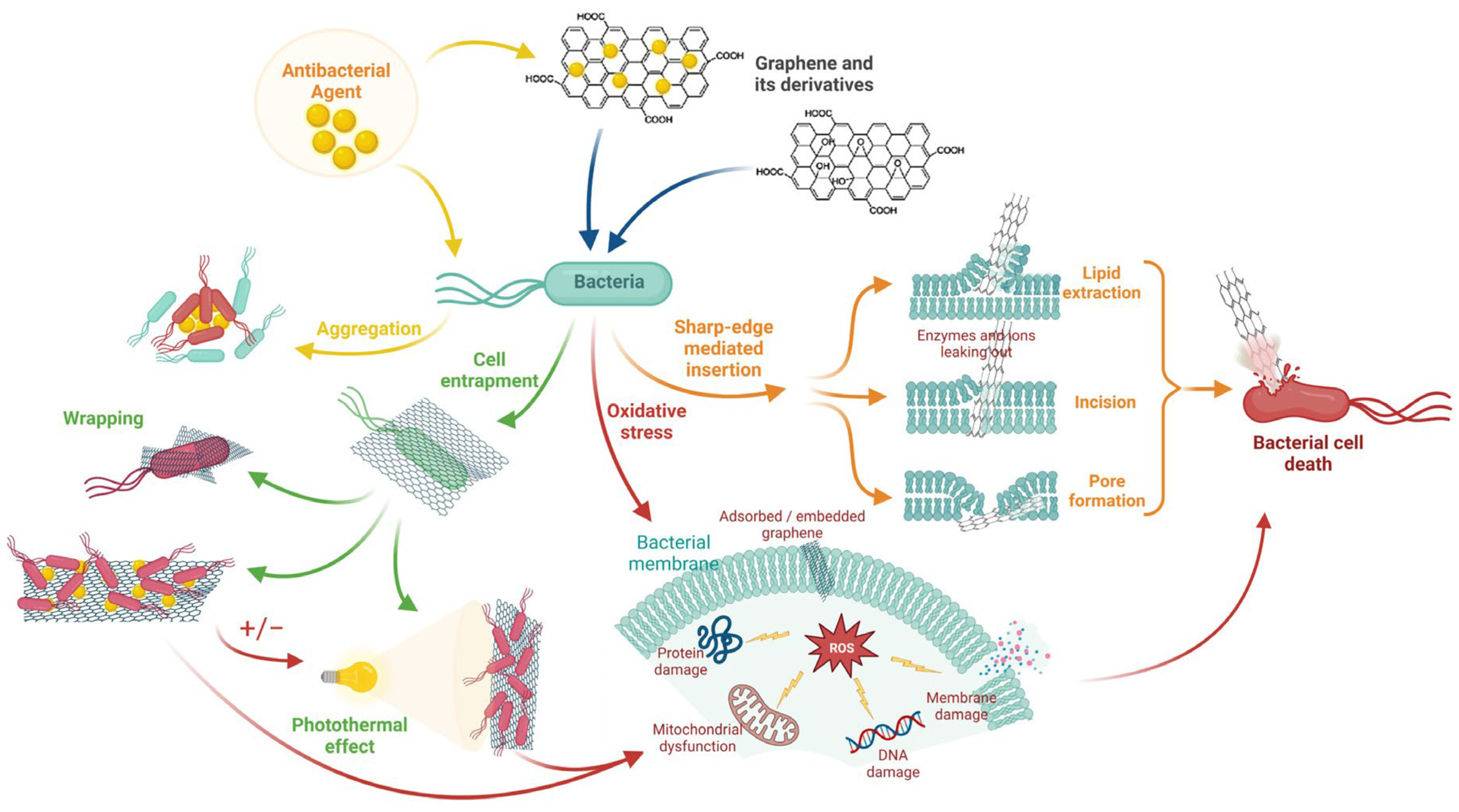
3.4. Antibacterial Properties
3.5. Mechanical Properties
4. Dental Applications
4.1. Endodontic Materials
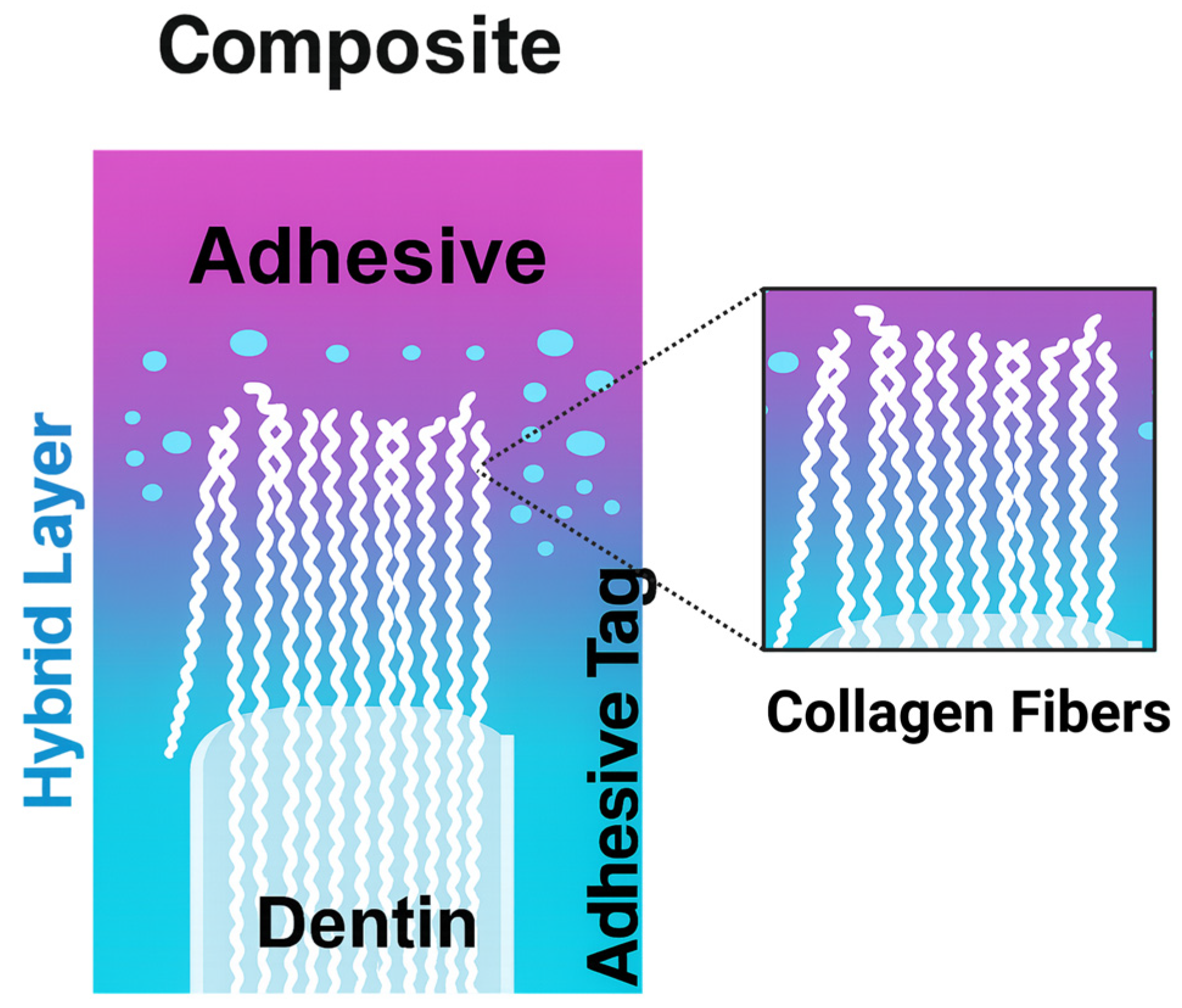
4.2. Adhesives
4.3. Restorative Materials
4.3.1. Direct Restorations
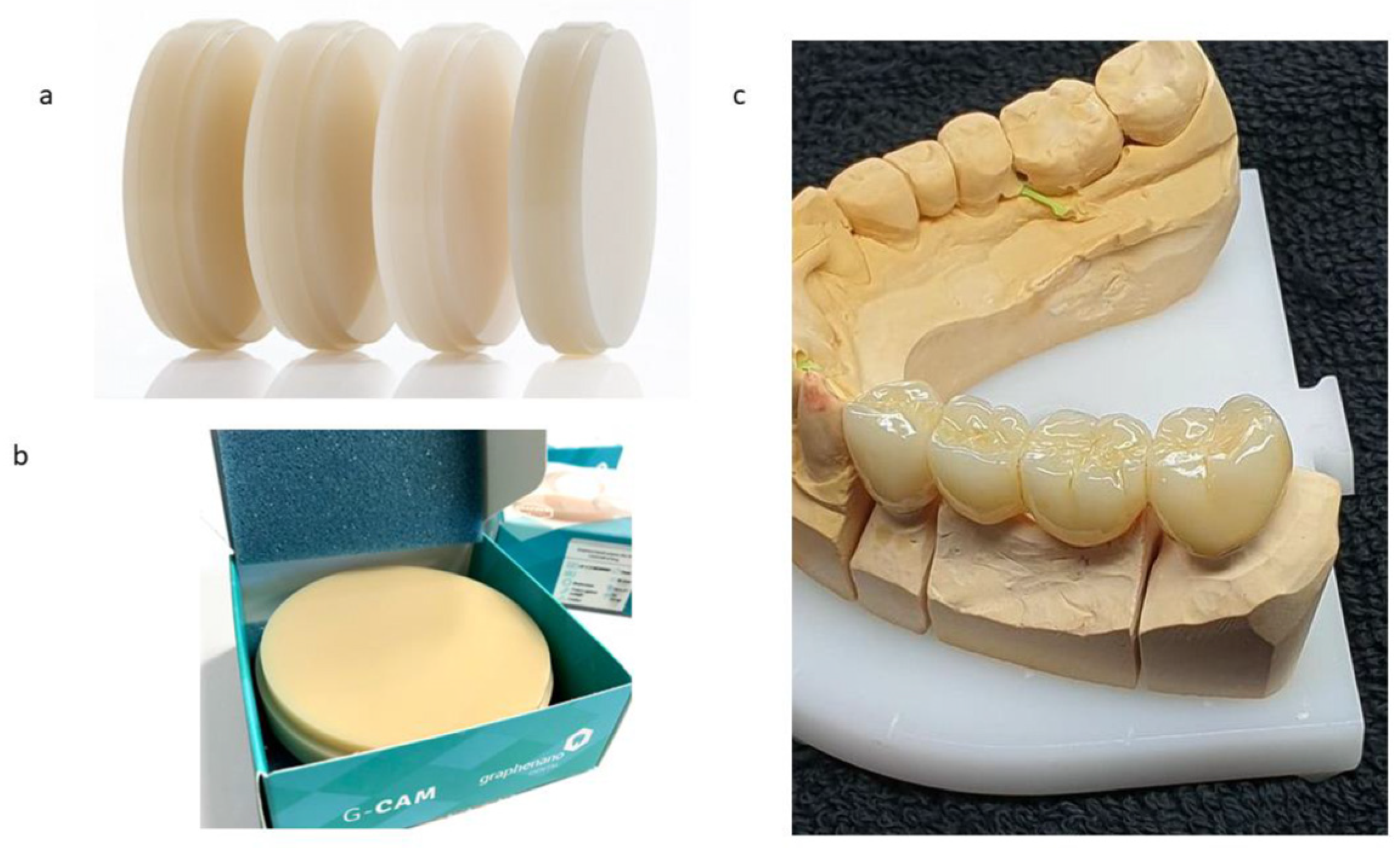
4.3.2. Indirect Restorations
4.4. Cements/Luting Agents
4.5. Protective Coatings
5. Conclusions
6. Future Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GRM | Graphene and related materials |
| ISO | International Organization for Standardization |
| TS | Technical Specification |
| SLG | Single-layer graphene |
| FLG | Few-layer graphene |
| MLG | Multi-layer graphene |
| GNPs | Graphene nanoplatelets |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| CVD | Chemical vapor deposition |
| LPE | Liquid-phase exfoliation |
| 2D | Two-dimensional (materials) |
| LCA | Life-cycle assessment |
| ID/IG | Ratio of D-band/G-band intensities in Raman spectroscopy (with ID = defect band intensity; IG = graphitic band intensity) |
| PMMA | Poly(methyl methacrylate) |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| TA | Tannic acid |
| TEM | Transmission electron microscopy |
| BF-STEM | Bright-field scanning transmission electron microscopy |
| DF-STEM | Dark-field scanning transmission electron microscopy |
| BF | Bright field |
| DF | Dark field |
| PDL | Periodontal ligament cells |
| F-actin | Filamentous actin |
| DNA | Deoxyribonucleic acid |
| BSA | Bovine serum albumin |
| PVA | Poly(vinyl alcohol) |
| PVP | Poly(vinylpyrrolidone) |
| f-FLG | Functionalized few-layer graphene |
| CNC | Cellulose nanocrystals |
| Trp | Tryptophan |
| His | Histidine |
| ROS | Reactive oxygen species |
| SWCNTs | Single-walled carbon nanotubes |
| DC | Degree of conversion |
| UV | Ultraviolet |
| L929 | Mouse fibroblast cell line L929 |
| GSCs | Gingival stromal cells |
| THP-1 | Human monocytic cell line THP-1 |
| IL-8 | Interleukin-8 |
| SLA | Stereolithography (3D printing) |
| DLP | Digital light processing (3D printing) |
| NCPs | Non-collagenous proteins |
| DPP | Dentin phosphoprotein |
| DSP | Dentin sialoprotein |
| DCP | Dicalcium phosphate |
| PCET | Proton-coupled electron transfer |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl (radical assay) |
| CTE | Coefficient of thermal expansion |
| CS | Calcium silicate |
| C–S–H | Calcium–silicate–hydrate |
| GNS | Graphene nanosheets |
| BIO | Biodentine |
| ECZ | Endocem Zr |
| 10-MDP | 10-Methacryloyloxydecyl dihydrogen phosphate |
| ZrO2 | Zirconium dioxide |
| HA | Hydroxyapatite |
| SiO2-Ag-Gr | Silica–silver–graphene composite label (as used) |
| bis-GMA | Bisphenol-A glycidyl methacrylate |
| RT | Resin tags |
| AL | Adhesive layer |
| RC | Resin composite |
| RBCs | Resin-based composites |
| UDMA | Urethane dimethacrylate |
| TEGDMA | Triethylene glycol dimethacrylate |
| CAD/CAM | Computer-aided design/computer-aided manufacturing |
| PICNs | Polymer-infiltrated ceramic networks |
| AM | Additive manufacturing |
| G-CAM | Trade name for a graphene-PMMA composite |
| G-PMMA | Graphene-modified PMMA (as labeled in figure) |
| GICs | Glass ionomer cements |
| RMGICs | Resin-modified glass ionomer cements |
| FG | Fluorinated graphene |
| NiTi | Nickel–titanium (nitinol) |
Appendix A
Appendix A.1
| Search | PubMed | WoS | |
|---|---|---|---|
| Per type of GRM | GO | (“graphene oxide”[tiab] AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (“graphene oxide”) [AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] |
| rGO | (“reduced graphene oxide”[tiab] OR rGO[tiab]) AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (“reduced graphene oxide” OR rGO) [AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] | |
