Sequential Spinodal Decompositions and Ordering Reactions in an As-Quenched Cr39Co18Fe18Ni18Al7 High-Entropy Alloy
Highlights
- Sequential spinodal decompositions and ordering reactions are the primary mechanisms of phase transformation
- The BCC phase decomposes into Ni-Al-lean BCC and Ni-Al-enriched BCC phases via spinodal decomposition during cooling
- Ni-Al-enriched BCC phases transform into B2 phases through ordering.
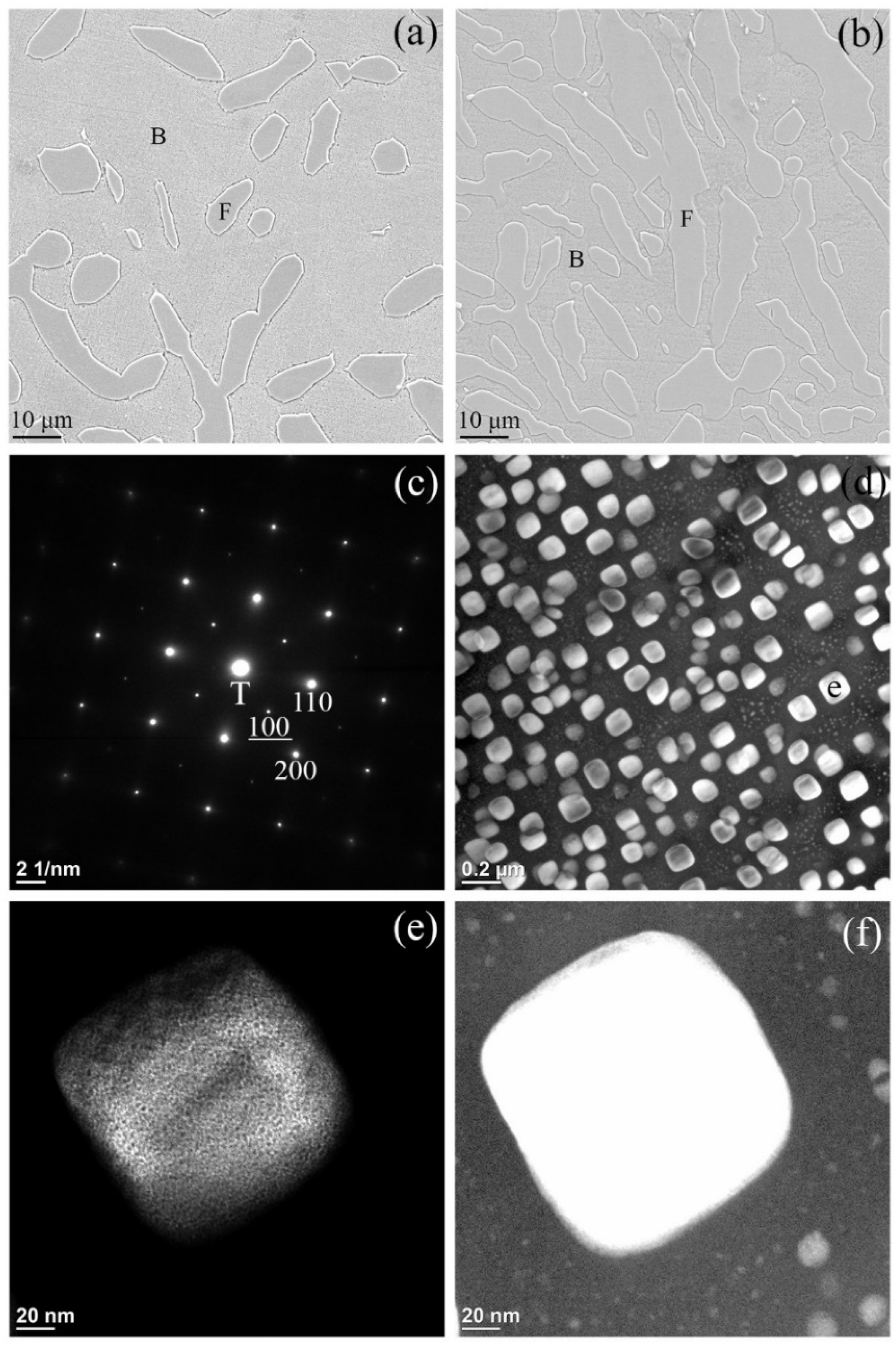
- Two different BCC/B2 microstructures with reciprocal phase distributions were observed.
