Unveiling the Effect of Scanning Speed on the Corrosion and Tribological Performance of Electron Beam Melted (EBM) Ti-6Al-4V-ELI Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
2.2. Tribological and Electrochemical Tests
2.3. Surface Morphology
2.4. Cell Culture and Adhesion on Ti-6Al-4V-ELI Disks
3. Results and Discussion
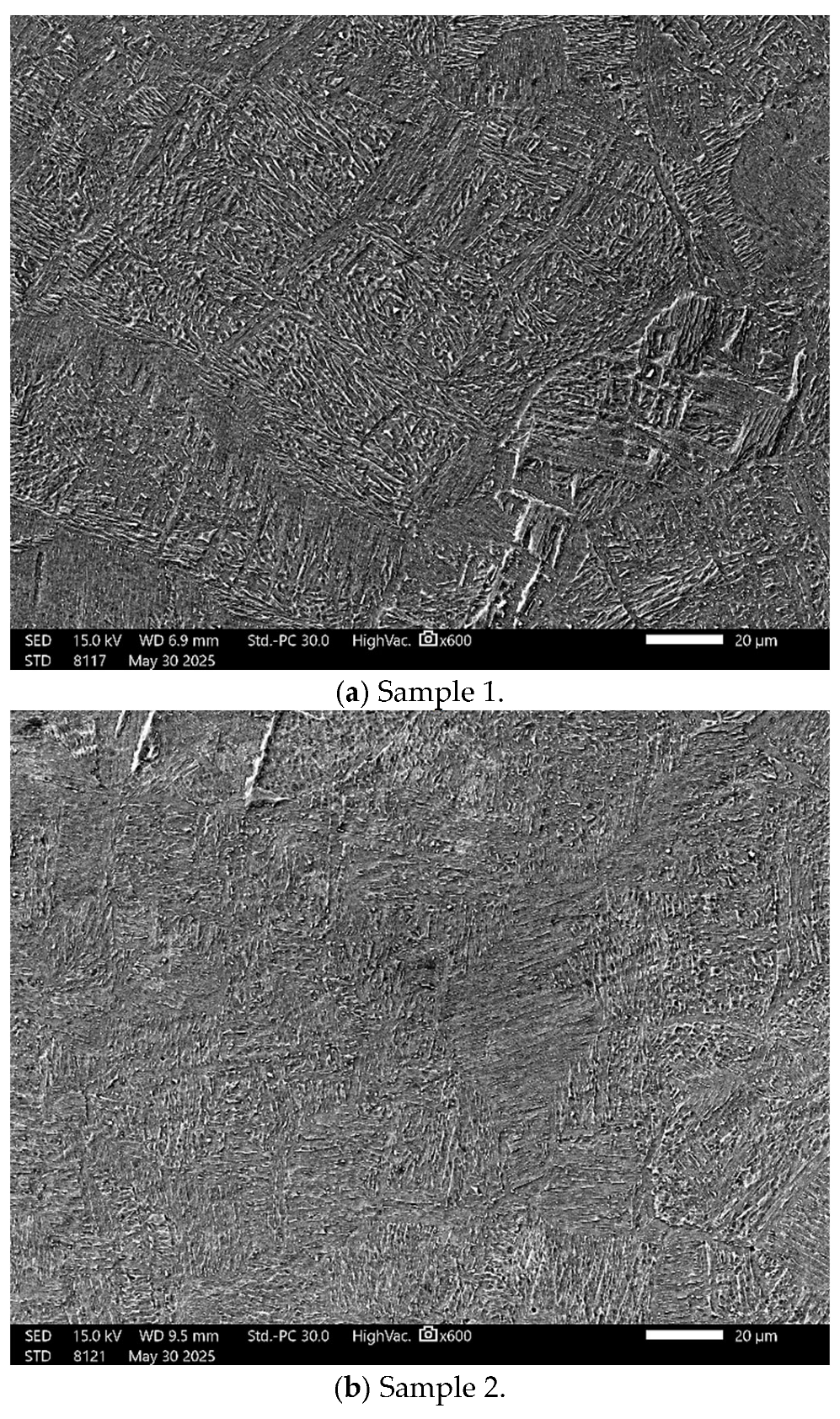
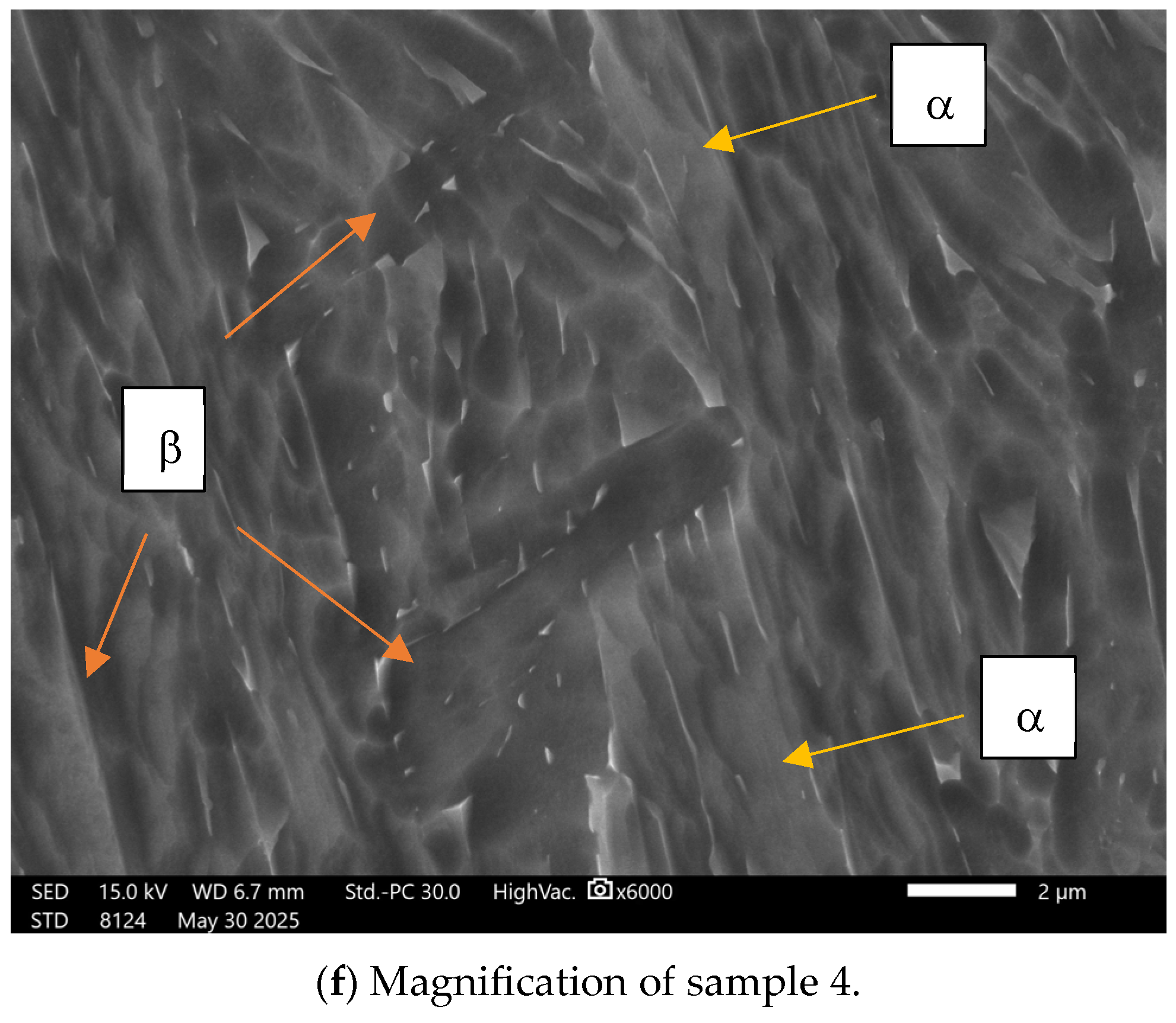
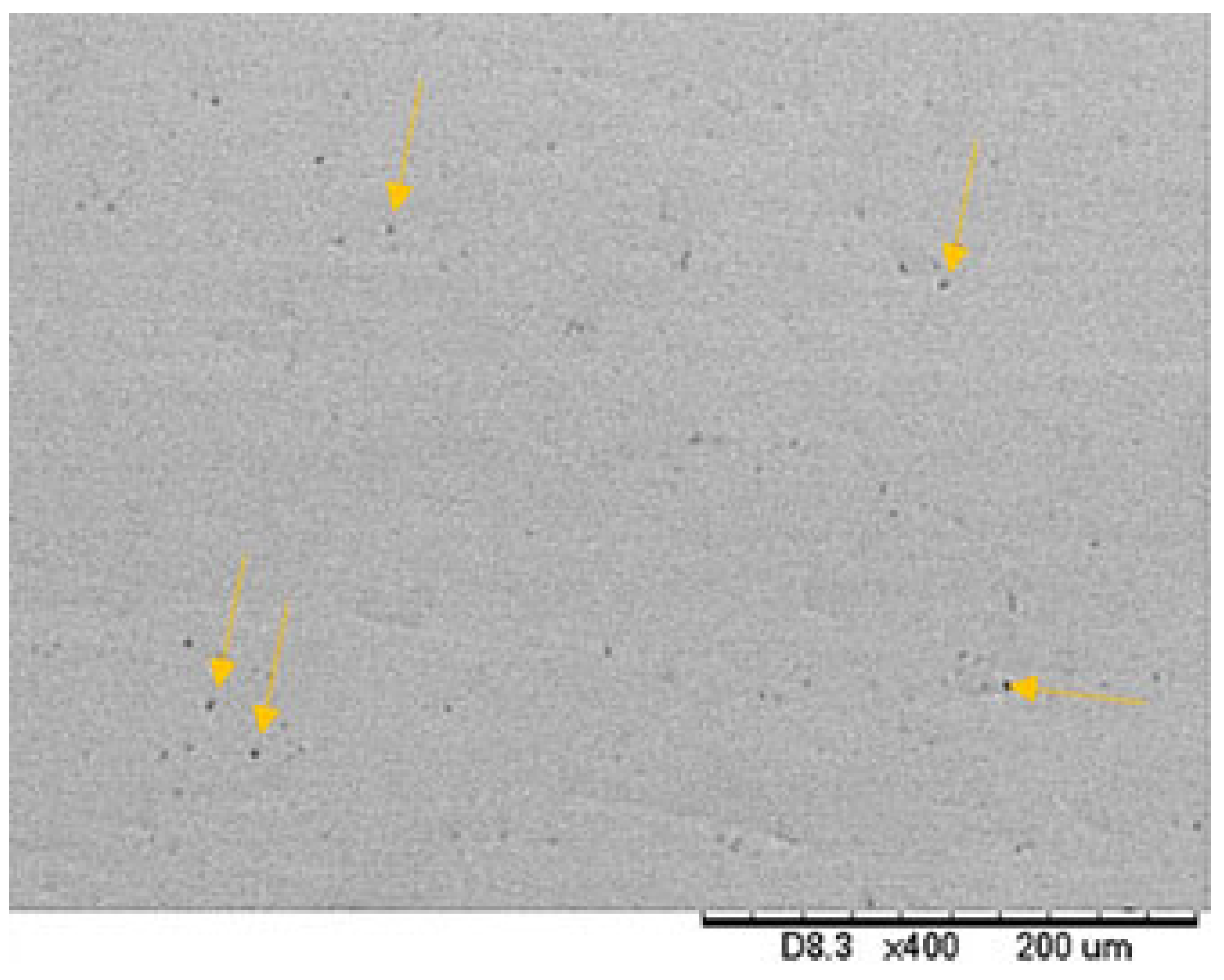
3.1. Topographical Characterization
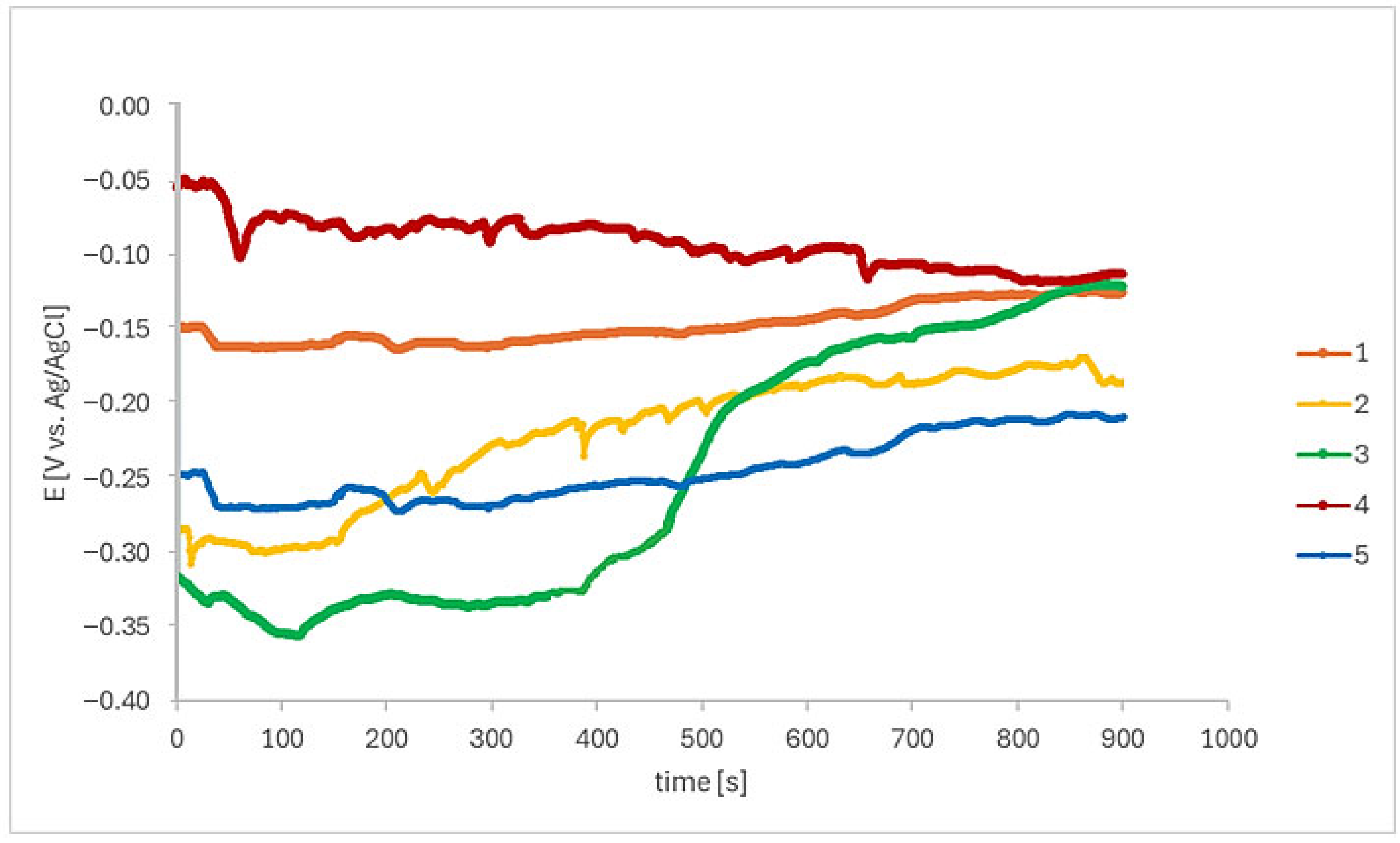
3.2. Electrochemical Tests
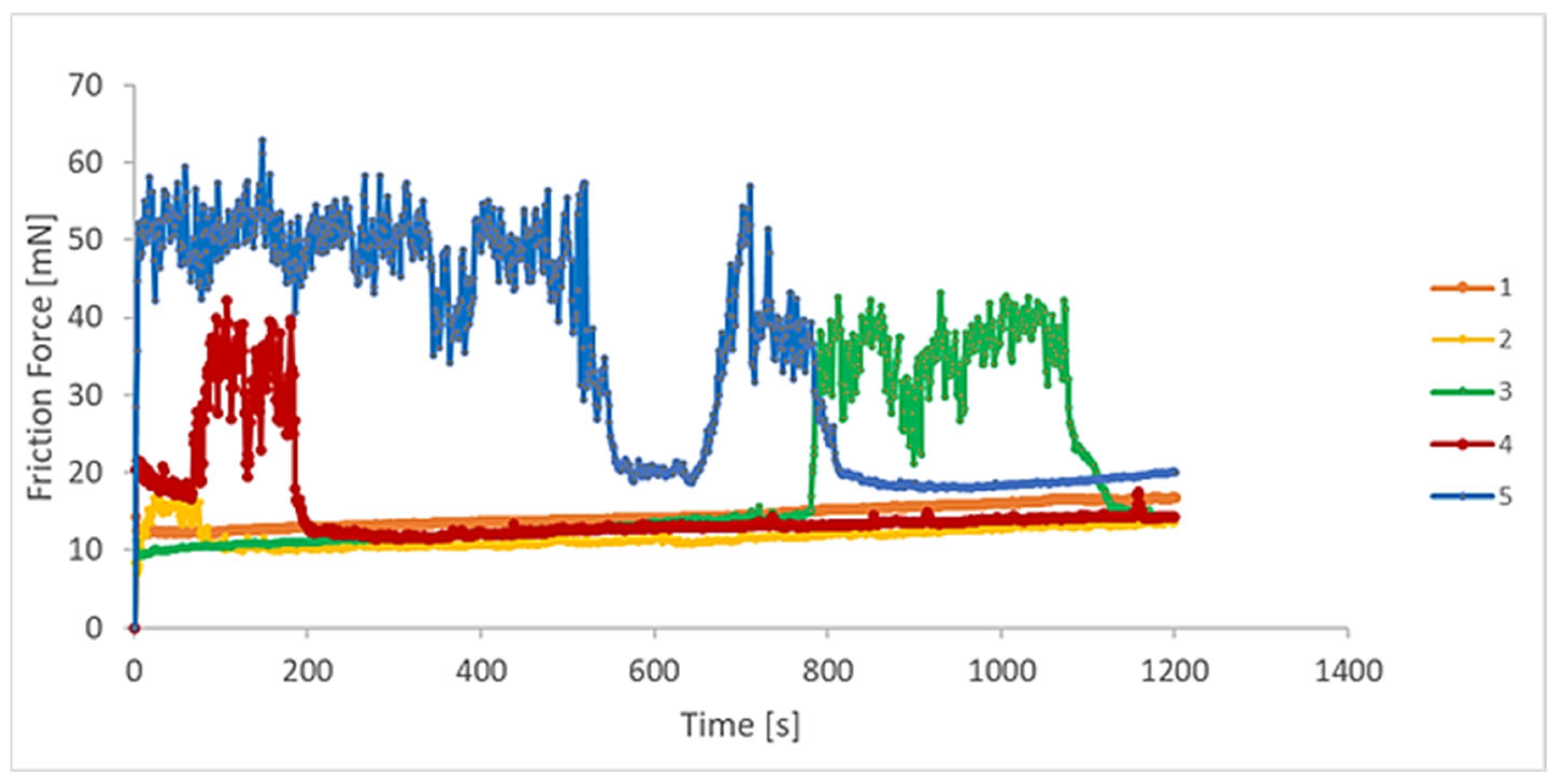
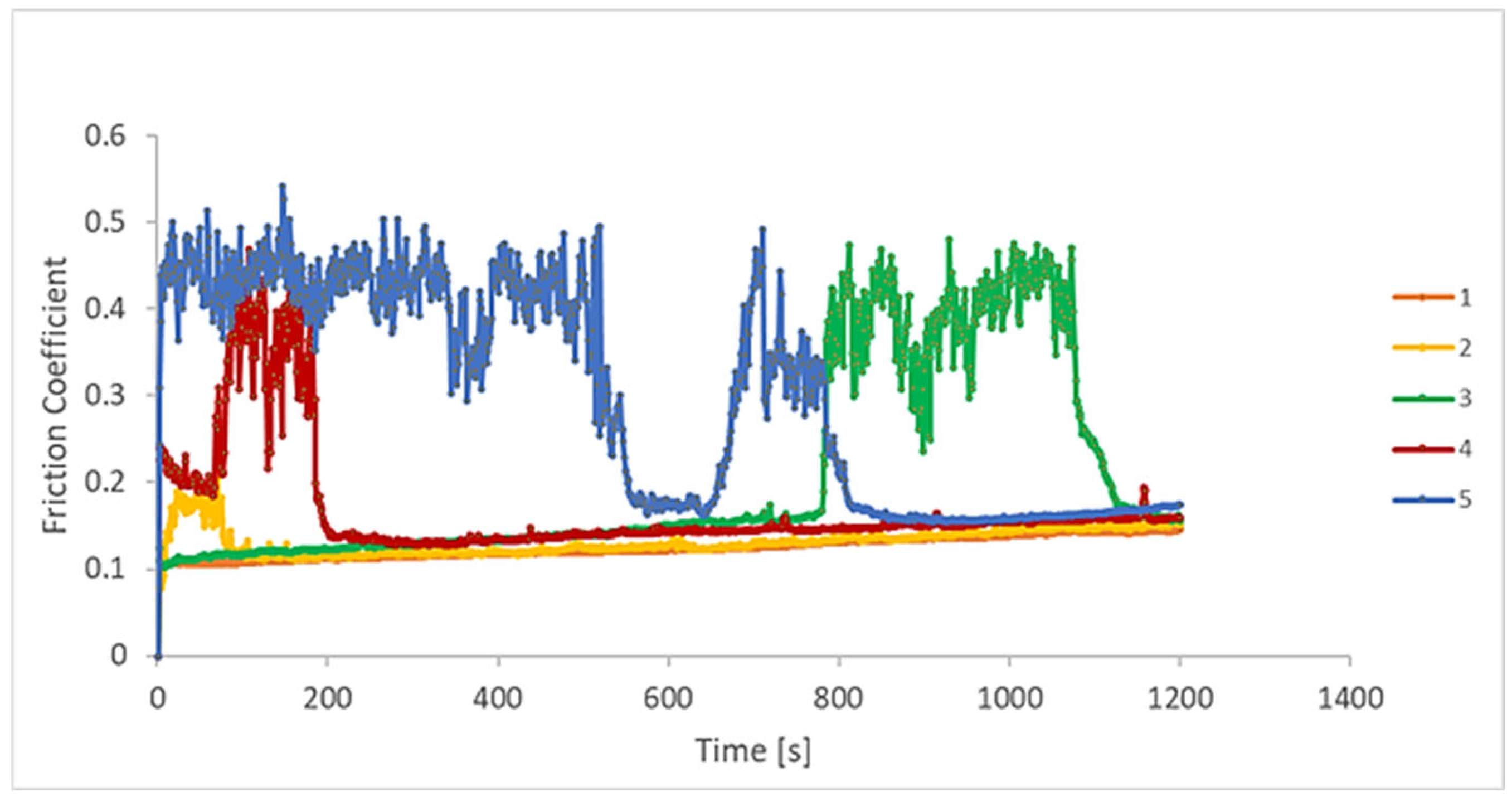
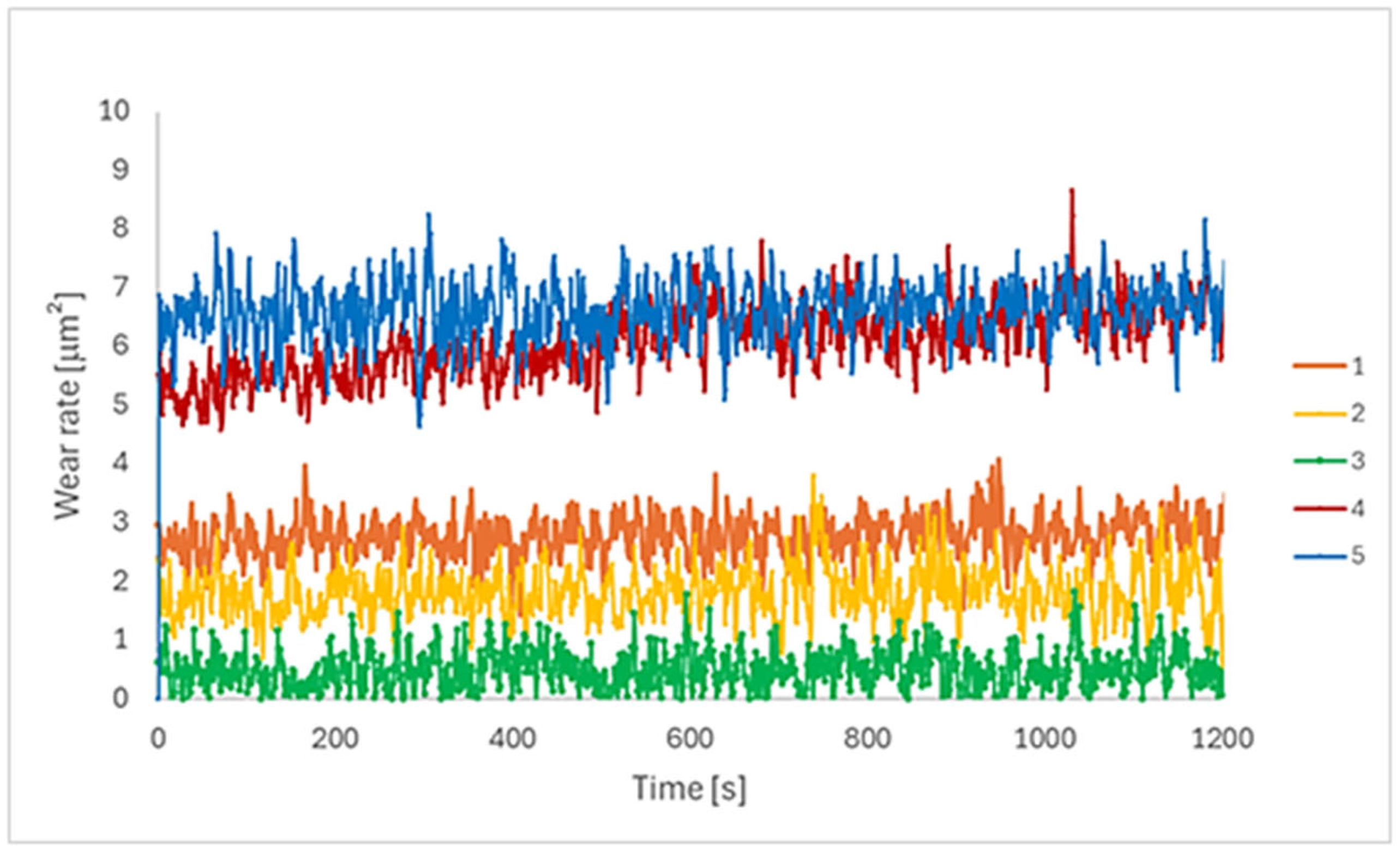
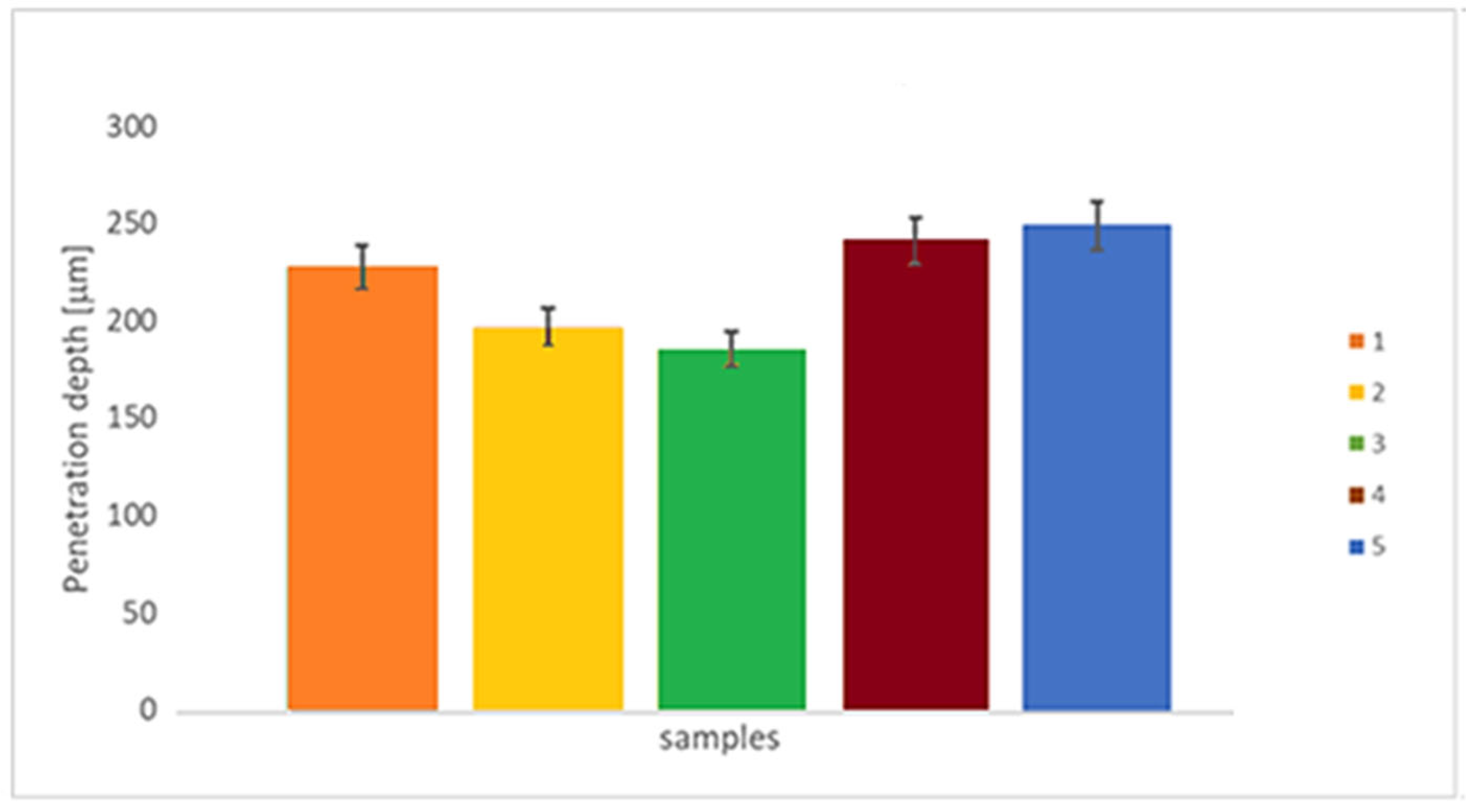
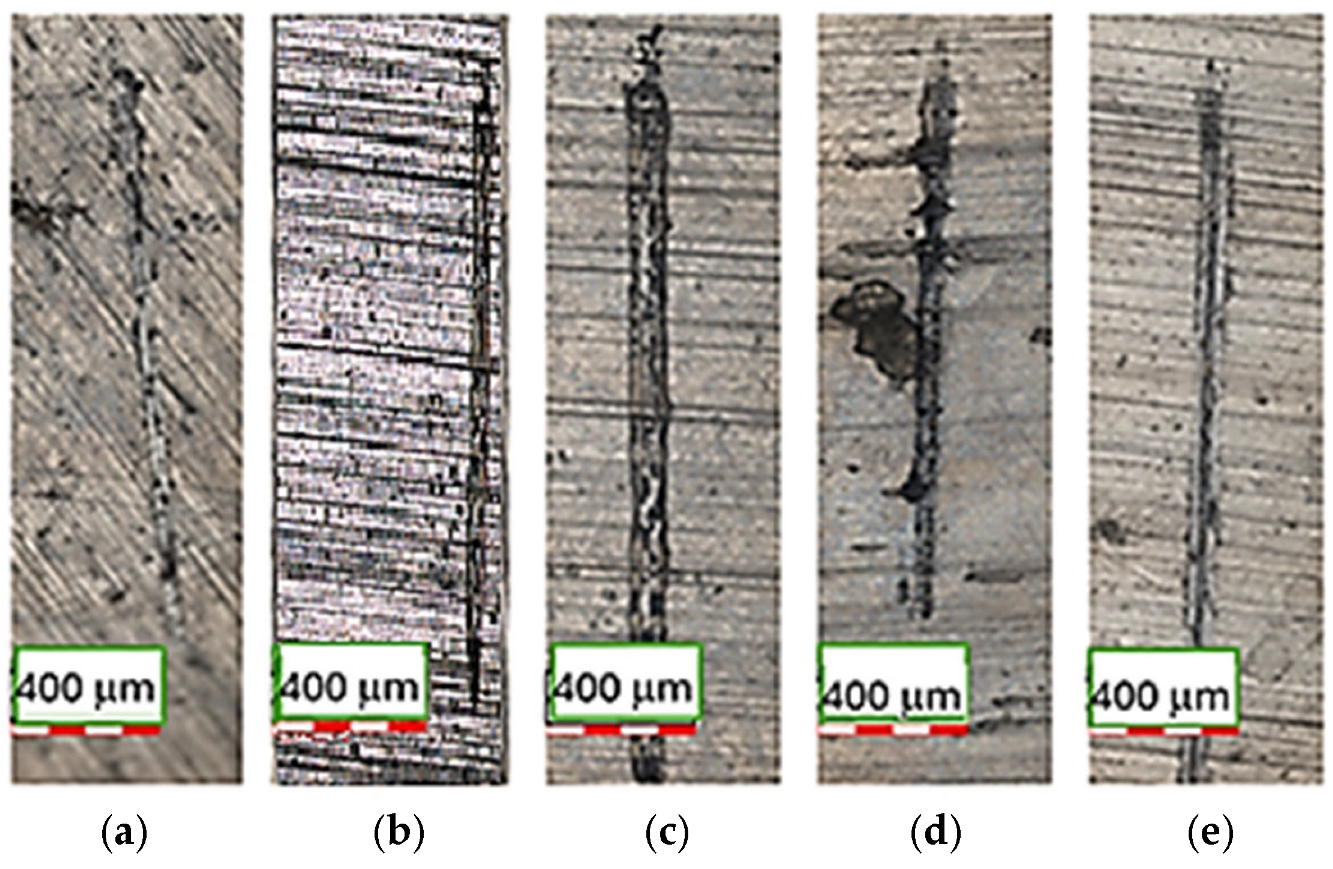
3.3. Tribological Behaviour
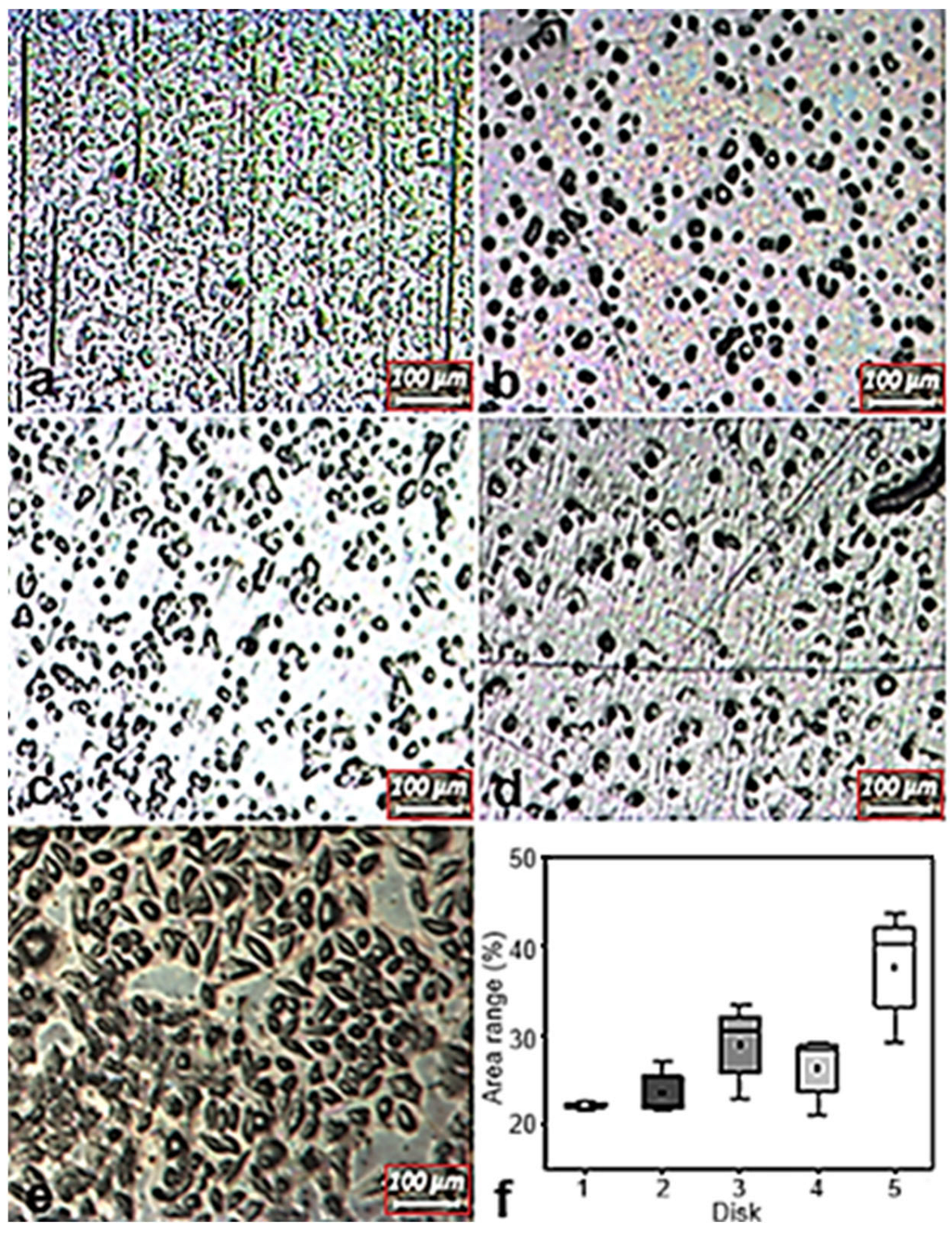
3.4. Cell Adhesion Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pieretti, E.F.; Pessine, E.J.; Correa, O.V.; Rossi, W.; Neves, M.D.M. Effect of Laser Parameters on the Corrosion Resistance of the ASTM F139 Stainless Steel. Int. J. Electrochem. Sci. 2015, 10, 1221–1232. [Google Scholar] [CrossRef]
- Zhang, L.C.; Liu, Y.; Li, S.; Hao, Y. Additive Manufacturing of Titanium Alloys by Electron Beam Melting: A Review. Adv. Eng. Mater. 2017, 20, 1700842. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Costa, I. Surface characterization of ASTM F139 stainless steel marked by laser and mechanical techniques. Electrochim. Acta 2013, 114, 838–843. [Google Scholar] [CrossRef]
- Kurtz, M.A.; Wessinger, A.C.; Mace, A.; Moreno-Reyes, A.; Gilbert, J.L. Additively manufactured Ti-29Nb-21Zr shows improved oxide polarization resistance versus Ti-6Al-4V in inflammatory simulating solution. J. Biomed. Mater. Res. A 2023, 111, 1538–1553. [Google Scholar] [CrossRef]