| SLG | (“single layer graphene”[tiab] OR “single-layer graphene”[tiab] OR SLG[tiab]) AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (“single layer graphene” OR “single-layer graphene” OR SLG) [AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] | |
| FLG | (“few layer graphene”[tiab] OR “few-layer graphene”[tiab] OR FLG[tiab]) AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (“few layer graphene” OR “few-layer graphene” OR FLG) [AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] | |
| MLG | (“Multi layer graphene”[tiab] OR “Multi-layer graphene”[tiab] OR MLG[tiab]) AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (“Multi layer graphene” OR “Mult-layer graphene” OR MLG) [AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] | |
| GNP | (“graphene nanoplatelet*”[tiab] OR GNP[tiab] OR GNPs[tiab]) AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (“graphene nanoplatelet” OR “graphene nanoplatelets” OR GNP OR GNPs) [AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] | |
| Graphene | graphene[tiab] NOT (“graphene oxide”[tiab] OR “reduced graphene oxide”[tiab] OR rGO[tiab] OR “graphene nanoplatelet*”[tiab] OR GNP[tiab] OR GNPs[tiab] OR “few layer graphene”[tiab] OR “few-layer graphene”[tiab] OR FLG[tiab]) AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (graphen*) NOT TS = (“graphene oxide” OR “reduced graphene oxide” OR rGO OR “graphene nanoplatelet” OR “graphene nanoplatelets” OR GNP OR GNPs OR “few layer graphene” OR “few-layer graphene” OR FLG) [AND TS = (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] | |
| Graphite | graphite[tiab] NOT (“graphene oxide”[tiab] OR “reduced graphene oxide”[tiab] OR rGO[tiab] OR “graphene nanoplatelet*”[tiab] OR GNP[tiab] OR GNPs[tiab] OR “few layer graphene”[tiab] OR “few-layer graphene”[tiab] OR FLG[tiab])AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = (graphit*)NOT TS = (“graphene oxide” OR “reduced graphene oxide” OR rGO OR “graphene nanoplatelet” OR “graphene nanoplatelets” OR GNP OR GNPs OR “few layer graphene” OR “few-layer graphene” OR FLG) [AND TS = (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)] |
Appendix A.2
| Search | PubMed | WoS | |
|---|---|---|---|
| Per Field | Biomolecule-mediated graphene integration in dental field | (graphene[tiab] OR “graphene oxide”[tiab] OR “reduced graphene oxide”[tiab] OR rGO[tiab] OR “few layer graphene”[tiab] OR “few-layer graphene”[tiab] OR FLG[tiab] OR “graphene nanoplatelet*”[tiab] OR GNP[tiab] OR GNPs[tiab] )AND( biomolecule*[tiab] OR protein*[tiab] OR peptide*[tiab] OR enzyme*[tiab] OR polysaccharide*[tiab] OR biopolymer*[tiab] OR “amino acid*”[tiab] OR DNA[tiab] OR RNA[tiab] OR “green synthes*”[tiab] OR biogenic[tiab] OR bioreduct*[tiab] OR “bio-reduct*”[tiab] OR “liquid phase exfoliation”[tiab] OR exfoliat*[tiab] )AND (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh] OR enamel[tiab] OR dentin[tiab] OR endodont*[tiab] OR periodont*[tiab] OR orthodont*[tiab] OR implant*[tiab] OR restorative[tiab]) | TS = ((graphen* OR “graphene oxide” OR “reduced graphene oxide” OR rGO OR “few layer graphene” OR “few-layer graphene” OR FLG OR “graphene nanoplatelet” OR “graphene nanoplatelets” OR GNP OR GNPs) AND (biomolecule* OR protein* OR peptide* OR enzyme* OR polysaccharide* OR biopolymer* OR “amino acid*” OR DNA OR RNA OR “green synthes*” OR biogenic OR bioreduct* OR “bio-reduct*” OR “liquid phase exfoliation” OR exfoliat*) AND (dentist* OR dental OR enamel OR dentin OR endodont* OR periodont* OR orthodont* OR implant* OR restorative)) |