- The observed BCC phase transformation pathway aids control of phase evolution in Al–Co–Cr–Fe–Ni HEAs.
- Reciprocal BCC/B2 distribution shows Ni–Al tuning can adjust alloy phase stability and properties.
- The results provide guidance for designing spinodal-strengthened Cr–Co–Fe–Ni–Al HEAs.
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
- i.
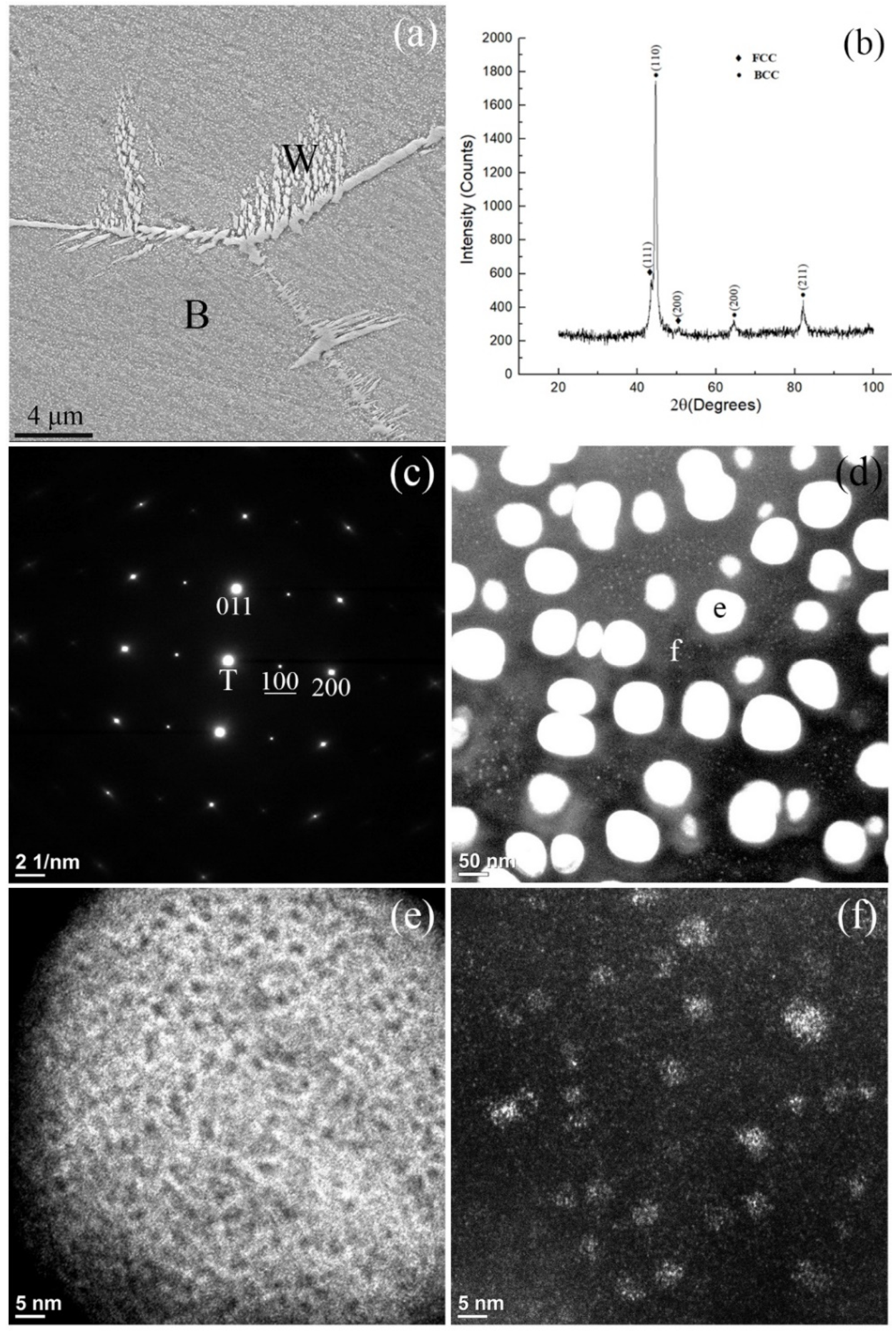
- The alloy quenched from various high temperatures (1100–1300 °C) consists predominantly of BCC and B2 phases with a minor fraction of FCC, and the fraction of BCC increases with higher annealing temperature.
