Effect of Microstructure on Oxidation Resistance and TGO Formation in FeCoNiCrAl HEA Coatings Deposited by Low-Temperature HVAF Spraying
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
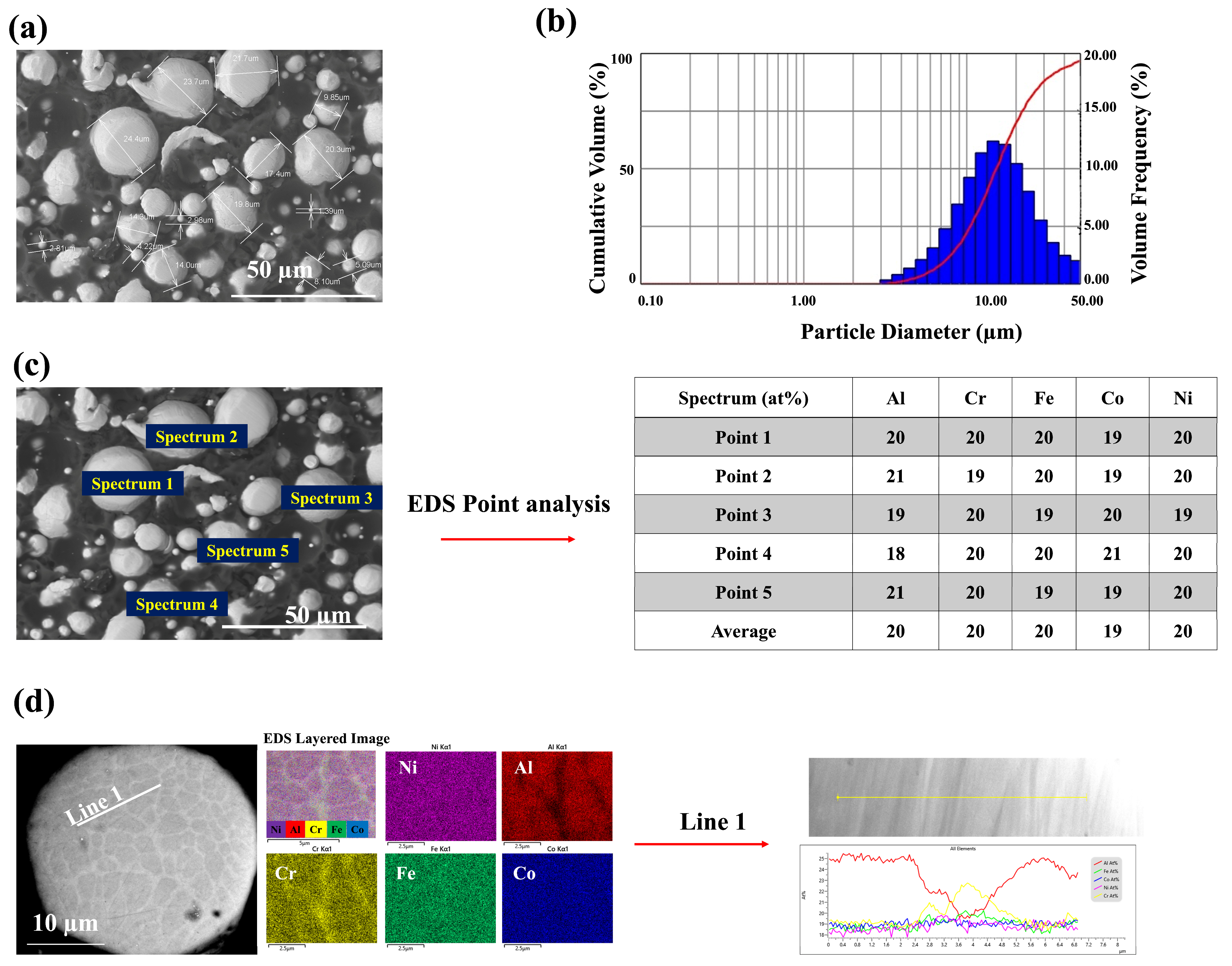
3.1. The Microstructure of the Coatings
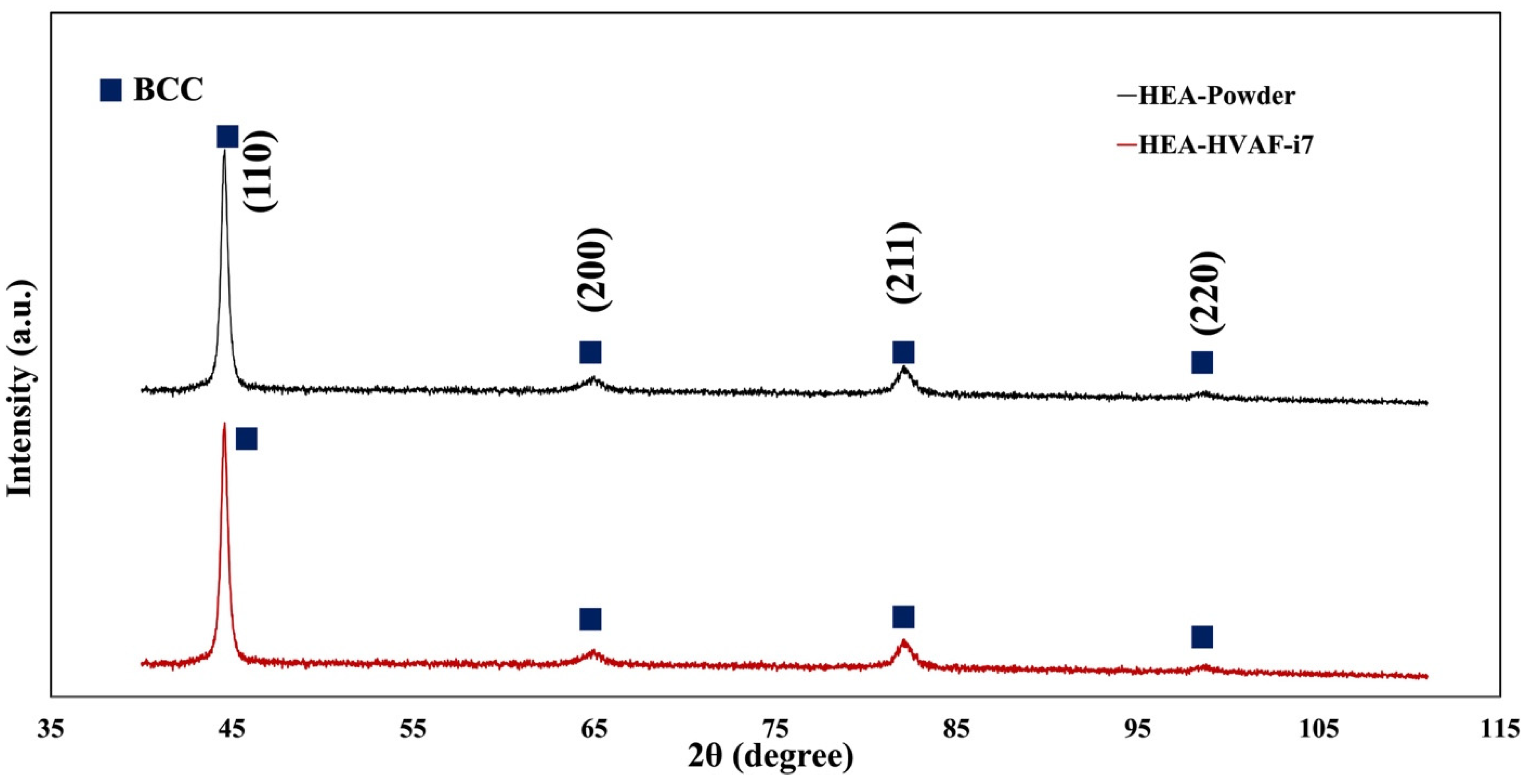