| Biomolecule-mediated graphene integration beyond the dental field | (graphene[tiab] OR “graphene oxide”[tiab] OR “reduced graphene oxide”[tiab] OR rGO[tiab] OR “few layer graphene”[tiab] OR “few-layer graphene”[tiab] OR FLG[tiab] OR “graphene nanoplatelet*”[tiab] OR GNP[tiab] OR GNPs[tiab]) AND (biomolecule*[tiab] OR protein*[tiab] OR peptide*[tiab] OR enzyme*[tiab] OR polysaccharide*[tiab] OR biopolymer*[tiab] OR “amino acid*”[tiab] OR DNA[tiab] OR RNA[tiab] OR “green synthes*”[tiab] OR biogenic[tiab] OR bioreduct*[tiab] OR “bio-reduct*”[tiab] OR “liquid phase exfoliation”[tiab] OR exfoliat*[tiab]) NOT (dentistry[tiab] OR dental*[tiab] OR “Dentistry”[Mesh]) | TS = ((graphen* OR “graphene oxide” OR “reduced graphene oxide” OR rGO OR “few layer graphene” OR “few-layer graphene” OR FLG OR “graphene nanoplatelet” OR “graphene nanoplatelets” OR GNP OR GNPs) AND (biomolecule* OR protein* OR peptide* OR enzyme* OR polysaccharide* OR biopolymer* OR “amino acid*” OR DNA OR RNAOR “green synthes*” OR biogenic OR bioreduct* OR “bio-reduct*” OR “liquid phase exfoliation” OR exfoliat*)) NOT TS = (dentist* OR dental) |
References
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Hutapea, J.A.A.; Manik, Y.G.O.; Ndruru, S.T.C.L.; Huang, J.; Goei, R.; Tok, A.I.Y.; Siburian, R. Comprehensive Review of Graphene Synthesis Techniques: Advancements, Challenges, and Future Directions. Micro 2025, 5, 40. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Li, B.; Feng, Y.; Tang, W. Correlation of Valence Electron Structure and Properties of Monolayer Graphene and MX2 (M=Mo, W.; X=S, Se, Te): Empirical Electron Theory (EET) Investigation. Phys. E Low-Dimens. Syst. Nanostructures 2025, 165, 116124. [Google Scholar] [CrossRef]
- Moczko, L.; Reichardt, S.; Singh, A.; Zhang, X.; Jouaiti, E.; López, L.E.P.; Wolff, J.L.P.; Moghe, A.R.; Lorchat, E.; Singh, R.; et al. Symmetry-Dependent Dielectric Screening of Optical Phonons in Monolayer Graphene. Phys. Rev. X 2025, 15, 021043. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene Research and Their Outputs: Status and Prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, B.; Han, L. Graphene-Based Nanomaterials for Dental Applications: Principles, Current Advances, and Future Outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing Dental Pathogens Using Antibacterial Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Ayyakannu Sundaram, G. Graphene in Dentistry: Transformative Applications and Innovations. Eur. Arch. Paediatr. Dent. 2025, 26, 811–812. [Google Scholar] [CrossRef]
- De Toledo, P.T.A.; Nunes, G.P.; Ferreira, M.F.; Martins, T.P.; Peres, G.R.; Soares, D.G.; Esteves-Oliveira, M. Bonding Behavior of Graphene-Based Enhanced Restorative Materials: A Systematic Review and Meta-Analysis. Int. J. Adhes. Adhes. 2025, 141, 104046. [Google Scholar] [CrossRef]
- Hussein, L.A.; Mahmoud, M.R.; Hussein, M.O.; Rayyan, M.; Naguib, A.; Sayed, M. Impact of Graphene-Incorporated Nanofillers on Material Properties and Performance of Polymers Used for Prosthodontic Patients: A Systematic Review and Meta-Analysis. BMC Oral Health 2025, 25, 1420. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Saini, R.S.; Kuruniyan, M.S.; Mosaddad, S.A.; Heboyan, A. Graphene and Its Modifications for Enhanced Adhesion in Dental Restoratives: A Molecular Docking and Dynamics Study. Sci. Rep. 2025, 15, 9455. [Google Scholar] [CrossRef]
- Kovtun, A.; Treossi, E.; Mirotta, N.; Scidà, A.; Liscio, A.; Christian, M.; Valorosi, F.; Boschi, A.; Young, R.J.; Galiotis, C.; et al. Benchmarking of Graphene-Based Materials: Real Commercial Products versus Ideal Graphene. 2D Mater. 2019, 6, 025006. [Google Scholar] [CrossRef]
- He, Y.; Andrade, A.F.; Ménard-Moyon, C.; Bianco, A. Biocompatible 2D Materials via Liquid Phase Exfoliation. Adv. Mater. 2024, 36, 2310999. [Google Scholar] [CrossRef]
- ISO/TS 80004-13; Nanotechnologies-Vocabulary_Part 13: Graphene and Related Two-Dimensional (2D) Materials. International Organization for Standardization: Geneva, Switzerland, 2024.
- Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G. Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical Applications of Graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Graphene-Oxide Peptide-Containing Materials for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 10174. [Google Scholar] [CrossRef]
- Bahmei, F.; Bahramifar, N.; Ghasemi, S.; Younesi, H.; Weil, M. Comparison of Environmental Impacts in the Production of Graphene from Biomass Waste and the Hummers’ Method. J. Clean. Prod. 2025, 497, 145145. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Amiri, A.; Naraghi, M.; Ahmadi, G.; Soleymaniha, M.; Shanbedi, M. A Review on Liquid-Phase Exfoliation for Scalable Production of Pure Graphene, Wrinkled, Crumpled and Functionalized Graphene and Challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Jin, L.; Liu, L.; Mo, Y.; Li, X.; Mo, Z.; Liu, Z.; You, S.; Zhu, H. Research Progress of the Liquid-Phase Exfoliation and Stable Dispersion Mechanism and Method of Graphene. Front. Mater. 2019, 6, 325. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S. Biomolecule-Assisted Exfoliation and Dispersion of Graphene and Other Two-Dimensional Materials: A Review of Recent Progress and Applications. Nanoscale 2016, 8, 15389–15413. [Google Scholar] [CrossRef]
- Ba, H.; Truong-Phuoc, L.; Pham-Huu, C.; Luo, W.; Baaziz, W.; Romero, T.; Janowska, I. Colloid Approach to the Sustainable Top-Down Synthesis of Layered Materials. ACS Omega 2017, 2, 8610–8617. [Google Scholar] [CrossRef]
- Wang, Y.; Di, S.; Yu, J.; Wang, L.; Li, Z. Recent Advances of Graphene–Biomacromolecule Nanocomposites in Medical Applications. J. Mater. Chem. B 2023, 11, 500–518. [Google Scholar] [CrossRef]
- Yadav, S.; Shukla, V.K.; Sharma, P.; Sharma, N. Navigating Dental Device Regulations: Current Landscape and Future Prospects. Biomed. Mater. Devices 2025, 4, 467–488. [Google Scholar] [CrossRef]
- Ojrzyńska, M.; Jamroz, J.; Maciałowicz, M.; Wilczyński, K.; Daniszewska, A.; Antonowicz, J.; Zdrojek, M. Quality and Thickness Control of Graphene Nanoplatelets during Large-Scale Production Using Liquid Phase Exfoliation. Mater. Today Commun. 2025, 42, 111543. [Google Scholar] [CrossRef]
- Chemello, G.; Radnik, J.; Hodoroaba, V.-D. XPS–SEM/EDS Tandem Analysis for the Elemental Composition of Functionalized Graphene Nanoplatelets. ACS Omega 2025, 10, 54601–54607. [Google Scholar] [CrossRef]
- Lozano-Chico, M.; Fernández-d’Arlas, B.; Matias-Alkaiaga, M.; Eceiza, A.; Iturrondobeitia, M.; Ugarte, L. Water-Based and Tannin-Assisted Liquid-Phase Exfoliation for a Sustainable Production of Graphene. Sustain. Mater. Technol. 2024, 40, e00956. [Google Scholar] [CrossRef]
- Bu, Y.; Cabulong, R.B.; Kim, B.S. Plant Extract-Based Liquid Phase Exfoliation Enables One-Step Green Production of Two-Dimensional Heterostructure Nanohybrids Capable of Dramatic Improvement in Polymer Properties. Green. Chem. 2024, 26, 3488–3506. [Google Scholar] [CrossRef]
- Salunke, B.K.; Kim, B.S. Facile Synthesis of Graphene Using a Biological Method. RSC Adv. 2016, 6, 17158–17162. [Google Scholar] [CrossRef]
- Ben Ammar, T.; Kharouf, N.; Vautier, D.; Ba, H.; Sudheer, N.; Lavalle, P.; Ball, V. Colloidal Few Layered Graphene–Tannic Acid Preserves the Biocompatibility of Periodontal Ligament Cells. Beilstein J. Nanotechnol. 2025, 16, 664–677. [Google Scholar] [CrossRef]
- Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity. Nanomaterials 2021, 11, 2963. [Google Scholar] [CrossRef]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Handique, J.G.; Baruah, J.B. Polyphenolic Compounds: An Overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel Composite Films Based on Sodium Alginate and Gallnut Extract with Enhanced Antioxidant, Antimicrobial, Barrier and Mechanical Properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Rather, L.J.; Islam, S.U.; Shabbir, M.; Bukhari, M.N.; Shahid, M.; Khan, M.A.; Mohammad, F. Ecological Dyeing of Woolen Yarn with Adhatoda Vasica Natural Dye in the Presence of Biomordants as an Alternative Copartner to Metal Mordants. J. Environ. Chem. Eng. 2016, 4, 3041–3049. [Google Scholar] [CrossRef]
- Manchala, S.; Tandava, V.S.R.K.; Jampaiah, D.; Bhargava, S.K.; Shanker, V. Novel and Highly Efficient Strategy for the Green Synthesis of Soluble Graphene by Aqueous Polyphenol Extracts of Eucalyptus Bark and Its Applications in High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11612–11620. [Google Scholar] [CrossRef]
- Ismail, Z. Photo-Fenton-Inspired Deoxygenation of Tea Polyphenol–Graphene by Household Bleach. Carbon Lett. 2020, 30, 449–456. [Google Scholar] [CrossRef]
- Akbar, N.; Kanwal, S.; Ahmed, M.S.; Kanwal, S.; Jamil, M.A.; Ezugwu, S.; Saleem, M.; Khisro, S.N.; Javed, M.; Ahmed, N.; et al. Biosynthesis of Graphene and Investigation of Antibacterial Activity of Graphene-Parthenium Hysterophorous Nanocomposite. Braz. Arch. Biol. Technol. 2021, 64, e21210180. [Google Scholar] [CrossRef]
- Janowska, I.; Truong-Phuoc, L.; Ba, H.; Pham-Huu, C. Nanocomposites Nanomatériau/Système Polymoléculaire Colloïdaux, Et Méthodes de Préparation; ICPEES: Strasbourg, France, 2018. [Google Scholar]
- Johansson, L.-S.; Tammelin, T.; Campbell, J.M.; Setälä, H.; Österberg, M. Experimental Evidence on Medium Driven Cellulose Surface Adaptation Demonstrated Using Nanofibrillated Cellulose. Soft Matter 2011, 7, 10917. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zhang, T.; Wang, Y.; Wu, J.; Ran, F. Achieving Fast Ionic Transport and High Mechanical Properties of Cellulose-Based Solid-State Electrolyte via a Cationic Chain-Extended Effect for Zinc Metal Batteries. Langmuir 2025, 41, 3455–3463. [Google Scholar] [CrossRef]
- Carrasco, P.M.; Montes, S.; García, I.; Borghei, M.; Jiang, H.; Odriozola, I.; Cabañero, G.; Ruiz, V. High-Concentration Aqueous Dispersions of Graphene Produced by Exfoliation of Graphite Using Cellulose Nanocrystals. Carbon 2014, 70, 157–163. [Google Scholar] [CrossRef]