- ii.
- The hardness increases with annealing temperature, aligning well with the increased volume fraction of the BCC phase at higher temperature.
- iii.
- Within the high-temperature BCC grains, BCC and B2 phases form as the final phases in the matrix and in the fine particles, respectively, after quenching.
- iv.
- Nanoparticles consisting of B2 and BCC phases precipitated homogeneously via spinodal decomposition and subsequent ordering reactions in both the matrix and fine particles.
- v.
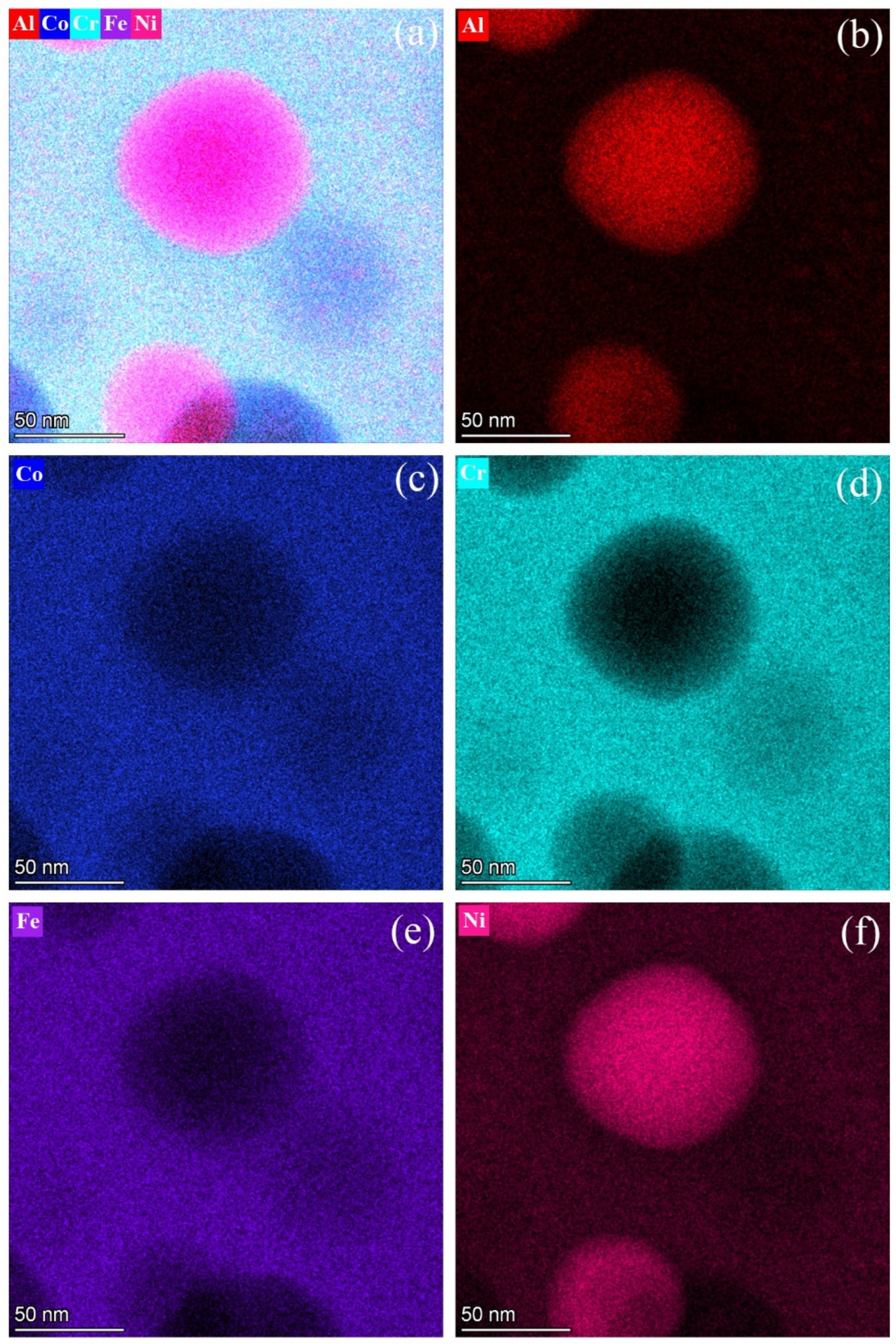
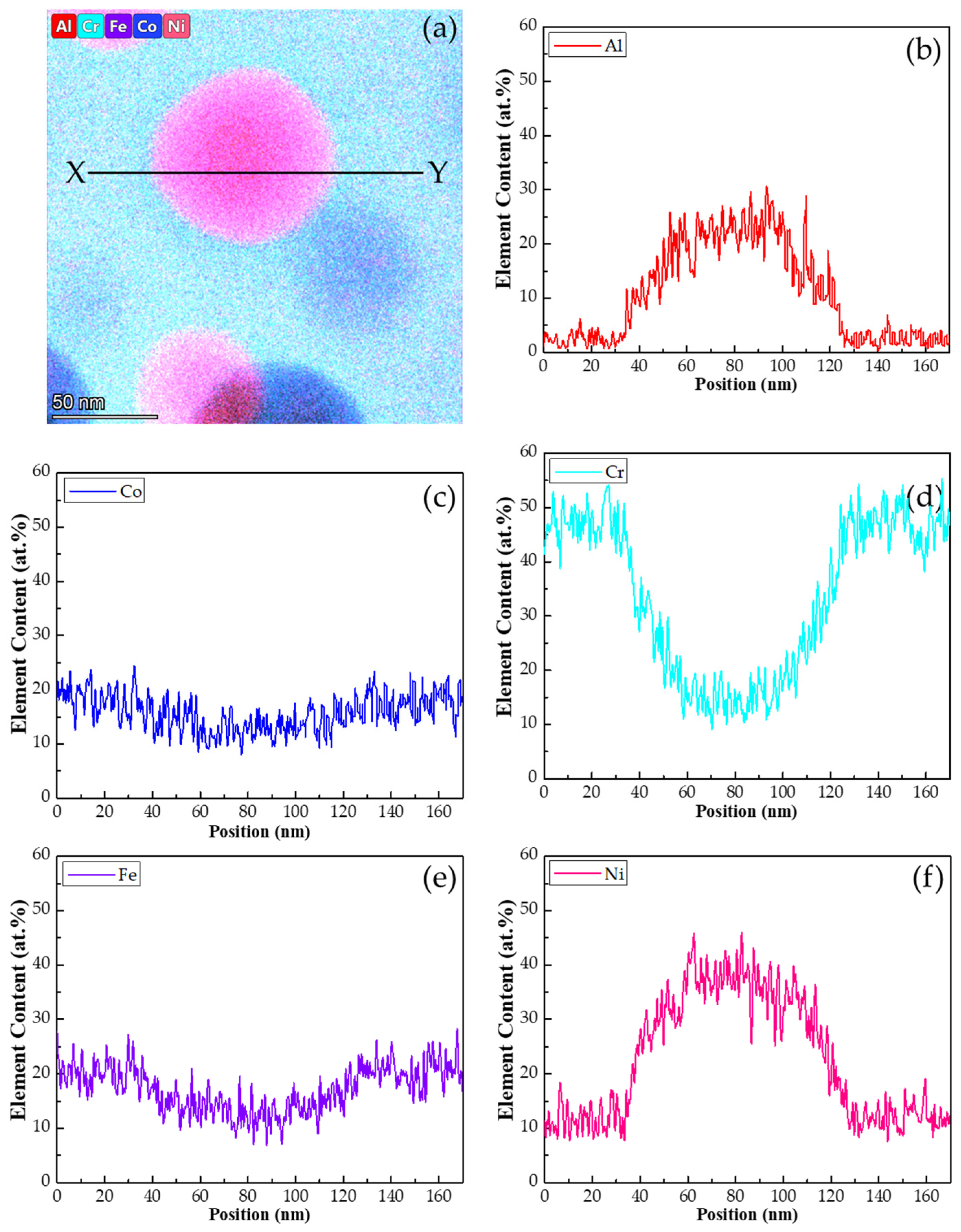
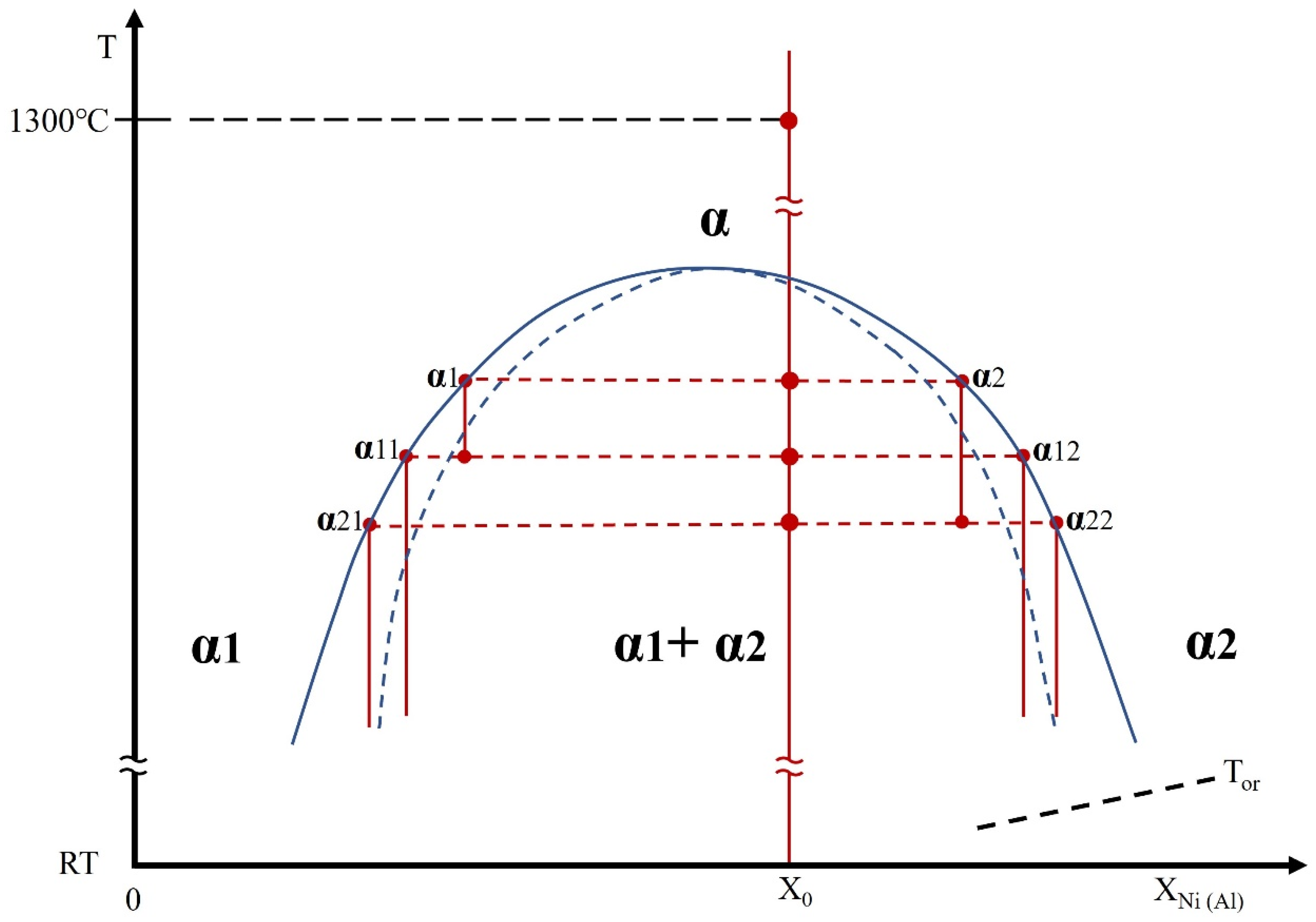
- A miscibility gap is evidenced by the co-existence of the two BCC phases produced from the spinodal decompositions. The matrix BCC phase, is depleted in Ni and Al and enriched in Co, Cr and Fe, whereas the fine particles in the matrix are enriched in Ni and Al and lean in Co, Cr and Fe.
- vi.
- Upon quenching, the two BCC phases underwent independent spinodal decompositions to produce new product BCC phases that may have further decomposed sequentially into additional BCC phases.
- vii.
- The BCC phases with high Ni and Al concentrations transformed into B2 phases as the temperature decreased below Tor.
- viii.
- Both the BCC matrix and the fine B2 particles contained nanoparticles consisting of the other phases, formed through sequential spinodal decompositions and ordering reactions that occurred during quenching.
- ix.