- Assis, S.L.; Costa, I. Electrochemical evaluation of Ti-13Nb-13Zr, Ti-6Al-4V and Ti-6Al-7Nb alloys for biomedical application by long-term immersion tests. Mater. Corros. 2007, 58, 329–333. [Google Scholar] [CrossRef]
- Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Žnidaršič, M.; Herting, G.; Milošev, I.; Wallinder, I.O. Mechanistic insight on the combined effect of albumin and hydrogen peroxide on surface oxide composition and extent of metal release from Ti6Al4V. J. Biomed. Mater. Res. B Appl. Biomater 2019, 107, 858–867. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Electrochemical and biological characterization of Ti-Nb-Zr-Si alloy for orthopedic applications. Sci. Rep. 2023, 13, 2312. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Fangaia, S.I.G.; Messias, A.; Valente, A.J.M.; Guerra, F.A.D.R.A.; Nicolau, P.M.G. Evaluation of the Tribocorrosion Behavior of Ti-6Al-4V Biomedical Alloy in Simulated Oral Environments. Processes 2024, 12, 1283. [Google Scholar] [CrossRef]
- Lavrys, S.; Pohrelyuk, I.; Veselivska, H.; Skrebtsov, A.; Kononenko, J.; Marchenko, Y. Corrosion behavior of near-alpha titanium alloy fabricated by additive manufacturing. Mater. Corr. 2022, 73, 2063–2070. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Baltazar-Zamora, M.A.; Méndez-Ramírez, C.T.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; Flores-De los Ríos, J.P.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Fernandes Santos, P.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Trenggono, A.; Champagne, S.; Hermawan, H.; Narushima, T. Improvement of microstructure, mechanical and corrosion properties of biomedical Ti-Mn alloys by Mo addition. Mater. Des. 2016, 110, 414. [Google Scholar] [CrossRef]