- Kasim, N.F.A.; Idris, W.W.F.; Abdullah, A.H.; Yusoh, K.; Ismail, Z. The Preparation of Graphene Ink from the Exfoliation of Graphite in Pullulan, Chitosan and Alginate for Strain-Sensitive Paper. Int. J. Biol. Macromol. 2020, 153, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Brljak, N.; Slocik, J.M.; Rao, R.; Knecht, M.R.; Walsh, T.R. Graphene Exfoliation Using Multidomain Peptides. J. Mater. Chem. B 2024, 12, 4824–4832. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Xing, C.; Yu, A.; Yu, J.; Chen, P. Aromatic Amino Acid-Dependent Surface Assembly of Amphiphilic Peptides for One-Step Graphite Exfoliation and Graphene Functionalization. J. Phys. Chem. Lett. 2024, 15, 6611–6620. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Suleimenova, A.; Alves, J.F.; Frasco, M.F.; Barquinha, P.; Sales, M.G.F. Ultrasound-Assisted Protein-Enhanced Graphene Synthesis for Rapid Electrochemical Antibody Sensing. Microchem. J. 2025, 209, 112647. [Google Scholar] [CrossRef]
- Laaksonen, P.; Kainlauri, M.; Laaksonen, T.; Shchepetov, A.; Jiang, H.; Ahopelto, J.; Linder, M.B. Interfacial Engineering by Proteins: Exfoliation and Functionalization of Graphene by Hydrophobins. Angew. Chem. Int. Ed. 2010, 49, 4946–4949. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Musaibah, A.S.O.; Divakar, D.D.; Eldwakhly, E. Graphene Oxide-based Experimental Silane Primers Enhance Shear Bond Strength between Resin Composite and Zirconia. Eur. J. Oral Sci. 2019, 127, 570–576. [Google Scholar] [CrossRef]
- Simsikova, M.; Sikola, T. Interaction of Graphene Oxide with Proteins and Applications of Their Conjugates. J. Nanomed. Res. 2017, 5, 00109. [Google Scholar] [CrossRef]
- Van Der Schueren, B.; El Marouazi, H.; Mohanty, A.; Lévêque, P.; Sutter, C.; Romero, T.; Janowska, I. Polyvinyl Alcohol-Few Layer Graphene Composite Films Prepared from Aqueous Colloids. Investigations of Mechanical, Conductive and Gas Barrier Properties. Nanomaterials 2020, 10, 858. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in Medical and Pharmaceutical Applications: Perspectives and Challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Song, Z.; Boulbet-Friedelmeyer, L.; Motay, M.; Del Rio Castillo, A.E.; Ba, H.; Soltani, R.; Drummond, C.; Lafue, Y.; Penicaud, A.; Bonaccorso, F.; et al. A Comparative Study on Cytotoxicity of Different Types of Commercial Single- and Few-Layer Graphenes on Human Monocytes and Macrophages. Carbon 2025, 244, 120685. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Vallés, C.; Mateos, R.; Vera-López, S.; Kinloch, I.A.; Andrés, M.P.S. Influence of Surfactants of Different Nature and Chain Length on the Morphology, Thermal Stability and Sheet Resistance of Graphene. Soft Matter 2018, 14, 6013–6023. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; Deng, Z.; Xu, Y. Comparative Studies on the Stabilization of Whey Protein Dispersions by Using Various Polysaccharides: Used to Improve the Stability of Liquid Inflammatory Bowel Disease Formula Food for Special Medical Purposes. Int. J. Food Sci. Technol. 2025, 60, vvaf210. [Google Scholar] [CrossRef]
- Silveri, F.; Della Pelle, F.; Rojas, D.; Bukhari, Q.U.A.; Ferraro, G.; Fratini, E.; Compagnone, D. (+)-Catechin-Assisted Graphene Production by Sonochemical Exfoliation in Water. A New Redox-Active Nanomaterial for Electromediated Sensing. Microchim. Acta 2021, 188, 369. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-Based Materials Biocompatibility: A Review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential Nano-Bio Interactions and Toxicity Effects of Pristine versus Functionalized Graphene. Nanoscale 2011, 3, 2461. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, L.; Li, M.; Zhao, R.; Yang, X.; Ji, T.; Gu, Z.; Yin, J.-J.; Gao, X.; Nie, G. Deciphering the Underlying Mechanisms of Oxidation-State Dependent Cytotoxicity of Graphene Oxide on Mammalian Cells. Toxicol. Lett. 2015, 237, 61–71. [Google Scholar] [CrossRef]
- Janjić, K.; Valentova, A.; Arellano, S.; Unterhuber, A.; Krause, A.; Oberoi, G.; Unger, E.; Tabrizi, H.A.S.; Schedle, A. The Impact of Print Orientation and Graphene Nanoplatelets on Biaxial Flexural Strength and Cytotoxicity of a 3D Printable Resin for Occlusal Splints. Dent. Mater. 2024, 40, 1742–1752. [Google Scholar] [CrossRef]
- Ahmad, K.H.; Mohamad, Z.; Khan, Z.I. Influence of Graphene Nanoplatelets and Post-Curing Conditions on the Mechanical and Viscoelastic Properties of Stereolithography 3D-Printed Nanocomposites. Polymers 2024, 16, 2721. [Google Scholar] [CrossRef]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef]
- Sauro, S.; Carvalho, R.M.; Ferracane, J. The Rise of Advanced Bioactive Restorative Materials: Are They Redefining Operative Dentistry? Dent. Mater. 2025, 41, 1411–1429. [Google Scholar] [CrossRef]
- Abozaid, D.; Azab, A.; Bahnsawy, M.A.; Eldebawy, M.; Ayad, A.; Soomro, R.; Elwakeel, E.; Mohamed, M.A. Bioactive Restorative Materials in Dentistry: A Comprehensive Review of Mechanisms, Clinical Applications, and Future Directions. Odontology 2025. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.-S.; Park, J.-C.; Hong, S.W.; Han, D.-W. Reduced Graphene Oxide-Coated Hydroxyapatite Composites Stimulate Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Al-Sarawi, S.; Losic, D.; Mazumdar, J.; Clark, J.; Gronthos, S.; O’Hare Doig, R. Biodegradable and Biocompatible Graphene-based Scaffolds for Functional Neural Tissue Engineering: A Strategy Approach Using Dental Pulp Stem Cells and Biomaterials. Biotechnol. Bioeng. 2021, 118, 4217–4230. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, H.; Zhang, H.; Wu, L.; Chen, G.; Shao, H.; Wang, S.; He, X.; Zheng, S.; Cao, C.Y.; et al. Synergistic Effects of Graphene Quantum Dots and Carbodiimide in Promoting Resin–Dentin Bond Durability. Dent. Mater. 2021, 37, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Çelikel, P.; Şimşek Derelioğlu, S.; Şengül, F.; Okkay, U. The Role of Graphene and BiodentineTM on Proliferation and Odontoblastic Differentiation of Pulp Stem Cells. Curr. Res. Dent. Sci. 2024, 34, 291–296. [Google Scholar] [CrossRef]
- Korkmaz, Y.; Imhof, T.; Kämmerer, P.W.; Bloch, W.; Rink-Notzon, S.; Möst, T.; Weber, M.; Kesting, M.; Galler, K.M.; Deschner, J. The Colocalizations of Pulp Neural Stem Cells Markers with Dentin Matrix Protein-1, Dentin Sialoprotein and Dentin Phosphoprotein in Human Denticle (Pulp Stone) Lining Cells. Ann. Anat.—Anat. Anz. 2022, 239, 151815. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, Y.; Song, S.-J.; Cha, J.; Hong, S.; Lim, Y.-J.; Jeong, S.; Han, D.-W.; Kim, B. Dicalcium Phosphate Coated with Graphene Synergistically Increases Osteogenic Differentiation In Vitro. Coatings 2017, 8, 13. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Owens, A.C.E.; Kulaots, I.; Chen, Y.; Kane, A.B.; Hurt, R.H. Antioxidant Chemistry of Graphene-Based Materials and Its Role in Oxidation Protection Technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar] [CrossRef]