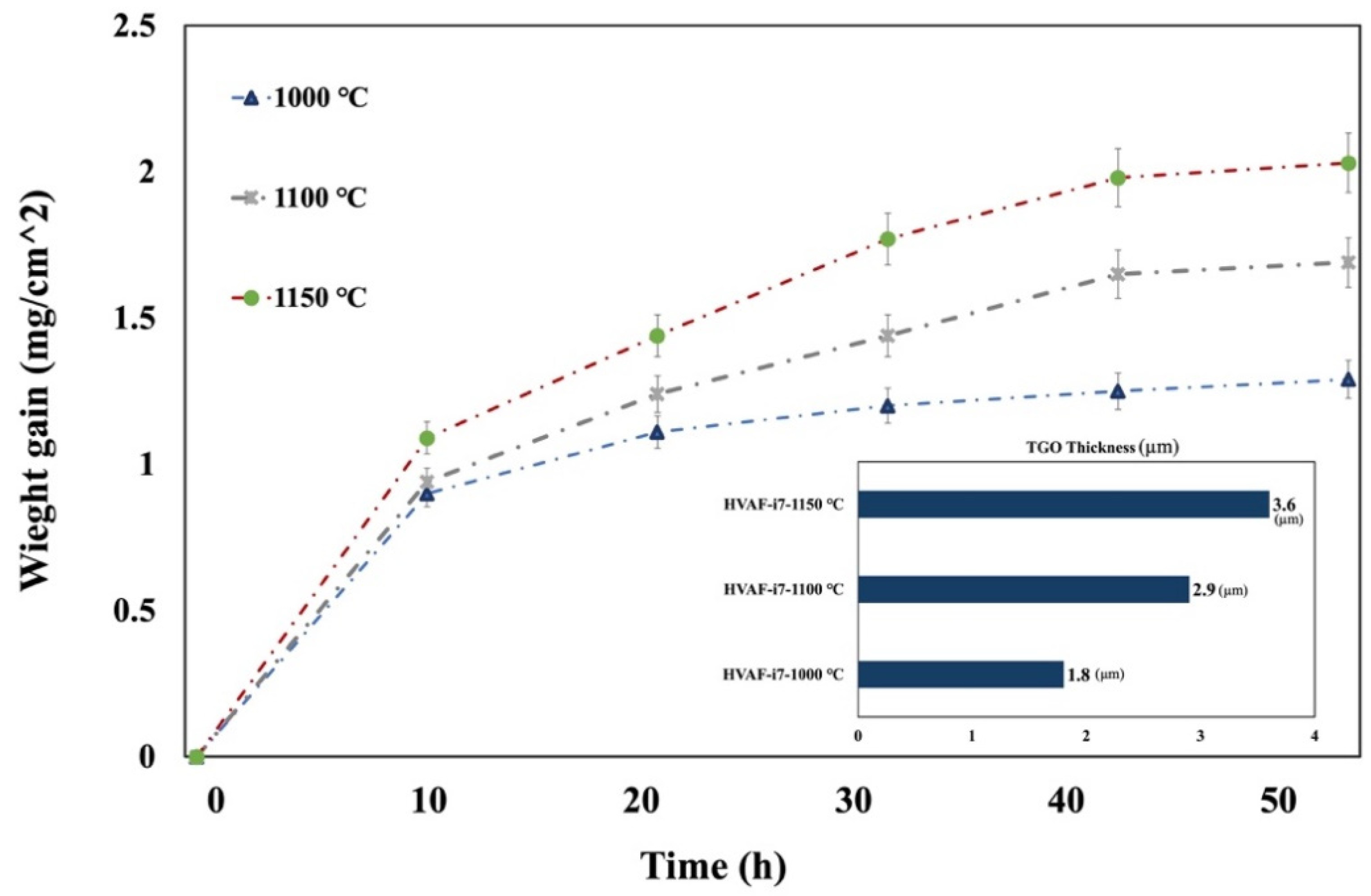
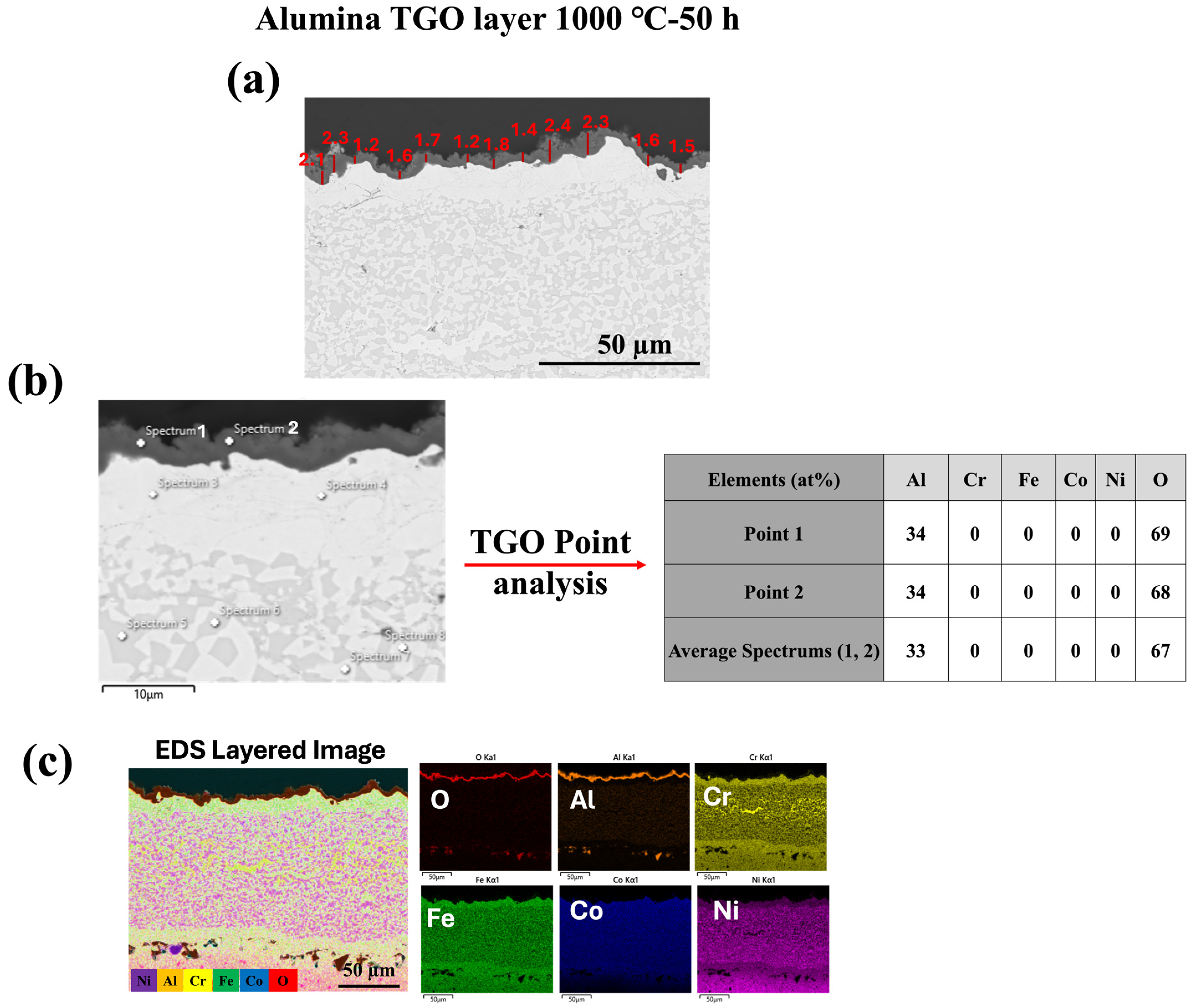
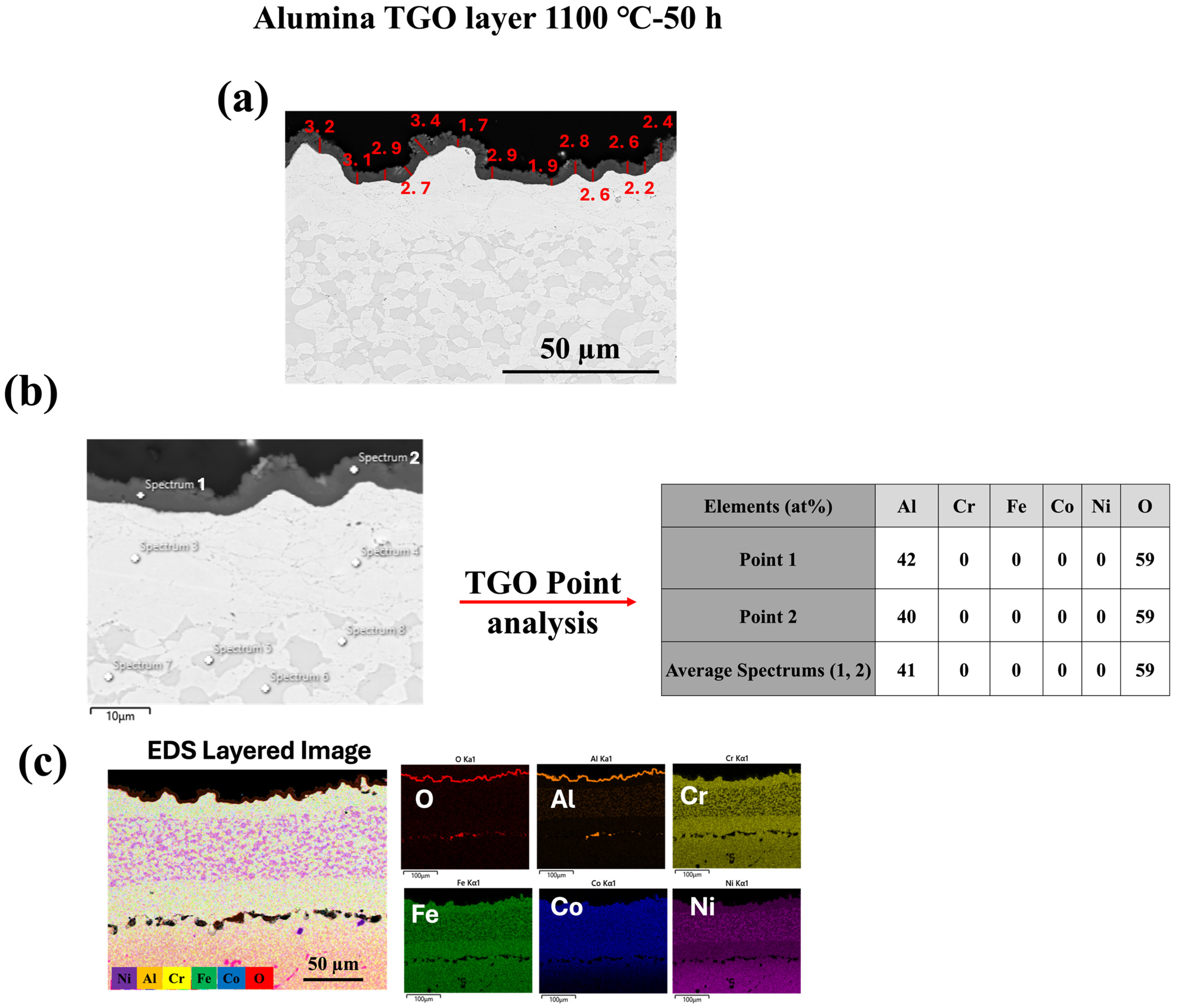