- The overall transformation sequence is governed by Ni–Al co-clustering and their strong tendency to order, which promote chemical partitioning and stabilize the resulting phases during cooling. Therefore, the co-clustering and ordering effects of Ni and Al played the dominant roles in controlling the phase transformations of the as-quenched HEA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arshad, M.; Bano, S.; Amer, M.; Janik, V.; Hayat, Q.; Bai, M. High-temperature oxidation and phase stability of AlCrCoFeNi high entropy alloy: Insights from in situ HT-XRD and thermodynamic calculations. Materials 2024, 17, 3579. [Google Scholar] [PubMed]
- Wang, Y.; Li, B.; Ren, M.; Yang, C.; Fu, H. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar]
- Tong, C.J.; Chen, Y.L.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar]
- Wang, W.R.; Wang, W.L.; Yeh, J.W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast,-homogenized, and-deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar]
- Meshi, L.; Linden, Y.; Munitz, A.; Salhov, S.; Pinkas, M. Retardation of the σ phase formation in the AlCoCrFeNi multi-component alloy. Mater. Charact. 2019, 148, 171–177. [Google Scholar]
- Shun, T.T.; Du, Y.-C. Microstructure and tensile behaviors of FCC Al0.3CoCrFeNi high entropy alloy. J. Alloys Compd. 2009, 479, 157–160. [Google Scholar]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase separation in equiatomic AlCoCrFeNi high-entropy alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar]
- Bloomfield, M.; Christofidou, K.; Mignanelli, P.; Reponen, A.M.; Stone, H.; Jones, N. Phase stability of the AlxCrFeCoNi alloy system. J. Alloys Compd. 2022, 926, 166734. [Google Scholar]