- Dos Santos, F.K.F.; Júnior, A.A.M.P.; Filho, A.L.N.; Fonseca, C.J.N.; Isidorio, D.K.M.; Araújo, F.D.A.; Oliveira, P.H.A.; Veiga Júnior, V.F.D. Graphene and Natural Products: A Review of Antioxidant Properties in Graphene Oxide Reduction. Int. J. Mol. Sci. 2024, 25, 5182. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Zhang, Y.; Qu, S.; Li, B.; Lin, J.; Lu, X.; Xie, C. Injectable Bioadhesive and Lubricating Hydrogel with Polyphenol Mediated Single Atom Nanozyme for Rheumatoid Arthritis Therapy. Nat. Commun. 2025, 16, 2768. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Tan, W.; Mao, S.; Pan, H.; Ye, X.; Donlao, N.; Tian, J. Polyphenol-Based Functional Materials: Structural Insights, Composite Strategies, and Biomedical Applications. Adv. Sci. 2025, 12, e08924. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Yoon, H.H.; Seong, H.; Seo, D.K.; Choi, S.W.; Ryu, J.; Kang, K.-S.; Jeon, S.R. Preventive Effects of Nano-Graphene Oxide against Parkinson’s Disease via Reactive Oxygen Species Scavenging and Anti-Inflammation. BMB Rep. 2023, 56, 202–207. [Google Scholar] [CrossRef]
- Afkhami, F.; Chen, Y.; Walsh, L.J.; Peters, O.A.; Xu, C. Application of Nanomaterials in Endodontics. BME Front. 2024, 5, 0043. [Google Scholar] [CrossRef]
- Byström, A.; Sundqvist, G. Bacteriologic Evaluation of the Effect of 0.5 Percent Sodium Hypochlorite in Endodontic Therapy. Oral Surg. Oral Med. Oral Pathol. 1983, 55, 307–312. [Google Scholar] [CrossRef]
- Waltimo, T.; Trope, M.; Haapasalo, M.; Ørstavik, D. Clinical Efficacy of Treatment Procedures in Endodontic Infection Control and One Year Follow-Up of Periapical Healing. J. Endod. 2005, 31, 863–866. [Google Scholar] [CrossRef]
- Peters, O.A.; Boessler, C.; Paqué, F. Root Canal Preparation with a Novel Nickel-Titanium Instrument Evaluated with Micro-Computed Tomography: Canal Surface Preparation over Time. J. Endod. 2010, 36, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Iandolo, A.; Amato, M.; Dagna, A.; Poggio, C.; Abdellatif, D.; Franco, V.; Pantaleo, G. Intracanal Heating of Sodium Hypochlorite: Scanning Electron Microscope Evaluation of Root Canal Walls. J. Conserv. Dent. 2018, 21, 569. [Google Scholar] [CrossRef]
- Iandolo, A.; Abdellatif, D.; Amato, M.; Pantaleo, G.; Blasi, A.; Franco, V.; Neelakantan, P. Dentinal Tubule Penetration and Root Canal Cleanliness Following Ultrasonic Activation of Intracanal-heated Sodium Hypochlorite. Aust. Endod. J. 2020, 46, 204–209. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Dental Materials with Antibiofilm Properties. Dent. Mater. 2014, 30, e1–e16. [Google Scholar] [CrossRef]
- Apostu, A.M.; Sufaru, I.-G.; Tanculescu, O.; Stoleriu, S.; Doloca, A.; Ciocan Pendefunda, A.A.; Solomon, S.M. Can Graphene Pave the Way to Successful Periodontal and Dental Prosthetic Treatments? A Narrative Review. Biomedicines 2023, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Martuci, R.; Oliveira, S.J.; Martuci, M.; Reis-Campos, J.; Figueiral, M.H. Antimicrobial Effect of Graphene in Dentistry: A Scoping Review. Dent. J. 2025, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qiao, S.; Zhang, X.; Li, Y.; Zhang, Y.; Wei, S.; Shi, J.; Lai, H. Graphene-Reinforced Titanium Enhances Soft Tissue Seal. Front. Bioeng. Biotechnol. 2021, 9, 665305. [Google Scholar] [CrossRef]
- Pereira, R.; Aguiar, F.H.B.; Lins, R.B.E.; Mainairdi, M.C.A.J.; Silva, B.G.; Ferretti, M.A.; Rischka, K. Graphene–Catechol Dental Sealant: Antibacterial and Mechanical Evaluation. Adv. Eng. Mater. 2025, 27, 2500312. [Google Scholar] [CrossRef]
- Narváez-Romero, A.M.; Rodríguez-Lozano, F.J.; Pecci-Lloret, M.P. Graphene-Based Materials for Bone Regeneration in Dentistry: A Systematic Review of In Vitro Applications and Material Comparisons. Nanomaterials 2025, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Remanan, S.; Das, T.K.; Das, N.C. Graphene as a Reinforcement in Thermoset Resins. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; pp. 317–341. ISBN 978-0-12-821639-2. [Google Scholar]
- Zhang, P.; Ma, L.; Fan, F.; Zeng, Z.; Peng, C.; Loya, P.E.; Liu, Z.; Gong, Y.; Zhang, J.; Zhang, X.; et al. Fracture Toughness of Graphene. Nat. Commun. 2014, 5, 3782. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, W.; Yin, Z.; Li, F. Laser-Assisted Graphene Tooth 3D Printing Process. CN Patent CN201710000035.9, 1 January 2017. [Google Scholar]
- Santos-Junior, A.; De Castro Pinto, L.; Mateo-Castillo, J.; Pinheiro, C. Success or Failure of Endodontic Treatments: A Retrospective Study. J. Conserv. Dent. 2019, 22, 129. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Tayyar, M.; Darmani, H. Cytotoxicity Evaluation of Various Resin Based Root Canal Sealers. Int. Endod. J. 2010, 43, 148–153. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Gorduysus, M.; Shinoda-Ito, Y.; Yamamoto, T.; Nishina, Y.; Takashiba, S.; Arias, Z. Graphene Oxide-Based Endodontic Sealer: An in Vitro Study. Acta Med. Okayama 2022, 76, 715–721. [Google Scholar] [PubMed]
- Elmergawy, F.H.; Fathy, I.A.; Abulwafa, Z.M.; Elzayat, G.A.; Seif, H.M. Fluorinated Graphene-Modified Biodentine: An in Vitro Study on Its Ion Release, Cell Growth, Differentiation Potential, and Compressive Strength. BMC Oral Health 2025, 25, 1716. [Google Scholar] [CrossRef]
- Jagtap, T.A.; Sonvane, B.A.; Handal, G.; Bhangare, J.N.; Saraf, K.V.; Mulay, A. Comparative Analysis of Physicocomechanical Properties of MTA and Biodentine with Addition of Graphene Oxide to MTA and Biodentine: An In-Vitro Study. J. Pharm. Bioallied Sci. 2025, 17, S608–S610. [Google Scholar] [CrossRef]
- Saoji, H.; Rao, D. Effect of Graphene Incorporation on the Initial Setting Time of MTA and Biodentine: An In Vitro Pilot Study. J. Pharm. Bioallied Sci. 2025, 17, S2824–S2826. [Google Scholar] [CrossRef]