- Williams, D.F. Corrosion of Implant Materials. Mater. Sci. 1976, 6, 237–266. [Google Scholar] [CrossRef]
- Gibbons, D.F. Biomedical Materials. Biophys. Bioeng. 1975, 4, 367–375. [Google Scholar] [CrossRef]
- Bertolini, R.; Ghiotti, A.; Bruschi, S. Wear Behavior of Ti6Al4V Surfaces Functionalized through Ultrasonic Vibration Turning. J. Mater. Eng. Perform. 2021, 30, 7597–7608. [Google Scholar] [CrossRef]
- Hammood, A.S.; Thair, L.; Altawaly, H.D.; Parvin, N. Tribocorrosion Behaviour of Ti–6Al–4V Alloy in Biomedical Implants: Effects of Applied Load and Surface Roughness on Material Degradation. J. Bio-Tribo-Corr. 2019, 5, 85–98. [Google Scholar] [CrossRef]
- Seo, D.I.; Lee, J.B. Localized corrosion and repassivation behaviors of additively manufactured titanium alloys in simulated biomedical solutions. npj Mater. Degrad. 2023, 7, 44. [Google Scholar] [CrossRef]
- Boraei, N.F.E.; Ibrahim, M.A.M.; Rehim, S.S.A.E.; Elshamy, I.H. Electrochemical corrosion behavior of β-Ti alloy in a physiological saline solution and the impact of H2O2 and albumin. J. Solid State Electrochem. 2024, 28, 2243–2256. [Google Scholar] [CrossRef]
- Ednie, L.; Antonysamy, A.A.; Parimi, L.; Mani, M.; Thomas, M.; Lancaster, R.J. Understanding the fatigue behaviour of Ti–6Al–4V manufactured via various additive processes. J. Mater. Res. Technol. 2024, 31, 1337–1354. [Google Scholar] [CrossRef]