- Wang, Q.; Ma, Y.; Jiang, B.; Li, X.; Shi, Y.; Dong, C.; Liaw, P.K. A cuboidal B2 nanoprecipitation-enhanced body-centered-cubic alloy Al0.7CoCrFe2Ni with prominent tensile properties. Scr. Mater. 2016, 120, 85–89. [Google Scholar] [CrossRef]
- Joseph, J.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Understanding the mechanical behaviour and the large strength/ductility differences between FCC and BCC AlxCoCrFeNi high entropy alloys. J. Alloys Compd. 2017, 726, 885–895. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-entropy alloys: Potential candidates for high-temperature applications—An overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Liu, X.; Dong, T.; Zhao, X.; Yang, F.; Guo, F. AlxCoCrFeNi high entropy alloys with superior hot corrosion resistance to Na2SO4+ 25% NaCl at 900 °C. Corros. Sci. 2021, 187, 109479. [Google Scholar] [CrossRef]
- Xia, S.; Yang, X.; Yang, T.; Liu, S.; Zhang, Y. Irradiation resistance in AlxCoCrFeNi high entropy alloys. JOM 2015, 67, 2340–2344. [Google Scholar] [CrossRef]
- Xia, S.; Gao, M.; Zhang, Y. Abnormal temperature dependence of impact toughness in AlxCoCrFeNi system high entropy alloys. Mater. Chem. Phys. 2018, 210, 213–221. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Rao, J.; Diao, H.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.; Zhou, Y. Secondary phases in AlxCoCrFeNi high-entropy alloys: An in-situ TEM heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Zhao, M.; Jiang, Q. Effect of aluminum contents on microstructure and properties of AlxCoCrFeNi alloys. J. Alloys Compd. 