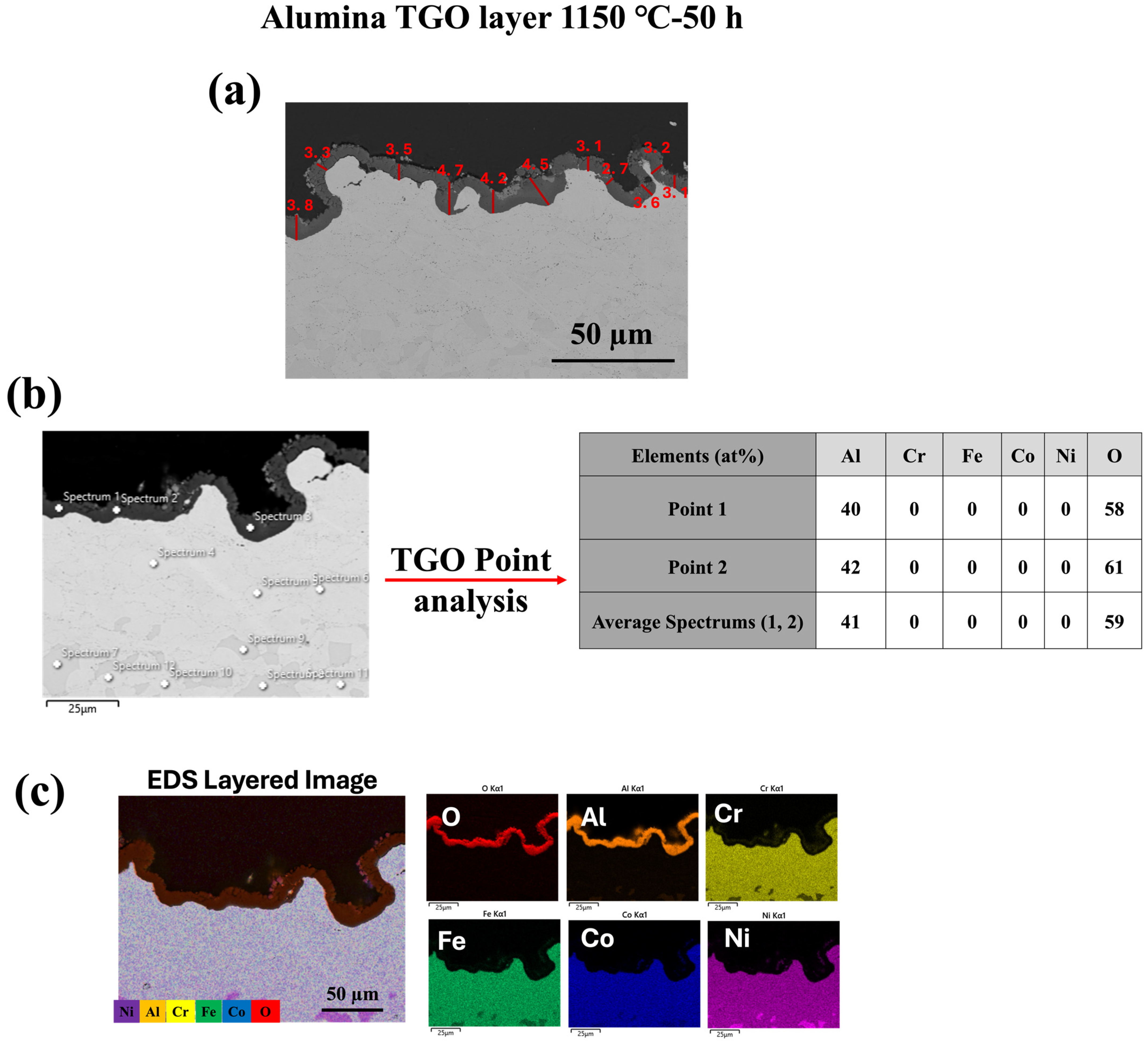
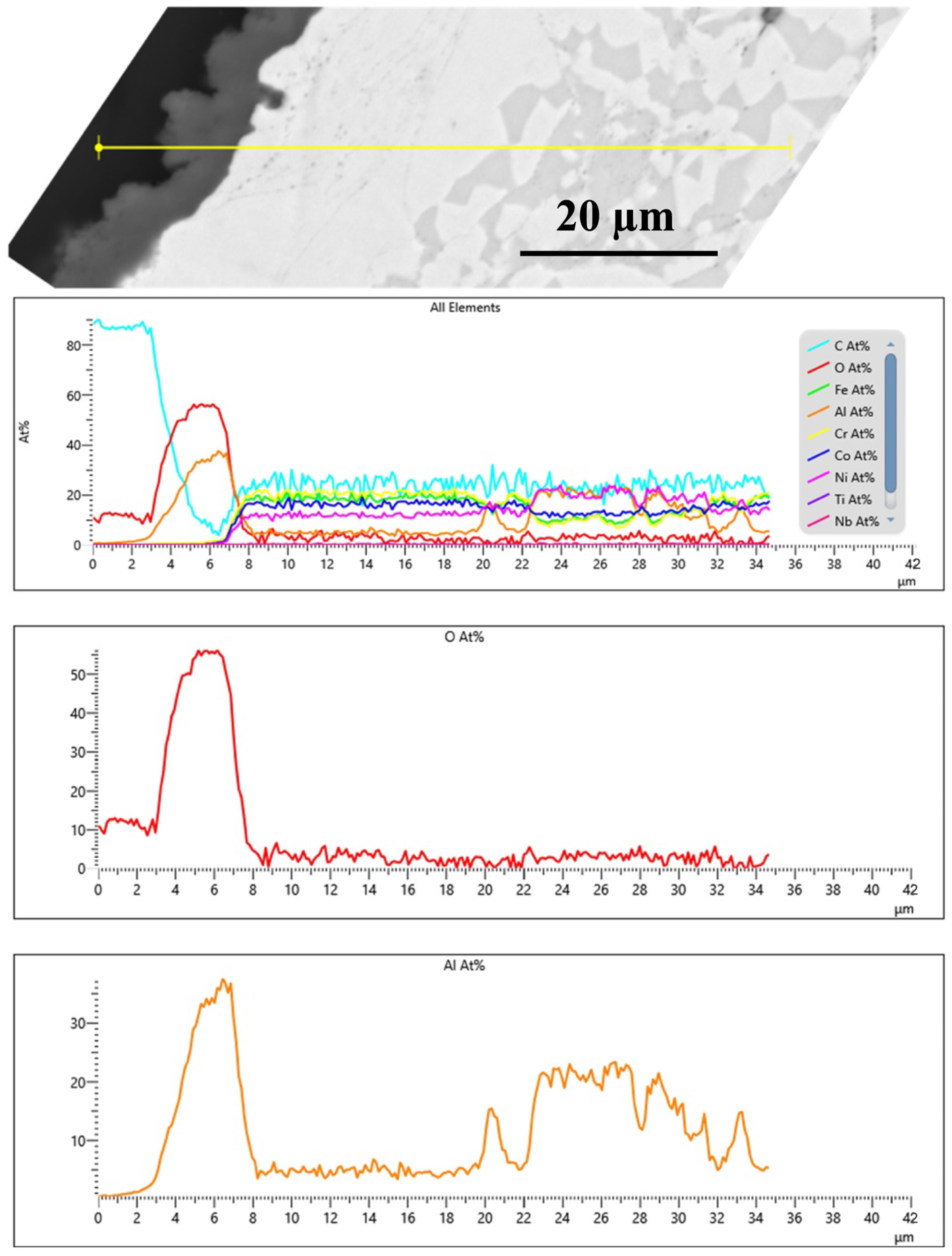
3.2. TGO Microstructure and Growth Kinetics
4. Conclusions
- (1)
- Utilizing the HVAF-i7 gun, we achieved a highly dense microstructure comparable to that of cold spray techniques, highlighting the efficacy of the fabrication method.