- Mohazzab, B.F.; Jaleh, B.; Fattah-Alhosseini, A.; Mahmoudi, F.; Momeni, A. Laser surface treatment of pure titanium: Microstructural analysis, wear properties, and corrosion behavior of titanium carbide coatings in Hank’s physiological solution. Surf. Inter. 2020, 20, 100597. [Google Scholar] [CrossRef]
- Pou, P.; Riveiro, A.; Val, J.; Comesaña, R.; Penide, J.; Arias-González, F.; Sotoa, R.; Lusquiños, F.; Pou, J. Laser surface texturing of titanium for bioengineering applications. Procedia Manuf. 2017, 13, 694–701. [Google Scholar] [CrossRef]
- Sharma, D.; Kamran, M.; Paraye, N.K.; Anant, R. Insights into the wear behaviour of electron beam melted Ti–6Al–4V alloy in the as-built and the heat-treated conditions. J. Manufact. Process 2021, 71, 669–678. [Google Scholar] [CrossRef]
- Gayathri, Y.K.B.; Kumar, R.L.; Ramalingam, V.V.; Priyadharshini, G.S.; Kumar, K.S.; Prabhu, T.R. Additive Manufacturing of Ti-6Al-4V alloy for Biomedical Applications. J. Bio-Tribo-Corr. 2022, 8, 98. [Google Scholar]
- Toptan, F.; Alves, A.C.; Carvalho, O.; Bartolomeu, F.; Pinto, A.M.P.; Silva, F.; Miranda, G. Corrosion and tribocorrosion behaviour of Ti6Al4V produced by selective laser melting and hot pressing in comparison with the commercial alloy. J. Mater. Process. Tech. 2019, 226, 239–245. [Google Scholar] [CrossRef]
- Han, Y.; Xing, X.; Zhou, L.; Huang, S.; Lin, Z.; Hong, G.; Chen, J. GL13K-modified titanium regulates osteogenic differentiation via the NF-κB pathway. Int. Immunopharmacol. 2024, 126, 111279. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Somlo, K.; Poulios, K.; Funch, C.V.; Niordson, C.F. Anisotropic tensile behaviour of additively manufactured Ti-6Al-4V simulated with crystal plasticity. Mech. Mater. 2021, 162, 104034. [Google Scholar] [CrossRef]