2010, 504, S515–S518. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Effects of AL addition on microstructure and mechanical properties of AlxCoCrFeNi High-entropy alloy. Mater. Sci. Eng. A 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gwalani, B.; Soni, V.; Ramanujan, R.; Banerjee, R. Influence of Cr substitution and temperature on hierarchical phase decomposition in the AlCoFeNi high entropy alloy. Sci. Rep. 2018, 8, 15578. [Google Scholar]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Zhao, Q.; Chen, Y.; Yang, K.; Wang, Q. An overview on fatigue of high-entropy alloys. Materials 2023, 16, 7552. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Sistla, Y.S. Thermal stability assessment of mixed phase AlCoCrFeNi high entropy alloy: In silico studies. Phys. Condens. Matter 2025, 712, 417319. [Google Scholar] [CrossRef]
- Kenedy, G.R.; Chemeli, K.R.; Cheng, W.C. The Observation of Cellular Precipitation in an Ni36Co18Cr20Fe19Al7 High-Entropy Alloy after Quenching and Annealing. Materials 2022, 15, 6613. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Strumza, E.; Hayun, S. Comprehensive study of phase transitions in equiatomic AlCoCrFeNi high-entropy alloy. J. Alloys Compd. 2021, 856, 158220. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, R.; Tan, X.; Quan, X.; Song, B.; Liu, Q. The precipitation behavior in Al0.3CoCrFeNi high-entropy alloy affected by deformation and annealing. Metals 2023, 13, 157. [Google Scholar] [CrossRef]