- (2)
- The microstructure and chemistry of the coating, along with the growth of the thermally grown oxide (TGO) layer, were investigated across different temperatures, revealing a dense, uniform, and thin alumina TGO layer after 50 h at 1000 °C, 1100 °C, and 1150 °C. It was observed that with increasing temperature, the TGO layer increased, albeit not significantly.
- (3)
- The oxidation resistance of the HEA coating was significantly enhanced by the dense microstructure achieved via HVAF-i7, which contributed to the controlled growth of the TGO layer and the formation of a continuous alumina scale. While no direct benchmark bond coat was included for comparison, the observed microstructural characteristics and TGO behavior align with the known performance benefits associated with dense coatings produced through HVAF processes.
- (4)
- The absence of reactive elements contributed to the spalling of the alumina TGO layer at higher temperatures, which will be further explored in future research.
- (5)
- Analysis of deposit efficiency (79%) and porosity (0.2%) indicated that the best deposition efficiency and lowest porosity were achieved with medium air and fuel pressure, resulting in a dense, uniform, and random solid solution phase.
- (6)
- Investigation of the microstructure revealed a relatively thin inter-diffusion layer compared with the coating thickness, suggesting a negligible impact on oxidation behavior.
- (7)
- EDS mapping and area analysis confirmed the presence of a random solid solution phase and uniform element distribution after coating, highlighting the efficacy of the HVAF technique and the high entropy properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbazi, H.; Vakilifard, H.; Nair, R.B.; Liberati, A.C.; Lima, R.S.; Stoyanov, P.; Moreau, C. High Entropy Alloy Bond Coats for Thermal Barrier Coatings: A Review. J. Therm. Spray Technol. 2023, 33, 430–446. [Google Scholar] [CrossRef]
- Vakilifard, H.; Shahbazi, H.; Liberati, A.C.; Saraswathy, R.B.N.; Lima, R.S.; Pugh, M.D.; Moreau, C. High Entropy Oxides as Promising Materials for Thermal Barrier Topcoats: A Review. J. Therm. Spray Technol. 2024, 33, 447–470. [Google Scholar] [CrossRef]
- Zhang, P.; Peng, R.L.; Li, X.-H.; Johansson, S.; Xiao, P. Performance of MCrAlX Coatings Oxidation, Hot Corrosion and Interdiffusion; Linköping University, Institutionen för Ekonomisk och Industriell Utveckling, and Linköping University, Tekniska Fakulteten: Linköping, Sweden, 2019. [Google Scholar]
- Peng, R.L.; Zhang, P.; Hai, Z. Durable MCrAlX Coatings For Demanding Applications in Gasturbines KME 703. 2018. Available online: www.energiforsk.se (accessed on 16 February 2025).
- Ghadami, F.; Aghdam, A.S.R.; Ghadami, S. Microstructural Characteristics and Oxidation Behavior of the Modified MCrAlX Coatings: A Critical Review. Vacuum 2021, 185, 109980. [Google Scholar] [CrossRef]
- Zhang, P. Oxidation Behaviour of MCrAlX Coatings: Effect of Surface Treatment and an Al-Activity Based Life Criterion; Linköping University Electronic Press: Linköping, Sweden, 2018. [Google Scholar]
- Yu, J.; Mu, R.; Qu, S.; Cai, Y.; Shen, Z. The Effect of Heat Treatment at 1200 °C on Microstructure and Mechanical Properties Evolution of AlCoCrFeNiSiYHf High Entropy Alloys. Vacuum 2022, 200, 111039. [Google Scholar] [CrossRef]
- Srivastava, M.; Jadhav, M.S.; Chethan; Chakradhar, R.; Singh, S. Investigation of HVOF Sprayed Novel Al1.4Co2.1Cr0.7Ni2.45Si0.2Ti0.14 HEA Coating as Bond Coat Material in TBC System. J. Alloy. Compd. 2022, 924, 166388. [Google Scholar] [CrossRef]
- Lu, Y.P.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.J.; Wang, T.M.; Wen, B.; Wang, Z.J.; Jie, J.C.; Cao, Z.Q.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-Entropy Alloys by Mechanical Alloying: A Review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Zhang, Y.; Liu, S.-H.; Li, C.-J.; Li, C.-X. High-Temperature Oxidation Behavior of CuAlNiCrFe High-Entropy Alloy Bond Coats Deposited Using High-Speed Laser Cladding Process. Surf. Coatings Technol. 2020, 398, 126093. [Google Scholar] [CrossRef]
- Mooraj, S.; Chen, W. A Review on High-Throughput Development of High-Entropy Alloys by Combinatorial Methods. J. Mater. Inform. 2023, 3, 4. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, L.; Cui, Y.; Geng, K.; Manladan, S.M.; Luo, Z. High-Temperature Oxidation Behavior of FeCoCrNiAlx High-Entropy Alloy Coatings. Mater. Res. Express 2019, 6, 126552. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Butler, T.M. High Entropy Alloys: Oxidation. In Encyclopedia of Materials: Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2021; pp. 522–532. [Google Scholar]