- Ponader, S.; Vairaktaris, E.; Heinl, P.; Wilmowsky, C.V.; Rottmair, A.; Körner, C.; Singer, R.F.; Holst, S.; Schlegel, K.A.; Neukam, F.W.; et al. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J. Biomed. Mater. Res. Part A 2007, 84, 1111–1119. [Google Scholar] [CrossRef]
- Aswar, S.J.; Chakule, R.R.; Baviskar, D.; Khandare, N.H.; Kharche, Y.A.; Deshmukh, D.M.; Dakhole, M.Y.; Vishwanatha, S.; Aden, A.A. Enhancing surface finish and increasing fatigue resistance of Ti6Al4V produced through electron beam melting via chemical machining. J. Mater. Sci. Mater. Eng. 2025, 20, 44. [Google Scholar] [CrossRef]
- Li, J.; Li, S.J.; Hao, Y.L.; Yang, R. Electrochemical characterization of nanostructured Ti–24Nb–4Zr–8Sn alloy in 3.5% NaCl solution. Int. J. Hydrogen Energy 2014, 39, 17452–17459. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.Y.; Zhang, H.B. Enhancing the surface integrity and corrosion resistance of Ti-6Al-4V titanium alloy through cryogenic burnishing. Int. J. Adv. Manuf. Technol. 2017, 88, 2785–2793. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Mueller, Y.; Forster, D.; Virtanen, S. Electrochemical characterisation of Passive Films on Ti Alloys Under Simulated Biological Conditions. Electrochim. Acta 2002, 47, 1923. [Google Scholar] [CrossRef]
- Hou, X.; Ren, Q.; Yang, Y.; Cao, X.; Hu, J.; Zhang, C.; Deng, H.; Yu, D.; Li, K.; Lan, W. Effect of temperature on the electrochemical pitting corrosion behavior of 316L stainless steel in chloride-containing MDEA solution. J. Nat. Gas Sci. Eng. 2021, 86, 103718. [Google Scholar] [CrossRef]
- Jafarzadegan, M.; Ahmadian, F.; Salarvand, V.; Kashkooli, S. Investigation of microstructure and corrosion resistance of AISI 304 stainless steel joint with ER308 and ERNiCr-3 filler metals by GTAW. Metall. Res. Technol. 2020, 117, 507. [Google Scholar] [CrossRef]