- Park, H.; Haftlang, F.; Heo, Y.U.; Seol, J.B.; Wang, Z.; Kim, H.S. Periodic spinodal decomposition in double–strengthened medium–entropy alloy. Nat. Commun. 2024, 15, 5757. [Google Scholar]
- Findik, F. Improvements in spinodal alloys from past to present. Mater. Des. 2012, 42, 131–146. [Google Scholar] [CrossRef]
- Bai, K.; Ng, C.K.; Lin, M.; Cheng, B.; Zeng, Y.; Wuu, D.; Lee, J.J.; Teo, S.L.; Ng, S.R.; Tan, D.C.C. Unexpected spinodal decomposition in as-cast eutectic high entropy alloy Al30Co10Cr30Fe15Ni15. Mater. Des. 2023, 236, 112508. [Google Scholar] [CrossRef]
- Wani, I.; Bhattacharjee, T.; Sheikh, S.; Lu, Y.; Chatterjee, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Ultrafine-grained AlCoCrFeNi2.1 eutectic high-entropy alloy. Mater. Res. Lett. 2016, 4, 174–179. [Google Scholar] [CrossRef]
- Tsai, M.H.; Tsai, K.Y.; Tsai, C.W.; Lee, C.; Juan, C.C.; Yeh, J.W. Criterion for sigma phase formation in Cr-and V-containing high-entropy alloys. Mater. Res. Lett. 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, B.; Li, C.; Wang, Q.; Dong, C.; Liaw, P.K.; Xu, F.; Sun, L. The BCC/B2 morphologies in AlxNiCoFeCr high-entropy alloys. Metals 2017, 7, 57. [Google Scholar] [CrossRef]
- Borkar, T.; Chaudhary, V.; Gwalani, B.; Choudhuri, D.; Mikler, C.V.; Soni, V.; Alam, T.; Ramanujan, R.V.; Banerjee, R. A combinatorial approach for assessing the magnetic properties of high entropy alloys: Role of Cr in AlCoxCr1–xFeNi. Adv. Eng. Mater. 2017, 19, 1700048. [Google Scholar] [CrossRef]
- Lim, K.R.; Lee, K.S.; Lee, J.S.; Kim, J.Y.; Chang, H.J.; Na, Y.S. Dual-phase high-entropy alloys for high-temperature structural applications. J. Alloys Compd. 2017, 728, 1235–1238. [Google Scholar] [CrossRef]