- Murty, B.S. High-Entropy Alloys; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal Spray High-Entropy Alloy Coatings: A Review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Anupam, A.; Kumar, S.; Chavan, N.M.; Murty, B.S.; Kottada, R.S. First Report on Cold-Sprayed AlCoCrFeNi High-Entropy Alloy and Its Isothermal Oxidation. J. Mater. Res. 2019, 34, 796–806. [Google Scholar]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.; Mu, R.; Zhao, X.; Guo, F. Y/Hf-Doped AlCoCrFeNi High-Entropy Alloy with Ultra Oxidation and Spallation Resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Mehner, T.; Rymer, L.-M.; Björklund, S.; Joshi, S.; Lampke, T. Microstructure and Corrosion Properties of AlCrFeCoNi High-Entropy Alloy Coatings Prepared by HVAF and HVOF. J. Therm. Spray Technol. 2022, 31, 247–255. [Google Scholar]
- Ossiansson, M.; Gupta, M.; Löbel, M.; Lindner, T.; Lampke, T.; Joshi, S. Assessment of CrFeCoNi and AlCrFeCoNi High-Entropy Alloys as Bond Coats for Thermal Barrier Coatings. J. Therm. Spray Technol. 2022, 31, 1404–1422. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Li, L.; Huang, A.; Zhang, H.; Zhang, X.; Lu, J.; Zhao, X. On the Oxidation and Interdiffusion Behavior of an AlCoCrFeNiY High-Entropy Alloy Bond Coat on a Directionally Solidified Ni-Based Superalloy. J. Mater. Sci. Technol. 2023, 186, 64–78. [Google Scholar] [CrossRef]
- Shahbazi, H.; Lima, R.S.; Stoyanov, P.; Moreau, C. Enhancing the Optimized HEA Bond Coating in TBC Systems via HVAF Technique. In Proceedings of the Thermal Spray 2024: Proceedings from the International Thermal Spray Conference, Milan, Italy, 29 April–1 May 2024; ASM International: Almere, The Netherlands, 2024; pp. 594–610. [Google Scholar]
- Sigaroodi, M.R.J.; Poursaeidi, E.; Rahimi, J.; Jamalabad, Y.Y. Heat Treatment Effect on Coating Shock Resistance of Thermal Barrier Coating System with Different Types of Bond Coat. J. Eur. Ceram. Soc. 2023, 43, 3658–3675. [Google Scholar] [CrossRef]
- Jadhav, M.; Singh, S.; Srivastava, M.; Kumar, G.V. An Investigation on High Entropy Alloy for Bond Coat Application in Thermal Barrier Coating System. J. Alloys Compd. 2019, 783, 662–673. [Google Scholar]
- Manap, A.; Nakano, A.; Ogawa, K. The Protectiveness of Thermally Grown Oxides on Cold Sprayed CoNiCrAlY Bond Coat in Thermal Barrier Coating. J. Therm. Spray Technol. 2012, 21, 586–596. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO Growth Behaviour in TBCs with APS and HVOF Bond Coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar]
- Zhang, D.; Gong, S.; Xu, H.; Wu, Z. Effect of Bond Coat Surface Roughness on the Thermal Cyclic Behavior of Thermal Barrier Coatings. Surf. Coat. Technol. 2006, 201, 649–653. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, B.; Kim, H.; Lee, C. Isothermal Oxidation of Air Plasma Spray NiCrAlY Bond Coatings. Surf. Coat. Technol. 2002, 150, 297–308. [Google Scholar] [CrossRef]
- Shahbazi, H.; Heydarian, A. High Entropy Alloy Bond-Coat for TBCs. J. Therm. Spray Technol. 2023, 33, 430–446. [Google Scholar]
- Matikainen, V.; Koivuluoto, H.; Vuoristo, P.; Schubert, J.; Houdková, Š. Effect of Nozzle Geometry on the Microstructure and Properties of HVAF-Sprayed WC-10Co4Cr and Cr3C2-25NiCr Coatings. J. Therm. Spray Technol. 2018, 27, 680–694. [Google Scholar] [CrossRef]
- Torkashvand, K.; Gupta, M.; Björklund, S.; Marra, F.; Baiamonte, L.; Joshi, S. Influence of Nozzle Configuration and Particle Size on Characteristics and Sliding Wear Behaviour of HVAF-Sprayed WC-CoCr Coatings. Surf. Coat. Technol. 2021, 423, 127585. [Google Scholar]
- Diop, S.A.B.; Moreau, C.; Nasti, A.; Dolatabad, A. Oxidation of In-Flight Particles During HVAF—A Numerical Study. In Proceedings of the Thermal Spray 2023: Proceedings from the International Thermal Spray Conference, Québec City, QC, Canada, 22–25 May 2023; ASM International: Almere, The Netherlands, 2023; pp. 610–617. [Google Scholar]
- Lyphout, C.; Björklund, S. Internal Diameter HVAF Spraying for Wear and Corrosion Applications. J. Therm. Spray Technol. 2014, 24, 235–243. [Google Scholar]
- Asadiankouhidehkordi, G.; Liberati, A.C.; Ettouil, F.B.; Moreau, C. Inner Diameter High-Velocity Air Fuel (ID-HVAF) Spraying of Copper, Compared to Cold Spray. In Proceedings of the Thermal Spray 2023: Proceedings from the International Thermal Spray Conference, Québec City, QC, Canada, 22–25 May 2023; ASM International: Almere, The Netherlands, 2023; pp. 531–537. [Google Scholar]