- Reséndiz, L.R.; Cano, T.S.; Naeem, M.; Rehman, A.U.; Salamci, E.; Mendoza, V.T.; Duran, E.D.; Díaz, L.B.; Salamci, M.U. Mechanical and Electrochemical Properties Comparison of Additively Manufactured Ti-6Al-4V Alloys by Electron Beam Melting and Selective Laser Melting. J. Mater. Eng. Perform. 2024, 33, 9028–9038. [Google Scholar] [CrossRef]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Liramartínez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguín-Coca, J.; Estupiñán-López, F.; Almeraya-Calderón, F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; Bolfarini, C.; Reis, A.C. Comparative Analysis of Corrosion Resistance between Beta Titanium and Ti-6Al-4V Alloys: A Systematic Review. J. Trace Elem. Med. Biol. 2020, 62, 126618. [Google Scholar]
- Liu, Y.; Tan, G.; Tang, J.; Zhang, L.; Shen, G.; Gu, Z.; Jie, X. Enhanced corrosion and wear resistance of Zn–Ni/Cu–Al2O3 composite coating prepared by cold spray. J. Solid State Electrochem. 2023, 27, 439–453. [Google Scholar] [CrossRef]
- Okasaki, Y. Effect of friction on anodic polarization properties of metallic biomaterials. Biomaterials 2002, 23, 2071–2077. [Google Scholar] [CrossRef]
- Black, J. Systemic effects of biomaterials. Biomaterials 1984, 5, 11–18. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological response to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry sliding wear behavior of laser clad TiVCrAlSi high entropy alloy coatings on Ti-6Al-4V substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Doni, Z.; Alves, A.C.; Toptan, F.; Gomes, J.R.; Ramalho, A.; Buciumeanu, M.; Palaghian, L.; Silva, F.S. Dry