- Mehrali, M.; Moghaddam, E.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Latibari, S.T.; Metselaar, H.S.C.; Kadri, N.A.; Zandi, K.; Osman, N.A.A. Synthesis, Mechanical Properties, and in Vitro Biocompatibility with Osteoblasts of Calcium Silicate–Reduced Graphene Oxide Composites. ACS Appl. Mater. Interfaces 2014, 6, 3947–3962. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D.; Collins, F.; Wang, C.M.; Duan, W.H. Reinforcing Effects of Graphene Oxide on Portland Cement Paste. J. Mater. Civ. Eng. 2015, 27, A4014010. [Google Scholar] [CrossRef]
- Qutieshat, A.; Al-Hiyasat, A.; Islam, M. The Effect of Adding Graphene Oxide Nanoplatelets to Portland Cement: Potential for Dental Applications. J. Conserv. Dent. 2020, 23, 15. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Sh, K.; Ys, T.; Na, Y. The Effect of Different Dental Adhesive Systems on Hybrid Layer Qualities. Ann. Dent. Univ. Malaya 2014, 21, 29–37. [Google Scholar] [CrossRef]
- Rosa, V.; Rodríguez-Lozano, F.J.; Min, K. Graphene to Improve the Physicomechanical Properties and Bioactivity of the Cements. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 599–614. ISBN 978-0-08-102476-8. [Google Scholar]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles—An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef]
- Ilie, N.; Erich Serfözö, N.; Prodan, D.; Diegelmann, J.; Moldovan, M. Synthesis and Performance of Experimental Resin-Based Dental Adhesives Reinforced with Functionalized Graphene and Hydroxyapatite Fillers. Mater. Des. 2022, 221, 110985. [Google Scholar] [CrossRef]
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental Resin Composites: A Review on Materials to Product Realizations. Compos. Part B Eng. 2022, 230, 109495. [Google Scholar] [CrossRef]
- Prodan, D.; Moldovan, M.; Cuc, S.; Sarosi, C.; Petean, I.; Filip, M.; Carpa, R.; Doukeh, R.; Mirica, I.-C. Advanced Dentistry Biomaterials Containing Graphene Oxide. Polymers 2024, 16, 1743. [Google Scholar] [CrossRef] [PubMed]
- Kawala, M.; Smardz, J.; Adamczyk, L.; Grychowska, N.; Wieckiewicz, M. Selected Applications for Current Polymers in Prosthetic Dentistry—State of the Art. Curr. Med. Chem. 2019, 25, 6002–6012. [Google Scholar] [CrossRef]
- Amin, F.; Rahman, S.; Khurshid, Z.; Zafar, M.S.; Sefat, F.; Kumar, N. Effect of Nanostructures on the Properties of Glass Ionomer Dental Restoratives/Cements: A Comprehensive Narrative Review. Materials 2021, 14, 6260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, X.; Zhang, R.; Sun, L.; Zhang, Z.; Zhao, Y.; Zhang, T.; Yan, J.; Zhang, Y.; Wu, X.; et al. Recent Advances in Glass-ceramics: Performance and Toughening Mechanisms in Restorative Dentistry. J. Biomed. Mater. Res. 2024, 112, e35334. [Google Scholar] [CrossRef] [PubMed]
- Roman, T.; Naji, K.; Calinescu, M.D.; Bérangère, C.; Olivier, E. Who Are the Hybrid Ceramics? Bibliometric Review. Int. J. Prosthodont. 2025, 1–31. [Google Scholar] [CrossRef]
- Gawali, N.; Shah, P.P.; Gowdar, I.M.; Bhavsar, K.A.; Giri, D.; Laddha, R. The Evolution of Digital Dentistry: A Comprehensive Review. J. Pharm. Bioallied Sci. 2024, 16, S1920–S1922. [Google Scholar] [CrossRef]
- Huang, G.; Wu, L.; Hu, J.; Zhou, X.; He, F.; Wan, L.; Pan, S.-T. Main Applications and Recent Research Progresses of Additive Manufacturing in Dentistry. BioMed Res. Int. 2022, 2022, 5530188. [Google Scholar] [CrossRef]
- Punset, M.; Brizuela, A.; Pérez-Pevida, E.; Herrero-Climent, M.; Manero, J.M.; Gil, J. Mechanical Characterization of Dental Prostheses Manufactured with PMMA–Graphene Composites. Materials 2022, 15, 5391. [Google Scholar] [CrossRef]
- Bourgi, R.; Doumandji, Z.; Cuevas-Suárez, C.E.; Ben Ammar, T.; Laporte, C.; Kharouf, N.; Haikel, Y. Exploring the Role of Nanoparticles in Dental Materials: A Comprehensive Review. Coatings 2025, 15, 33. [Google Scholar] [CrossRef]
- Leung, G.K.-H.; Wong, A.W.-Y.; Chu, C.-H.; Yu, O.Y. Update on Dental Luting Materials. Dent. J. 2022, 10, 208. [Google Scholar] [CrossRef]
- Shi, N.; Guo, Y.; Liu, W.; Chen, Q.; Yang, J.; Hu, X.; Mohetaer, A.Y.M.; Liu, B.; Song, F. High-Strength Anticaries Fluorinated Graphene/ZnS:Mn2+ Mechanoluminescence-Based Glass Ionomer Cement for the Filling Treatment of Dental Caries. ACS Biomater. Sci. Eng. 2025, 11, 5306–5317. [Google Scholar] [CrossRef]
- Reddy, V.N.; Rehaman, T.; Gadekar, T.; Mareddy, A.R.; Done, V.; Kanugula, A. Evaluation of Tribiomechanical Properties of Fluorinated Graphene-Infused Atraumatic Restorative Glass Ionomer Cement. J. South Asian Assoc. Pediatr. Dent. 2025, 7, 137–142. [Google Scholar] [CrossRef]
- Srimaneepong, V.; Skallevold, H.E.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Sapkota, J. Graphene for Antimicrobial and Coating Application. Int. J. Mol. Sci. 2022, 23, 499. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tan, D.; Chen, X.; Liao, J.; Wu, L. Research on Graphene and Its Derivatives in Oral Disease Treatment. Int. J. Mol. Sci. 2022, 23, 4737. [Google Scholar] [CrossRef]
- Healy, B.; Yu, T.; Da Silva Alves, D.; Breslin, C.B. Review of Recent Developments in the Formulation of Graphene-Based Coatings for the Corrosion Protection of Metals and Alloys. Corros. Mater. Degrad. 2020, 1, 296–327. [Google Scholar] [CrossRef]
- Saikia, M.; Dutta, T.; Jadhav, N.; Kalita, D.J. Insights into the Development of Corrosion Protection Coatings. Polymers 2025, 17, 1548. [Google Scholar] [CrossRef]
- Wu, J.; Zuo, Y.; Xu, Z.; Wang, L.; Zou, J.; Jia, Z.; Wang, C.; Zhang, G. The Preparation of a GO/ZnO/nHAp Composite Coating and the Study of Its Performance Optimization for Pure Titanium Implants. Micromachines 2025, 16, 637. [Google Scholar] [CrossRef]
- Hithesh, M.C.; Mohana, K.N.S.; Harsha, Y.M.; Sreelakshmi, M.; Nayak, S.R. Development of Anti-Corrosion Coating Material by Inducting Functionalized Graphene Oxide into Acrylated Glucose—Vinyl Acetate Copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2025, 715, 136622. [Google Scholar] [CrossRef]
- Malhotra, R.; Han, Y.; Nijhuis, C.A.; Silikas, N.; Castro Neto, A.H.; Rosa, V. Graphene Nanocoating Provides Superb Long-Lasting Corrosion Protection to Titanium Alloy. Dent. Mater. 2021, 37, 1553–1560. [Google Scholar] [CrossRef]
- Raza, M.A.; Rehman, Z.U.; Ghauri, F.A.; Ahmad, A.; Ahmad, R.; Raffi, M. Corrosion Study of Electrophoretically Deposited Graphene Oxide Coatings on Copper Metal. Thin Solid Film. 2016, 620, 150–159. [Google Scholar] [CrossRef]