- Khamsepour, P.; Oberste-Berghaus, J.; Aghasibeig, M.; Moreau, C.; Dolatabadi, A. The Characteristics of In-Flight Ti-6Al-4V Particles and the Coatings Formed by the Inner-Diameter High-Velocity Air-Fuel (ID-HVAF) Process. In Proceedings of the Thermal Spray 2022: Proceedings from the International Thermal Spray Conference, Vienna, Austria, 4–6 May 2022; DVS Media GmbH: Düsseldorf, Germany, 2022; pp. 164–171. [Google Scholar]
- Mauer, G.; Rauwald, K.-H.; Sohn, Y.J.; Vaßen, R. The Potential of High-Velocity Air-Fuel Spraying (HVAF) to Manufacture Bond Coats for Thermal Barrier Coating Systems. J. Therm. Spray Technol. 2023, 33, 746–755. [Google Scholar]
- Mahalingam, S.; Yunus, S.M.; Manap, A.; Afandi, N.M.; Zainuddin, R.A.; Kadir, N.F. Crack Propagation and Effect of Mixed Oxides on TGO Growth in Thick La–Gd–YSZ Thermal Barrier Coating. Coatings 2019, 9, 719. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, Y.; Zhao, X.; Guo, F.; Xiao, P. Effect of Microstructure of a NiCoCrAlY Coating Fabricated by High-Velocity Air Fuel on the Isothermal Oxidation. Corros. Sci. 2019, 159, 108126. [Google Scholar]
- Luo, L.; Shan, X.; Zou, Z.; Zhao, C.; Wang, X.; Zhang, A.; Zhao, X.; Guo, F.; Xiao, P. A High Performance NiCoCrAlY Bond Coat Manufactured Using Laser Powder Deposition. Corros. Sci. 2017, 126, 356–365. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. Mechanically Alloyed High Entropy Alloys: Existing Challenges and Opportunities. J. Mater. Res. Technol. 2022, 17, 2431–2456. [Google Scholar] [CrossRef]
- Nowak, W.; Naumenko, D.; Mor, G.; Mor, F.; Mack, D.; Vassen, R.; Singheiser, L.; Quadakkers, W. Effect of Processing Parameters on MCrAlY Bondcoat Roughness and Lifetime of APS–TBC Systems. Surf. Coat. Technol. 2014, 260, 82–89. [Google Scholar] [CrossRef]
- Gupta, M.; Markocsan, N.; Li, X.-H.; Peng, R.L. Improving the Lifetime of Suspension Plasma Sprayed Thermal Barrier Coatings. Surf. Coat. Technol. 2017, 332, 550–559. [Google Scholar] [CrossRef]
- Hsu, W.L.; Yang, Y.C.; Chen, C.Y.; Yeh, J.W. Thermal Sprayed High-Entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 Coating with Improved Mechanical Properties and Oxidation Resistance. Intermetallics 2017, 89, 105–110. [Google Scholar]
- Hsu, W.L.; Murakami, H.; Yeh, J.W.; Yeh, A.C.; Shimoda, K. On the Study of Thermal-Sprayed Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 HEA Overlay Coating. Surf. Coat. Technol. 2017, 316, 71–74. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Qiao, D.; Man, J.; Yan, W.; Xue, B.; Bian, X.; Zeng, W. Comprehensive Understanding of the Effect of TGO Growth Modes on Thermal Barrier Coating Failure Based on a Simulation. Materials 2024, 17, 180. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, T.; Wang, Z.; Yu, J.; Guo, W.; Yang, Y. Effect of Thermal Growth Oxide Composition and Morphology on Local Stresses in Thermal Barrier Coatings. Materials 2022, 15, 8442. [Google Scholar] [CrossRef]
- Takahashi, R.J.; Assis, J.M.K.; Neto, F.P.; Reis, D.A.P. Heat Treatment for TGO Growth on NiCrAlY for TBC Application. Mater. Res. Express 2019, 6, 126442. [Google Scholar] [CrossRef]

| Parameters | Value |
|---|---|
| Fuel pressure (bar) | 7.72 |
| Air pressure (bar) (P1/P2) | 8.96 |
| Carrier gas flow rate (L/min) | 25 |
| Powder feed rate (g/min) | 120 |
| No. of passes | 5 |
| Preheat (passes) | 5 |
| Torch traverse speed (mm/s) | 1000 |
| Spray distance (mm) | 50.8 |
| Substrate temperature (FLIR systems) | 155 °C |
| Peak (hkl) | FWHM (Powder) (°) | FWHM (HVAF-i7 Coating) (°) | Difference (°) |
|---|---|---|---|
| (110) | 0.46124 | 0.46131 | 0.00007 |
| (200) | 1.123 | 1.123 | 0.00000 |
| (211) | 0.882 | 0.896 | 0.01400 |
| (220) | 1.684 | 1.703 | 0.01900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbazi, H.; Lima, R.S.; Stoyanov, P.; Moreau, C. Effect of Microstructure on Oxidation Resistance and TGO Formation in FeCoNiCrAl HEA Coatings Deposited by Low-Temperature HVAF Spraying. Materials 2025, 18, 1569. https://doi.org/10.3390/ma18071569
Shahbazi H, Lima RS, Stoyanov P, Moreau C. Effect of Microstructure on Oxidation Resistance and TGO Formation in FeCoNiCrAl HEA Coatings Deposited by Low-Temperature HVAF Spraying. Materials. 2025; 18(7):1569. https://doi.org/10.3390/ma18071569
Chicago/Turabian StyleShahbazi, Hossein, Rogerio S. Lima, Pantcho Stoyanov, and Christian Moreau. 2025. "Effect of Microstructure on Oxidation Resistance and TGO Formation in FeCoNiCrAl HEA Coatings Deposited by Low-Temperature HVAF Spraying" Materials 18, no. 7: 1569. https://doi.org/10.3390/ma18071569
APA StyleShahbazi, H., Lima, R. S., Stoyanov, P., & Moreau, C. (2025). Effect of Microstructure on Oxidation Resistance and TGO Formation in FeCoNiCrAl HEA Coatings Deposited by Low-Temperature HVAF Spraying. Materials, 18(7), 1569. https://doi.org/10.3390/ma18071569