sliding and tribocorrosion behaviour of hot-pressed CoCrMo biomedical alloy as compared with the cast CoCrMo and Ti6Al4V alloys. J. Mater. Des. 2013, 52, 47–57. [Google Scholar] [CrossRef]
- Pieretti, E.F.; Silva, L.C.E.; Ribeiro, M.S.; de Rossi, W.; Antunes, R.A.; Oliveira, M.C.L.; Piaggio, D.; Cruz, F.P.A.; da Paz, J.O.; das Neves, M.D.M. Triboelectrochemical Performance of Electron Beam–Melted Ti-6Al-4V-ELI for Biomedical Applications. Adv. Mater. Sci. Eng. 2025, 2025, 8059229. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Silva, F.S.; Carvalho, O.; Miranda, G. Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing, and conventional casting. Trans. Nonferr. Met. Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Podlipec, R.; Punzón-Quijorna, E.; Pirker, L.; Kelemen, M.; Vavpetič, P.; Kavalar, R.; Hlawacek, G.; Štrancar, J.; Pelicon, P.; Fokter, S.K. Revealing Inflammatory Indications Induced by Titanium Alloy Wear Debris in Periprosthetic Tissue by Label-Free Correlative High-Resolution Ion, Electron and Optical Microspectroscopy. Materials 2021, 14, 3048. [Google Scholar] [CrossRef]


| Chemical Composition [wt%] | ||
|---|---|---|
| Ti | Al | V |
| Bal. | 6.34 ± 0.05 | 3.98 ± 0.02 |
| Particle size distribution (ASTM B822) | ||
| D10 [mm] | D50 [mm] | D90 [mm] |
| 51.0 ± 0.03 | 69.0 ± 0.04 | 96.2 ± 0.03 |
| Flow rate [50 g·s−1] | Apparent density [g·cm−3] | Tap density [g·cm−3] |
| 25.0 ± 0.03 | 2.6 ± 0.01 | 2.8 ± 0.03 |
| Surface | Ra [μm] | Rq [μm] |
|---|---|---|
| 1 | 0.303 ± 0.172 | 0.425 ± 0.412 |
| 2 | 0.320 ± 0.175 | 0.440 ± 0.413 |
| 3 | 0.326 ± 0.115 | 0.518 ± 0.308 |