- Sesia, R.; Spriano, S.; Sangermano, M.; Ferraris, S. Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies. Metals 2023, 13, 1070. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ramezanzadeh, M.; Ramezanzadeh, B.; Bahlakeh, G. A Green Assisted Route for the Fabrication of a High-Efficiency Self-Healing Anti-Corrosion Coating through Graphene Oxide Nanoplatform Reduction by Tamarindus Indiaca Extract. J. Hazard. Mater. 2020, 390, 122147. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 9651:2025; Graphene-Related 2D Materials for Commercial Applications. International Organization for Standardization: Geneva, Switzerland, 2024.
- Dayi, B.; Küçükyıldız, E.N.; Taghizadehghalehjoughi, A. Evaluation of Cytotoxic Effect of Graphene Oxide Added to Mineral Trioxide Aggregate. J. Adv. Oral Res. 2023, 14, 21–28. [Google Scholar] [CrossRef]
- Camilleri, J. Characterization of Hydration Products of Mineral Trioxide Aggregate. Int. Endod. J. 2008, 41, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Yan, J.; Ning, X.; Zhang, Q.; Wu, Q.; Bi, L.; Zhang, Y.; Han, Y.; Guo, J. Antibacterial and Antibiofilm Properties of Graphene and Its Derivatives. Colloids Surf. B Biointerfaces 2021, 200, 111588. [Google Scholar] [CrossRef] [PubMed]
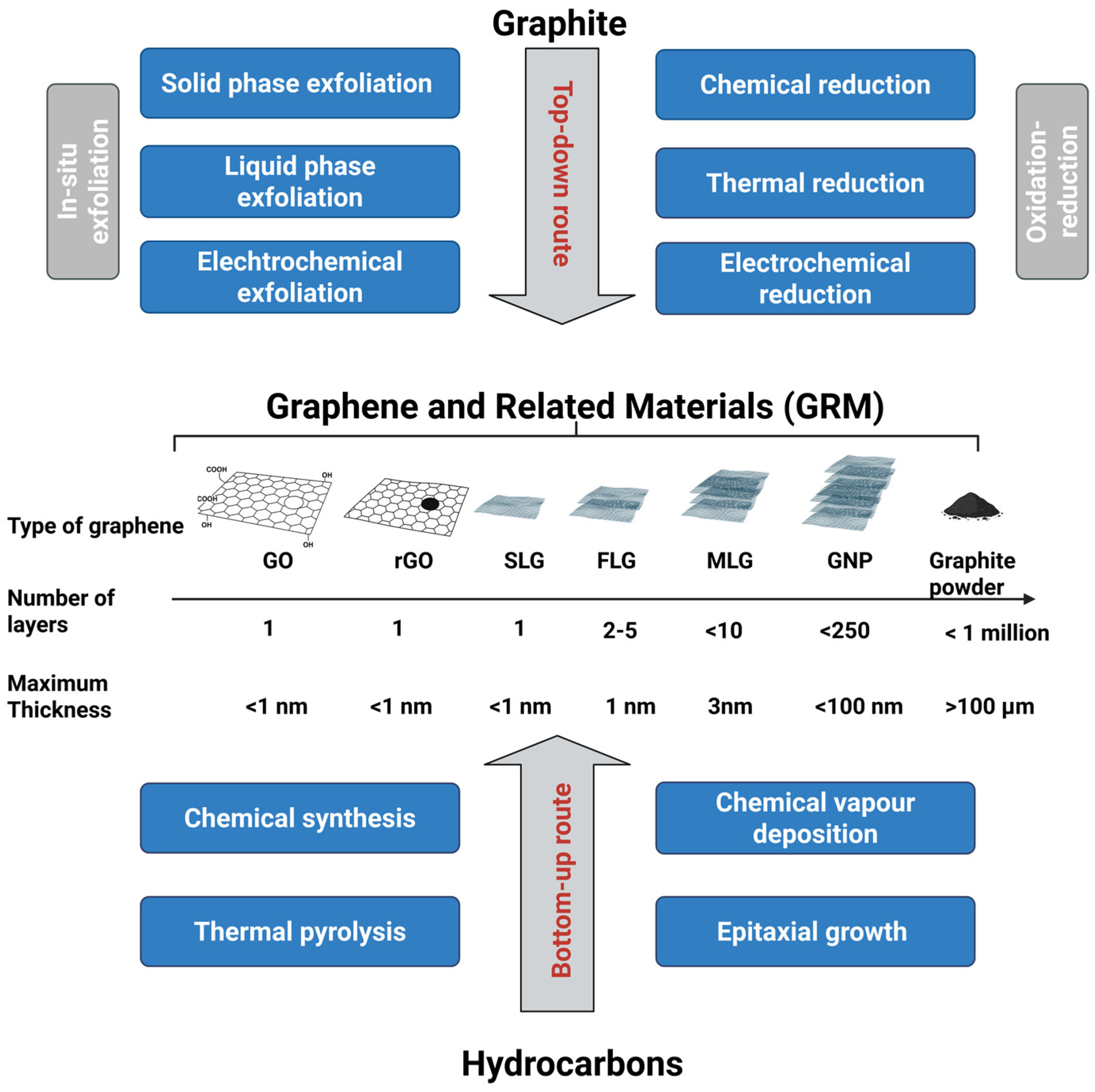
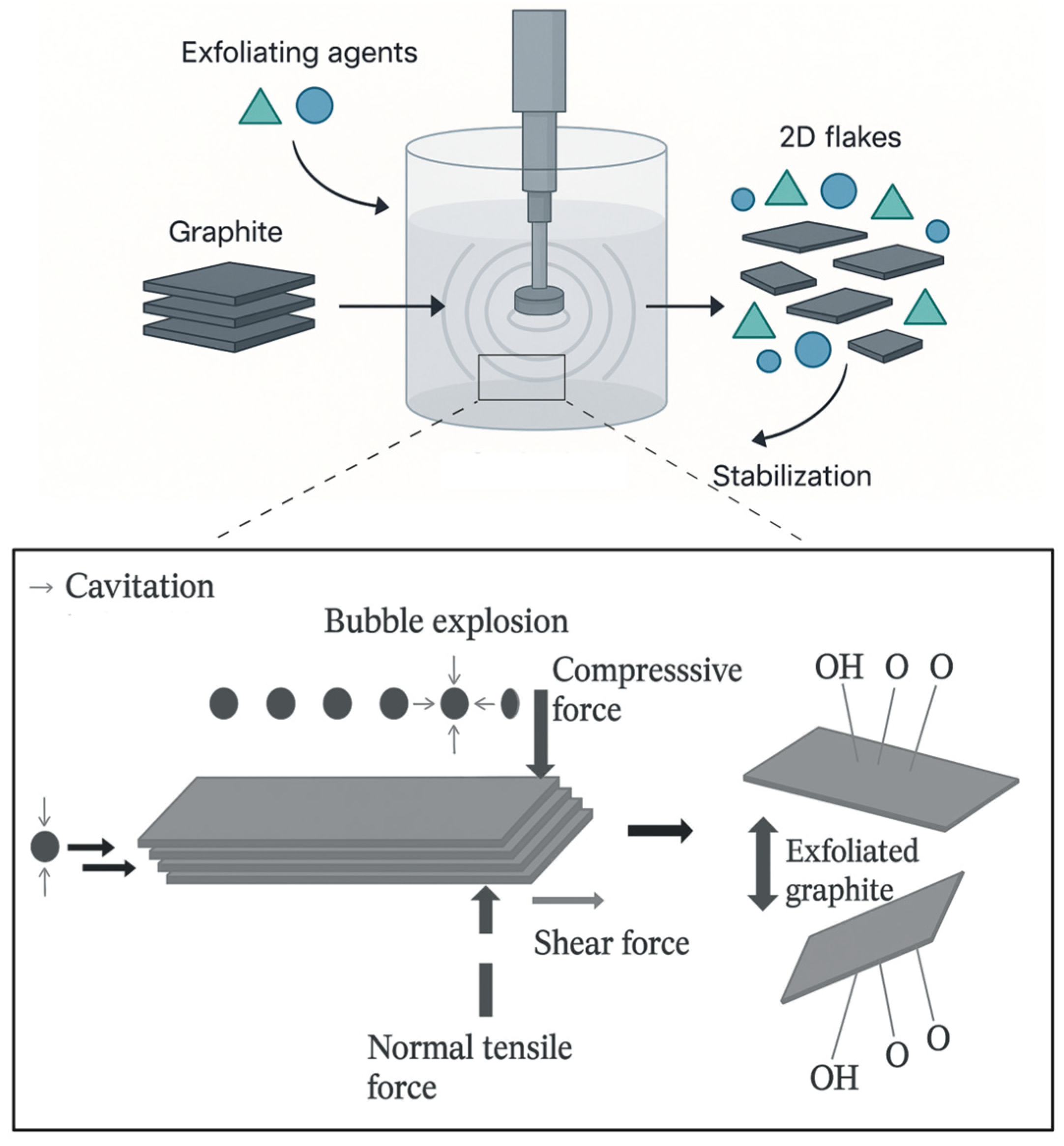



| Form | Key Strengths | Limitations | References |
|---|---|---|---|
| Single Layer Graphene (SLG) | • High specific surface area • Minimal structural disorder (very low ID/IG) • Very high carrier mobility and low sheet resistance • High crystallinity and maximal in-plane mechanical properties | • High cost • extremely sensitive to contamination • difficult to scale up due to substrate transfer and low yields | [6,16] |
| Few-Layer Graphene (FLG) | • High lattice order with high sp2 character • lower intrinsic defects than GO or rGO • Scalable via liquid exfoliation • Offers potential for functionalization • Colloidal stability. | • Lower electronic properties as the number of layers increases, but still of interest • Reduced specific surface area compared to monolayer SLG. | [16,21,22] |
| Multilayer Graphene (MLG) | • High structural stability • Lower intrinsic defects than GO/rGO • Graphitic behavior with moderate conductivity • Scalable via liquid exfoliation • Offers potential for functionalization. | • Reduced “2D character”: electrical, optical and mechanical anisotropy • Lower specific surface area | [6,16] |
| Graphene Nanoplatelets (GNP) | • High lattice order with high sp2 character • High specific surface area relative to bulk graphite • Good crystallinity | • Increased flake thickness • Structural defects at platelet edges • Risk of aggregation | [16,29] |
| Graphene Oxide (GO) | • High oxygen content (increase in O/C) → high chemical reactivity • Hydrophilic surface • Colloidal stability. | • High structural disorder (high ID/IG) • Loss of sp2 network continuity → low electrical conductivity • Low crystallinity • high defect density in the basal plane | [16,21] |
| Reduced Graphene Oxide (rGO) | • Higher sp2 restoration vs. GO → improved conductivity • Intermediate oxygen content (decrease in O/C) • Higher crystallinity than GO. | • Residual oxygen groups → incomplete graphitization • Structural disorder (High defect level) • Properties depend strongly on reduction process → variability. | [16,21] |
| Graphite | • Highly ordered crystallinity in bulk form • Very low defect density within basal planes • Excellent thermal stability • Strong π–π stacking and interlayer cohesion | • No accessible 2D behavior (electronic/optical/mechanical) due to higher Thickness • Very low specific surface area • Poor dispersibility; strong tendency to restack • Limited surface reactivity (low oxygen content) | [6,16] |
| Biomolecule | Nature | Graphene Form | Method | Properties | References |
|---|---|---|---|---|---|
| Gallnut extracts | Polyphenols/ Hydrolysable tannins | f-FLG 1 | LPE 2 | Antioxidant Antibacterial | [59] |
| Eucalyptus Bark extracts | Polyphenols | rGO 3 | Chemical Reduction | Long-term stability Good dispersibility | [40] |
| Black tea extracts (theaflavins/thearubigins) | Polyphenols | FLG | LPE | Additive (polymer reinforcement) | [41] |
| Tannic acid (TA) | Hydroxyl-rich polyphenol | FLG colloids (>1 g·L−1) | Shear-assisted exfoliation | Antioxidant good biocompatibility | [34] |
| Okra & baobab extracts | Plant extracts | FLG colloids (>1 g·L−1) | Shear-assisted exfoliation | Coating (conductive films/papers); Additive (ink base) | [43] |
| Cellulose CNC | Cationic amino polysaccharide | FLG | Bath sonication | Low resistivity; high strain sensitivity; stable colloids | [25,46] |
| Alginate, Chitosan | Polysaccharide/Anionic mannuronic/guluronic copolymer | FLG | Bath sonication | High yield; good stability | [47] |
| Aromatic-rich peptides (e.g., Trp 4-rich)/His 5-rich lipidated amphiphilic peptide | Peptides | FLG | LPE | Stable aqueous colloids; bio functional interfaces | [19,49] |
| BSA, hemoglobin, myoglobin (non-ionic proteins) | Proteins | Few- to multilayer graphene–protein nanocomposites (≤10 layers) | Aqueous ultrasonication ± shear | High-solid content; stable colloids | [25,43] |
| PVA 6/PVP 7 | Biocompatible polymers | FLG | LPE | stable colloids | [24,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ammar, T.; Roman, T.; Ba, H.; Ball, V.; Kharouf, N. Graphene and Related Materials: Properties and Applications in Dentistry. Materials 2025, 18, 5365. https://doi.org/10.3390/ma18235365
Ben Ammar T, Roman T, Ba H, Ball V, Kharouf N. Graphene and Related Materials: Properties and Applications in Dentistry. Materials. 2025; 18(23):5365. https://doi.org/10.3390/ma18235365
Chicago/Turabian StyleBen Ammar, Teissir, Tatiana Roman, Housseinou Ba, Vincent Ball, and Naji Kharouf. 2025. "Graphene and Related Materials: Properties and Applications in Dentistry" Materials 18, no. 23: 5365. https://doi.org/10.3390/ma18235365
APA StyleBen Ammar, T., Roman, T., Ba, H., Ball, V., & Kharouf, N. (2025). Graphene and Related Materials: Properties and Applications in Dentistry. Materials, 18(23), 5365. https://doi.org/10.3390/ma18235365