- Filatov, S. General concept of increasing crystal symmetry with an increase in temperature. Cryst. Rep. 2011, 56, 953–961. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Gupta, A.; Choudhari, A.; Rane, A.; Tiwari, A.; Sharma, P.; Gupta, A.; Sapale, P.; Tirumala, R.; Muthaiah, R.; Kumar, A. Advances in nickel-containing high-entropy alloys: From fundamentals to additive manufacturing. Materials 2024, 17, 3826. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korir, R.C.; Kenedy, G.R.; Cheng, W.-C.; Chen, S.-H. Sequential Spinodal Decompositions and Ordering Reactions in an As-Quenched Cr39Co18Fe18Ni18Al7 High-Entropy Alloy. Materials 2025, 18, 5364. https://doi.org/10.3390/ma18235364
Korir RC, Kenedy GR, Cheng W-C, Chen S-H. Sequential Spinodal Decompositions and Ordering Reactions in an As-Quenched Cr39Co18Fe18Ni18Al7 High-Entropy Alloy. Materials. 2025; 18(23):5364. https://doi.org/10.3390/ma18235364
Chicago/Turabian StyleKorir, Rosemary Chemeli, Gurumayum Robert Kenedy, Wei-Chun Cheng, and Shih-Hsun Chen. 2025. "Sequential Spinodal Decompositions and Ordering Reactions in an As-Quenched Cr39Co18Fe18Ni18Al7 High-Entropy Alloy" Materials 18, no. 23: 5364. https://doi.org/10.3390/ma18235364
APA StyleKorir, R. C., Kenedy, G. R., Cheng, W.-C., & Chen, S.-H. (2025). Sequential Spinodal Decompositions and Ordering Reactions in an As-Quenched Cr39Co18Fe18Ni18Al7 High-Entropy Alloy. Materials, 18(23), 5364. https://doi.org/10.3390/ma18235364