| 4 | 0.618 ± 0.181 | 0.711 ± 0.199 |
| 5 | 0.501 ± 0.173 | 0.587 ± 0.323 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieretti, E.F.; Piaggio, D.; Antunes, R.A.; de Oliveira, M.C.L.; da Silva, L.C.E.; Silva, C.R.; Yoshimura, T.M.; de Rossi, W.; Ribeiro, M.S.; das Neves, M.D.M. Unveiling the Effect of Scanning Speed on the Corrosion and Tribological Performance of Electron Beam Melted (EBM) Ti-6Al-4V-ELI Alloy. Materials 2025, 18, 5367. https://doi.org/10.3390/ma18235367
Pieretti EF, Piaggio D, Antunes RA, de Oliveira MCL, da Silva LCE, Silva CR, Yoshimura TM, de Rossi W, Ribeiro MS, das Neves MDM. Unveiling the Effect of Scanning Speed on the Corrosion and Tribological Performance of Electron Beam Melted (EBM) Ti-6Al-4V-ELI Alloy. Materials. 2025; 18(23):5367. https://doi.org/10.3390/ma18235367
Chicago/Turabian StylePieretti, Eurico Felix, Davide Piaggio, Renato Altobelli Antunes, Mara Cristina Lopes de Oliveira, Luís Carlos Elias da Silva, Camila Ramos Silva, Tania Mateus Yoshimura, Wagner de Rossi, Martha Simões Ribeiro, and Maurício David Martins das Neves. 2025. "Unveiling the Effect of Scanning Speed on the Corrosion and Tribological Performance of Electron Beam Melted (EBM) Ti-6Al-4V-ELI Alloy" Materials 18, no. 23: 5367. https://doi.org/10.3390/ma18235367
APA StylePieretti, E. F., Piaggio, D., Antunes, R. A., de Oliveira, M. C. L., da Silva, L. C. E., Silva, C. R., Yoshimura, T. M., de Rossi, W., Ribeiro, M. S., & das Neves, M. D. M. (2025). Unveiling the Effect of Scanning Speed on the Corrosion and Tribological Performance of Electron Beam Melted (EBM) Ti-6Al-4V-ELI Alloy. Materials, 18(23), 5367. https://doi.org/10.3390/ma18235367








